Introduction
Tumors are associated with local bleeding which
involves the activation of platelets during tumor development.
Lysophospholipids are released from the activated platelets and
subsequently converted to lysophosphatidic acid (LPA) by
lysophospholipase (1). Therefore,
LPA is considered to be highly expressed in tumors and regulate
various tumorigenic processes, such as metastasis. LPA has been
shown to induce diverse biological changes, including in
Ca2+ mobilization, cAMP accumulation, cell shape,
motility and proliferation in a variety of cell types (2–4).
Extracellular LPA has also been observed to be involved in certain
diseases (5–8) and have a positive role in the
progression of ovarian, breast, colon and gastric cancer (9–11).
These cellular responses to LPA are mediated by G protein-coupled
receptors, i.e., several subtypes of LPA receptors (LPARs). At
present, LPA1-6 receptors have been identified (3,4,12–17),
among which LPA1–3 are members of the endothelial differentiation
gene (Edg) family. LPA1–3 receptors have been investigated in the
progression of gastric cancer (18,19).
Immunohistochemical analysis of LPAR2 has shown that LPAR2
expression is a significant process in gastric cancer progression
(20), although the mechanism of
LPA-induced gastric cancer cell migration is not fully understood.
The present study reports that LPA stimulates the migration of
human gastric cancer cells (SGC-7901) and the LPAR2/Gq/11/p38
pathway regulates this migration.
Materials and methods
Cell culture and reagents
The human gastric cancer cell line SGC-7901 was
provided by Institute of Zoology of China (Beijing, China). Human
aortic smooth muscle cells (AoSMCs) were obtained from ATCC
(Manassas, VA, USA). The cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA) which was
supplemented with 10% (v/v) fetal bovine serum (Gibco) at 37°C in a
humidified atmosphere containing 5% CO2.
1-Oleoyl-sn-glycero-3-phosphate (LPA), fatty acid-free BSA and PTX
were obtained from Sigma (St. Louis, MO, USA). The p-p38 and p38
antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA) and Ki-16425 and YM-254890 were provided by Fumikazu
Okajima (Gunma University, Maebashi, Japan) as gifts.
Cell migration assays
Cell migration was measured using 24-well Transwell
plates (Corning, Tewksbury, MA, USA), with 8 μm-pore
polycarbonate membranes. The Transwell plates were coated with 1%
gelatin and the serum-free DMEM supplemented with LPA and 0.1%
fatty acid-free BSA in the lower chamber was used as a
lysophospholipid carrier. Cells (2×105/ml) suspended in
serum-free DMEM containing 0.1% fatty acid-free BSA were added to
the upper chamber and incubated for 12 h at 37°C. When the effects
of the LPA antagonists were examined, the cells were preincubated
for 10 min with antagonists before being loaded. Unmigrated cells
were removed from the top filter surface with a cotton swab and
fixed with 100% methanol for 10 min. Migrated cells were observed
to attach to the underside of the transwell plates and counted
under a light microscope using a ×200 objective after stainning
with 0.2% crystal violet. The experiments were repeated more than
three times for each condition and for each experiment, five random
fields were counted.
RNA interference
Cells (3×105) were incubated in a
six-well plate overnight. Transient shRNA transfection was
performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s instructions. Predesigned vectors
expressing control shRNA- or LPAR2-specific shRNA were purchased
from Inovogen (Inovogen, Beijing, China). The shRNA oligonucleotide
sequence of LPAR2 was 5′-AGTACTTCCTACTGTTGGC-3′. The transfected
cell clones were designated SGC-7901/shLPAR2 and
SGC-7901/shRNA-control and the LPAR2 expression was detected by
quantitative real-time PCR (RT-PCR) in these transfected cell
clones.
Quantitative RT-PCR
Total RNA was isolated with a total RNA isolation
kit (Bio Basic Inc., Markham, ON, Canada) according to the
manufacturer’s instructions. After DNase I (MBI Fermentas, Amherst,
NY, USA) treatment to remove possible traces of genomic DNA in the
RNA preparations, 5 μg total RNA was used in
reverse-transcription with the AMV First Strand cDNA Synthesis kit
(Bio Basic Inc.). The primers used in the reaction were: LPAR1
forward, 5′-TCCTGTCCCGCGCCAGGTACAC-3′; LPAR1 reverse,
5′-GGTGGTGAACACGCCCCAGAACT-3′; LPAR2 forward,
5′-ACCGCAGTGTGATGGCCGTG-3′; LPAR2 reverse,
5′-TAGGAGCGGCTGAGCAGGGG-3′; LPAR3 forward,
5′-GCCGTGGAGAGGCACATGTC-3′; LPAR3 reverse,
5′-TGGCGATGGCCCAGACAAGC-3′; GAPDH forward,
5′-TCAAGTGGGGCGATGCTGGC-3′; GAPDH reverse,
5′-TGGGGGCATCAGCAGAGGGG-3′. Quantitative RT-PCR was performed using
Hot Start Fluorescent PCR Core Reagent kits (Bio Basic Inc.). The
cycling conditions were: 94°C for 4 min, then 35 cycles at 94°C for
30 sec and 60°C for 30 sec. The mRNA level of the genes of interest
of each sample was normalized to that of the GAPDH mRNA and
presented as unit values of 2[Ct(GAPDH) - Ct(target
gene)]. Quantitative RT-PCR was performed in a Chromo4
detector (BioRad, Hercules, CA, USA).
Western blotting
For the western blotting of p38, the cells were
washed twice with ice-cold PBS and harvested from the dishes with a
cell scraper by adding a WIP lysis buffer. The recovered lysate was
incubated for 30 min on ice and centrifuged at 14,000 × g for 20
min to remove cell debris. The cell lysate was then subjected to
gel electrophoresis for western blotting of the phosphorylated p38
and total p38.
Statistical analysis
The Student’s t-test and one-way ANOVA using
Graphpad Instat 5 software were used for the statistical analyses.
P<0.05 was considered to indicate statistically significant
differences.
Results
LPA-induces SGC-7901 cell migration is
mediated by Gq-coupled receptor
LPA is a bioactive lysophospholipid that is known to
induce diverse cellular responses by LPA G protein-coupled
receptors (2). To detect the role
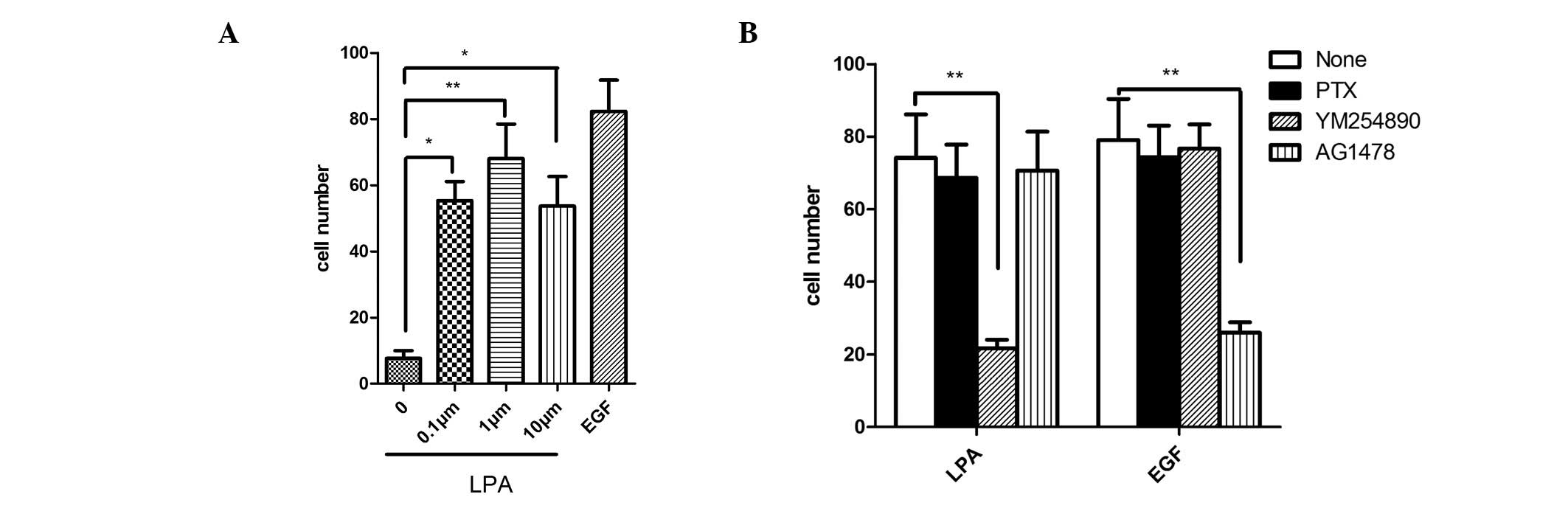
of LPA in cell migration, SGC-7901 cells were stimulated with LPA
at various concentrations (0.1, 1 and 10 μM). LPA was
observed to significantly increase cell migration and 1 μM
LPA was the most effective concentration (Fig. 1A). To identify which G protein is
involved in LPA-induced SGC-7901 cell migration, YM-254890, a
specific Gq protein inhibitor, was used in the cell migration
experiment. It was observed that YM-254890 markedly reduced the
LPA-induced cell migration. However, pertussis toxin (PTX) which
inhibits Gi protein activity and AG1487, a specific inhibitor of
epidermal growth factor (EGF) receptor, did not exhibit any effects
on the LPA-induced cell migration. As shown in Fig. 1B, these G protein inhibitors did not
affect EGF-induced cell migration, although AG1487 decreased the
migration. The results indicate that Gq appears to be involved in
LPA-induced cell migration but not by the EGF transactivation
pathway (Fig. 1B).
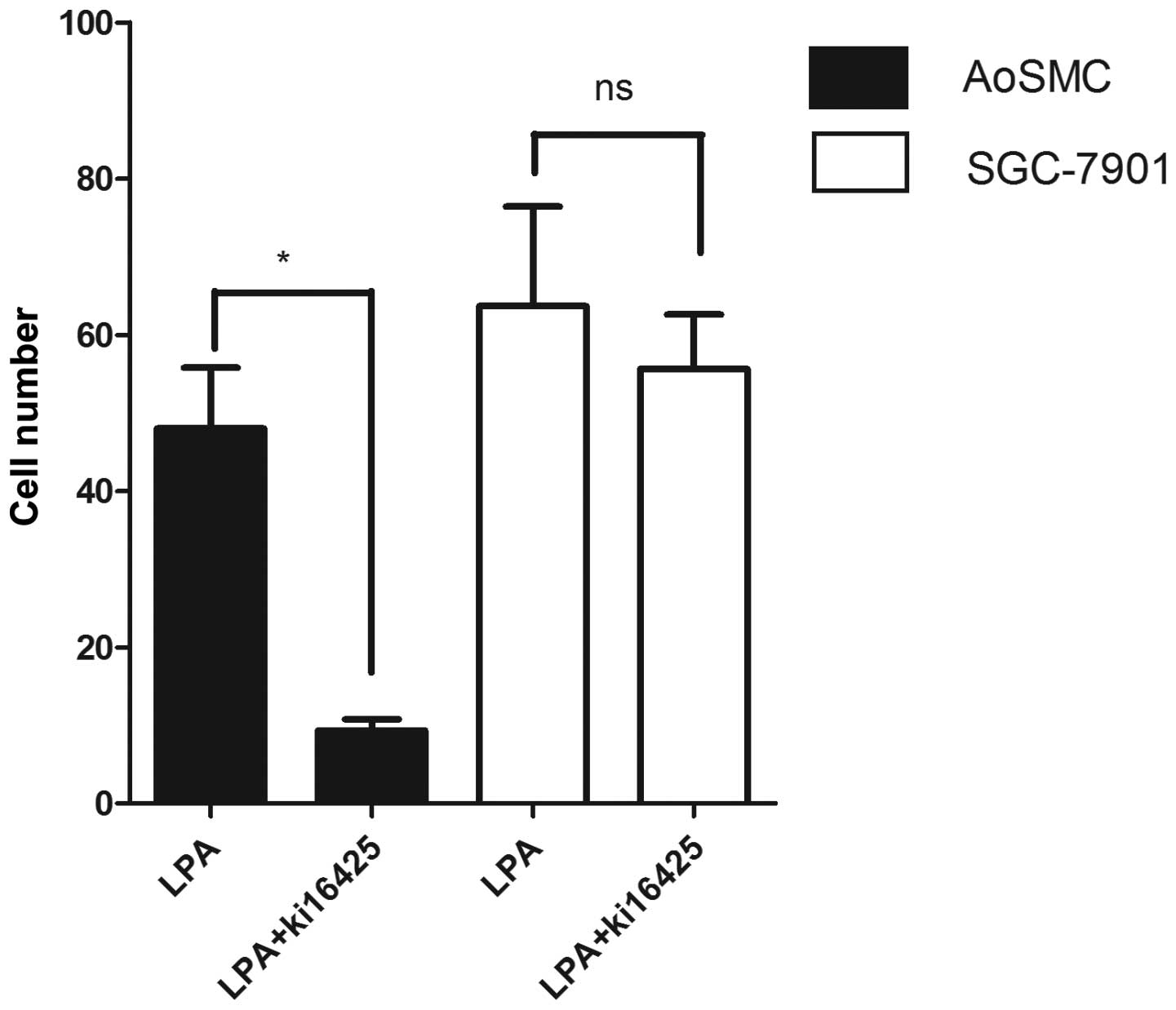
Antagonist for LPARs 1 and 3, Ki-16425,
does not affect the migration of SGC-7901 cells
To understand the signaling pathways stimulated by
LPA that lead to SGC-7901 cell migration, the role of Ki-16425 in
cell migration was investigated. Ki-16425 is known to act as an
antagonist of LPAR1 and LPAR3 (21). As shown in Fig. 2, the LPA-induced migration of the
AoSMCs in which LPAR1 is highly expressed (22) was reduced to the level of the
control in the presence of Ki-16425. However, Ki-16425 did not
suppress the LPA-induced migration of SGC-7901 cells. These results
suggest that the LPA-induced migration may not depend on LPAR1 and
LPAR3.
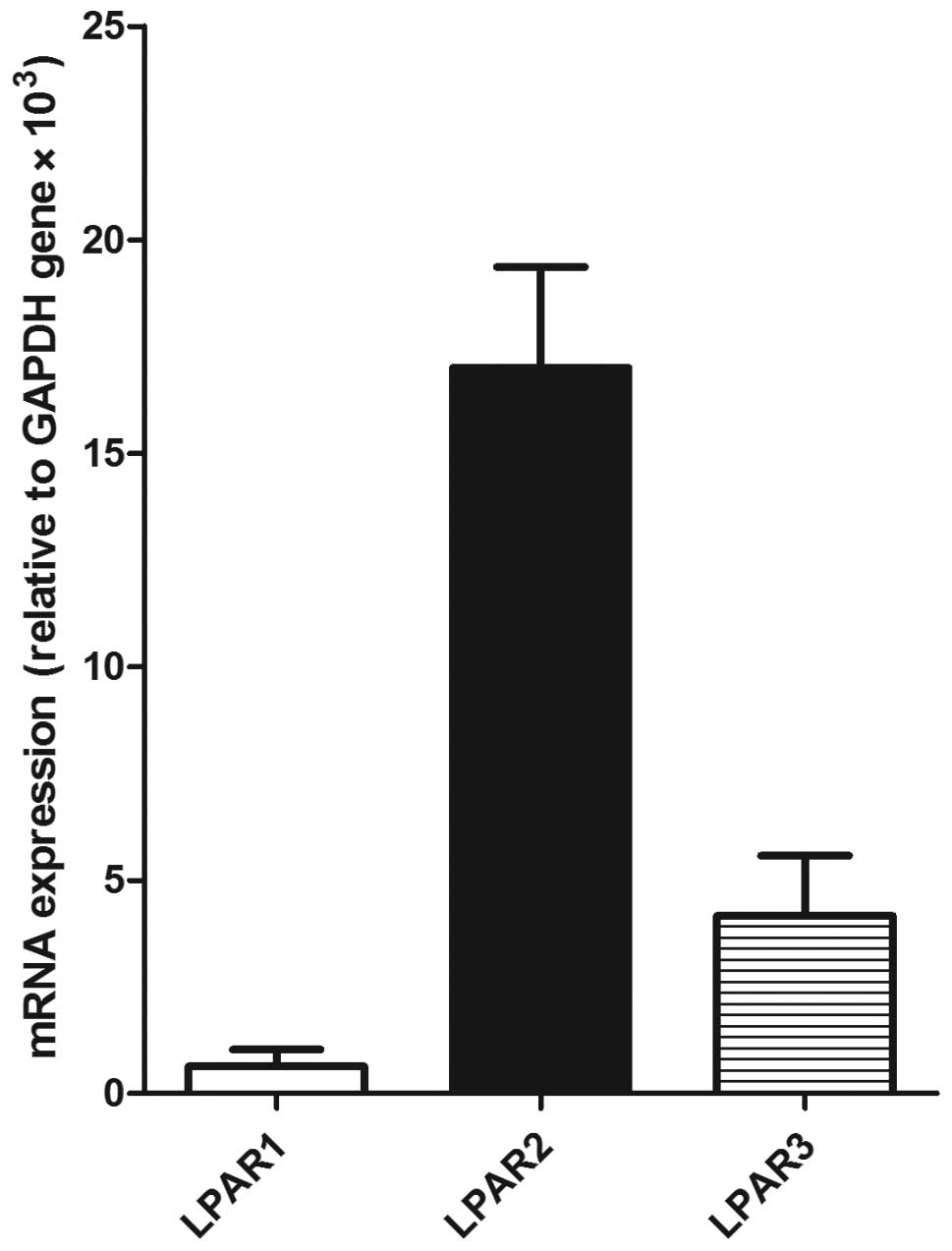
LPAR2 is highly expressed in SGC-7901
cells
To evaluate the expression of LPARs 1-3 in the
SGC-7901 cells, RT-PCR analysis was performed. LPAR2 was shown to
be highly expressed in the SGC-7901 cells and it was 27- and 4-fold
that of LPAR1 and LPAR3, respectively (Fig. 3). This result suggests that the
LPA-induced migration of SGC-7901 cells may be dependent on
LPAR2.
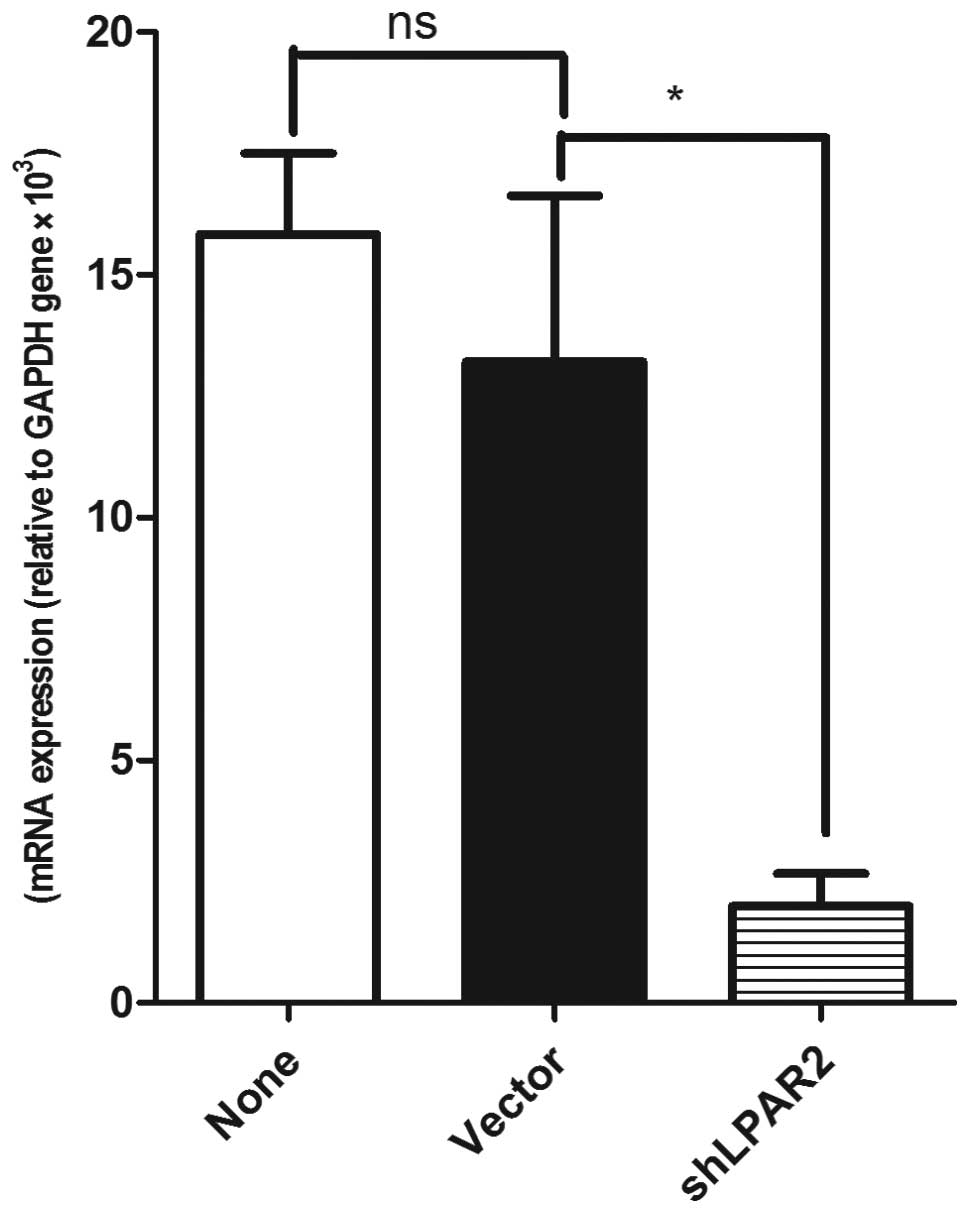
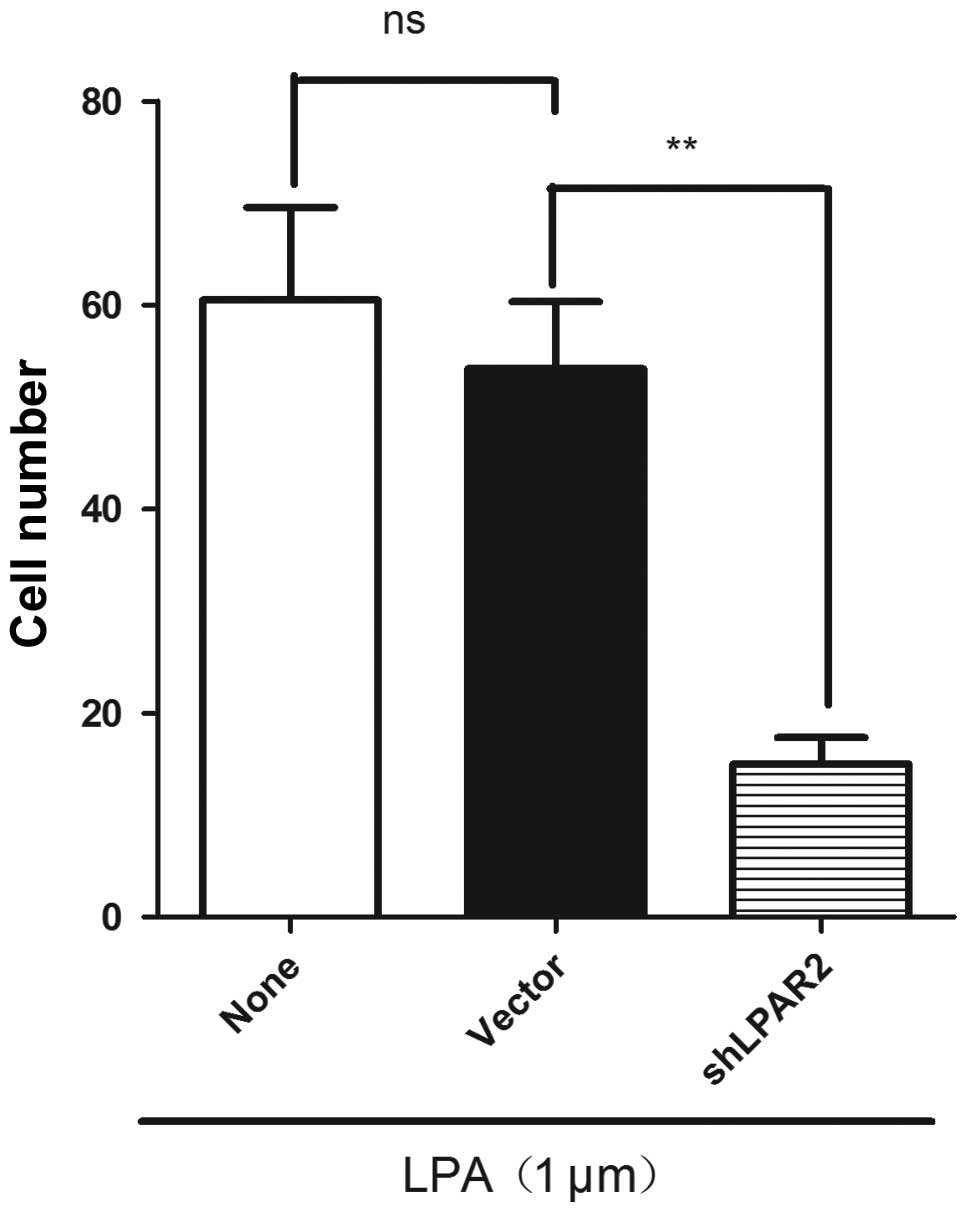
Silencing LPAR2 expression by shRNA
inhibits the LPA-induced cell migration of SGC-7901
As mentioned, LPAR2 was highly expressed in the
SGC-7901 cells, suggesting that LPAR2 may be important in
LPA-induced cell migration. To investigate the role of LPAR2 in the
LPA-induced cell migration, LPAR2 expression was silenced by
LPAR2-specific shRNAs in the SGC-7901 cells. RT-PCR analysis showed
that LPAR2 expression was decreased by 87.4% compared with the
control (Fig. 4). Migration
experiments showed that silencing LPAR2 expression significantly
decreased the LPA-induced migration of SGC-7901 cells compared with
the control SGC-7901 cells (Fig.
5). The results demonstrate that the LPA-induced migration of
SGC-7901 gastric cancer cells requires LPAR2.
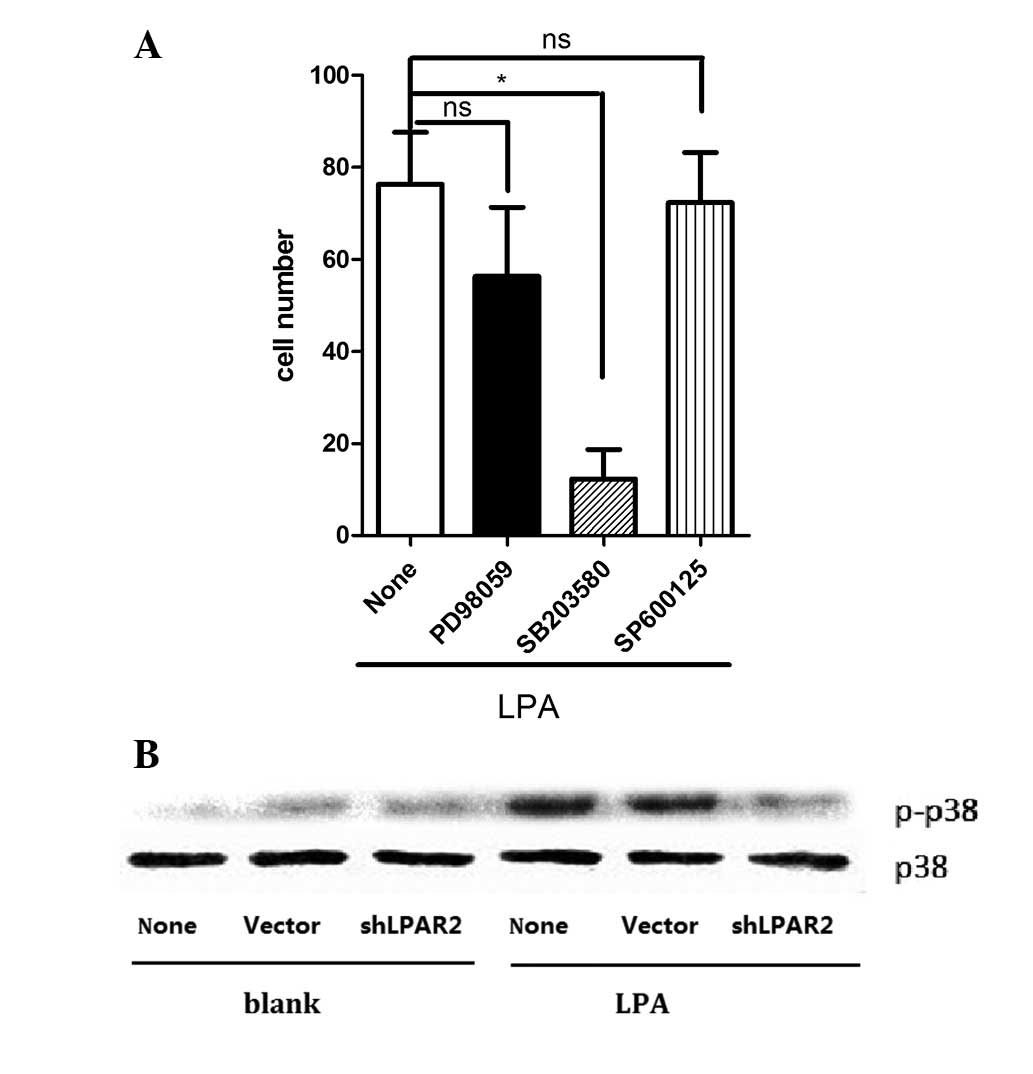
Phosphorylation of p38 is required for
the LPA-induced migration of SGC-7901 cells
To identify the mechanisms involved in LPA-induced
cell migration, the effects of specific inhibitors for various
kinases on cell migration were investigated. The presence of
SB203580, a p38 MAPK inhibitor, was observed to significantly
decrease the LPA-induced migration of SGC-7901 cells. However,
neither PD98059 (an inhibitor of ERK kinase) or SP600125 (a
JNK-MAPK inhibitor) affected LPA-induced cell migration (Fig. 6A). As shown in Fig. 6A, LPA activated the phosphorylation
of p38 MAPK and the p38 MAPK phosphorylation was 4-5-fold that of
control. The LPA-induced phosphorylation of p38 MAPK was attenuated
by pre-transfection with shLPAR2 (Fig.
6B).
Discussion
The enhanced migration observed in tumor cells is
often caused by external stimuli and the sequential participation
of cytoskeleton-related signaling molecules. However, the mechanism
between the LPAR and G protein subtypes has not been analyzed in
detail for LPA-induced migration in tumor cells. In the present
study, the potential role of LPAR2 in gastric cancer SGC-7901 cell
migration was evaluated. A previous study indicated that LPAR2 was
correlated with a higher rate of lymphatic and venous invasion,
lymphatic metastasis and the resulting tumor stage in diffuse-type
gastric cancer (20). As
chemotherapy supersedes radiation therapy as the standard
therapeutic approach for advanced gastric cancer, the search for
specific, effective and less toxic therapeutics becomes more
critical (23). The present study
identified LPAR2 as a potential new target. LPAR expression was
previously unknown in SGC-7901 cells and the present study
demonstrated that among the three principle LPARs, LPAR2 is
predominantly expressed by SGC-7901 cells. The effect of LPA on
gastric cancer migration with and without LPAR2 knockdown was then
evaluated and the effect of LPA on migration was shown to be
blocked in shLPAR2-transfected SGC-7901 cells. The inhibition of Gq
by a specific Gq protein inhibitor also reduced SGC-7901 migration
induced by LPA, indicating that Gq is responsible for LPA’s effect.
Moreover, LPA induced p38 MAPK activation in SGC-7901 cells, while
LPAR2 silencing reduced the effect of LPA.
The present study contributes to the use of specific
shLPAR2 to identify the mechanisms and functions of LPAR2 and to
investigate LPA’s regulatory effect on the gastric tumor
microenvironment. The effects of LPA are mediated by the activation
of the main three known LPARs and subsequent intracellular signal
transduction. LPAR1, LPAR2 and LPAR3 belong to the endothelial
differentiation gene family of G protein-coupled receptors
(24). Through these receptors, LPA
is implicated in numerous cellular processes, including cell
proliferation and migration (25–27).
The LPARs’ individual signaling pathways have yet to
be fully elucidated. LPAR2, in particular, has been shown to be
upregulated in a variety of cancer types, including colon, gastric,
ovary and endometrial cancer (20,28–30).
LPAR2 is implicated in numerous oncogenic pathways and has been
shown to transduce growth promoting signals in the LPA-rich
environments characteristic of aggressive cancers (28). LPAR2 shares high homology in amino
acid sequence with LPAR1 and LPAR3, with the exception of its
carboxyl terminal region (31).
This observation suggests that the cytoplasmic tails of the LPARs
may specify their individual LPA signaling function. LPAR2 has been
linked to specific receptor-interacting proteins such as TRIP-6,
through which it induces ovarian cancer cell migration (31). LPAR2 has also been shown to increase
LPA-mediated IL-6 and IL-8 production more efficiently than either
LPAR1 or LPAR3 (32). Notably, the
expression of the LPAR1 gene was observed to be significantly
increased in atherosclerotic plaques in an atherosclerosis animal
model. This finding demonstrates that the LPA-induced migration and
proliferation of vascular smooth muscle cells are mediated by LPAR1
(unpublished study). However, LPAR2 has a similar role in gastric
cancer cells, indicating that LPARs may have a cell specificity in
their distribution and function.
In conclusion, LPAR2 is markedly expressed in
SGC-7901 cells and, as a promising biomarker of gastric cancer, is
critical in gastric cancer cell migration (invasion) in LPA-rich
micro-environments. The pro-migratory effect of LPA is mediated by
LPAR2 coupling to Gq and the p38 activation cascade in SGC-7901
cells. The present findings suggest that LPAR2 may be a potential
target for the clinical treatment of gastric cancer. LPAR2
antagonists and inhibitors of its signaling pathway are potential
drugs for this purpose.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (31160184,
30760054).
References
|
1
|
Aoki J, Taira A, Takanezawa Y, et al:
Serum lysophosphatidic acid is produced through diverse
phospholipase pathways. J Biol Chem. 277:48737–48744. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Contos JJ, Ishii I and Chun J:
Lysophosphatidic acid receptors. Mol Pharmacol. 58:1188–1196.
2000.PubMed/NCBI
|
|
3
|
Moolenaar WH: Bioactive lysophospholipids
and their G protein-coupled receptors. Exp Cell Res. 253:230–238.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye X, Ishii I, Kingsbury MA and Chun J:
Lysophosphatidic acid as a novel cell survival/apoptotic factor.
Biochim Biophys Acta. 1585:108–113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Y, Gaudette DC, Boynton JD, et al:
Characterization of an ovarian cancer activating factor in ascites
from ovarian cancer patients. Clin Cancer Res. 1:1223–1232.
1995.PubMed/NCBI
|
|
6
|
Siess W, Zangl KJ, Essler M, et al:
Lysophosphatidic acid mediates the rapid activation of platelets
and endothelial cells by mildly oxidized low density lipoprotein
and accumulates in human atherosclerotic lesions. Proc Natl Acad
Sci USA. 96:6931–6936. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maschberger P, Bauer M, Baumann-Siemons J,
et al: Mildly oxidized low density lipoprotein rapidly stimulates
via activation of the lysophosphatidic acid receptor Src family and
Syk tyrosine kinases and Ca2+ influx in human platelets.
J Biol Chem. 275:19159–19166. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y, Xiao YJ, Baudhuin LM and Schwartz
BM: The role and clinical applications of bioactive lysolipids in
ovarian cancer. J Soc Gynecol Investig. 8:1–13. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Ye X, Mahanivong C, Bian D, Chun J
and Huang S: Signaling mechanisms responsible for lysophosphatidic
acid-induced urokinase plasminogen activator expression in ovarian
cancer cells. J Biol Chem. 280:10564–10571. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smicun Y, Gil O, Devine K and Fishman DA:
S1P and LPA have an attachment-dependent regulatory effect on
invasion of epithelial ovarian cancer cells. Gynecol Oncol.
107:298–309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Symowicz J, Adley BP, Woo MM, Auersperg N,
Hudson LG and Stack MS: Cyclooxygenase-2 functions as a downstream
mediator of lysophosphatidic acid to promote aggressive behavior in
ovarian carcinoma cells. Cancer Res. 65:2234–2242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Shen Z, Wiper DW, et al:
Lysophosphatidic acid as a potential biomarker for ovarian and
other gynecologic cancers. JAMA. 280:719–723. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
An S, Bleu T, Zheng Y and Goetzl EJ:
Recombinant human G protein-coupled lysophosphatidic acid receptors
mediate intracellular calcium mobilization. Mol Pharmacol.
54:881–888. 1998.PubMed/NCBI
|
|
14
|
Bandoh K, Aoki J, Hosono H, et al:
Molecular cloning and characterization of a novel human
G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol
Chem. 274:27776–27785. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hecht JH, Weiner JA, Post SR and Chun J:
Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid
receptor expressed in neurogenic regions of the developing cerebral
cortex. J Cell Biol. 135:1071–1083. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Im DS, Heise CE, Harding MA, et al:
Molecular cloning and characterization of a lysophosphatidic acid
receptor, Edg-7, expressed in prostate. Mol Pharmacol. 57:753–759.
2000.PubMed/NCBI
|
|
17
|
Noguchi K, Ishii S and Shimizu T:
Identification of p2y9/GPR23 as a novel G protein-coupled receptor
for lysophosphatidic acid, structurally distant from the Edg
family. J Biol Chem. 278:25600–25606. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Yu CP, Xia JT, et al: Sphingosine
kinase 1 is associated with gastric cancer progression and poor
survival of patients. Clin Cancer Res. 15:1393–1399. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shida D, Kitayama J, Yamaguchi H, et al:
Lysophosphatidic acid (LPA) enhances the metastatic potential of
human colon carcinoma DLD1 cells through LPA1. Cancer Res.
63:1706–1711. 2003.PubMed/NCBI
|
|
20
|
Yamashita H, Kitayama J, Shida D, et al:
Differential expression of lysophosphatidic acid receptor-2 in
intestinal and diffuse type gastric cancer. J Surg Oncol. 93:30–35.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohta H, Sato K, Murata N, et al: Ki16425,
a subtype-selective antagonist for EDG-family lysophosphatidic acid
receptors. Mol Pharmacol. 64:994–1005. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Damirin A, Tomura H, Komachi M, et al:
Role of lipo-protein-associated lysophospholipids in migratory
activity of coronary artery smooth muscle cells. Am J Physiol Heart
Circ Physiol. 292:H2513–H2522. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gibbs JB: Mechanism-based target
identification and drug discovery in cancer research. Science.
287:1969–1973. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi JW, Herr DR, Noguchi K, et al: LPA
receptors: subtypes and biological actions. Annu Rev Pharmacol
Toxicol. 50:157–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murray D, Horgan G, Macmathuna P and Doran
P: NET1-mediated RhoA activation facilitates lysophosphatidic
acid-induced cell migration and invasion in gastric cancer. Br J
Cancer. 99:1322–1329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramachandran S, Shida D, Nagahashi M, et
al: Lysophosphatidic acid stimulates gastric cancer cell
proliferation via ERK1-dependent upregulation of sphingosine kinase
1 transcription. FEBS Lett. 584:4077–4082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang R, Wang J, Ma S, Huang Z and Zhang
G: Requirement of Osteopontin in the migration and protection
against Taxol-induced apoptosis via the ATX-LPA axis in SGC7901
cells. BMC Cell Biol. 12:112011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goetzl EJ, Dolezalova H, Kong Y, et al:
Distinctive expression and functions of the type 4 endothelial
differentiation gene-encoded G protein-coupled receptor for
lysophosphatidic acid in ovarian cancer. Cancer Res. 59:5370–5375.
1999.PubMed/NCBI
|
|
29
|
Shida D, Watanabe T, Aoki J, et al:
Aberrant expression of lysophosphatidic acid (LPA) receptors in
human colorectal cancer. Lab Invest. 84:1352–1362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hope JM, Wang FQ, Whyte JS, et al: LPA
receptor 2 mediates LPA-induced endometrial cancer invasion.
Gynecol Oncol. 112:215–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu J, Lai YJ, Lin WC and Lin FT: TRIP6
enhances lysophosphatidic acid-induced cell migration by
interacting with the lysophosphatidic acid 2 receptor. J Biol Chem.
279:10459–10468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang X, Yu S, Bast RC, et al: Mechanisms
for lysophosphatidic acid-induced cytokine production in ovarian
cancer cells. J Biol Chem. 279:9653–9661. 2004. View Article : Google Scholar : PubMed/NCBI
|