Introduction
Epithelial-mesenchymal transition (EMT) is a
multistep biological process that enables a normal epithelial cell
to possess a mesenchymal phenotype (1). In cancer biology, EMT has received
considerable attention since a number of studies have recognized
EMT as a hallmark of cancer stemness as well as aggressiveness
(2). Alterations in cell behavior
caused by EMT, including potentiated migration and increased
resistance to apoptosis, have been demonstrated in previous studies
(1,2). EMT enables cells to escape
interactions and the spatial restrictions imposed by the basement
membrane and sustains the viability of the cells when in a detached
condition (3,4). Therefore, the transition was
previously hypothesized to be associated with the metastatic
potential of cancer cells (2,4).
Anoikis is a process of cell death which is induced in response to
the detachment of the cells from cell-cell and cell-basement
interactions. A number of studies have demonstrated that anoikis is
a critical process in the inhibition of cancer metastasis in
various solid tumors (5,6). In addition, EMT has been demonstrated
to be involved in anoikis resistance in melanoma and colon cancer
cells (7,8). Downregulation of E-cadherin, together
with upregulation of N-cadherin is known to be a key indicator of
the EMT process and these proteins are also associated with
acquisition of anoikis resistance (2,4,9,10).
Studies on EMT, as well as its association with
anoikis resistance in cancer cells originating from Thai
individuals, remain limited. In addition, Thailand has a high
incidence of lung cancer-related mortalities, the majority of which
are associated with cancer metastasis (11). In depth understanding of cancer cell
properties is likely to lead to improved precision and efficiency
in treating the disease. Therefore, the present study aimed to
investigate the expression of the EMT-related markers, E-cadherin
and N-cadherin, in cancer cells from a Thai patient. In addition,
the correlation of expression levels with anoikis and metastatic
characteristics was investigated and compared with those of
standard lung cancer cells. These results may improve the
development of therapeutic approaches.
Materials and methods
Clinical specimen and reagents
Pleural effusions were collected from a 76-year-old
male Thai patient with lung adenocarcinoma. Informed consent was
obtained from the patient and the study was approved by the ethics
committee of the Faculty of Medicine and the ethics committee of
the Faculty of Pharmaceutical Sciences (Chulalongkorn University,
Bangkok, Thailand). Human non-small cell lung cancer cells, A549,
H23 and H460, were obtained from the American Type Culture
Collection (Manassas, VA, USA). H23 and H460 cells were cultured in
RPMI-1640 medium while A549 cells were cultured in DMEM,
supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine
and 100 U/ml penicillin/streptomycin in a 5% CO2
environment at 37°C. Propidium iodide (PI) and Hoechst 33342 were
obtained from Sigma-Aldrich (St. Louis, MO, USA). Resazurin-based
cell viability reagent (Presto blue) was purchased from Invitrogen
Life Technologies (Carlsbad, CA, USA). Specific antibodies against
E-cadherin and N-cadherin were obtained from Cell Signaling
technology (Danvers, MA, USA) and β-actin antibody was obtained
from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Specimen preparation
Pleural effusion was centrifuged at 1,600 × g for 10
min at room temperature. The pellet was resuspended with 4 ml
sterile balanced salt solution and then centrifuged on a Ficoll
gradient (Ficoll-Paque™, GE Healthcare Biosciences, Pittsburgh, PA,
USA) at 400 × g for 40 min at 20°C to separate tumor cells from
erythrocytes. The layer of mononuclear cells was collected and
washed twice with 3 volumes of RPMI medium by centrifuging at 400 ×
g for 10 min at 20°C. The pellet was then resuspended and the cells
were cultured in ACL-4 medium supplemented with 5% FBS at 37°C with
5% CO2.
Anoikis assay
For anoikis evaluation, 6-well tissue culture plates
were coated with 200 μl poly 2-hydroxyethylmethacrylate
(poly-HEMA; Sigma-Aldrich) and left for 10 h in a laminar flow
hood. Cells were seeded in poly-HEMA-coated plates
(1×105 cells/ml) and incubated for various times up to
24 h at 37°C. Cell viability was assessed by the addition of 1:50
resazurin for 1 h at 37°C. Fluorescence intensity of resazurin
product (resorufin) was measured at 530 nm (excitation wavelength)
and 590 nm (emission wavelength) using a microplate reader. Cell
viability was calculated as a percentage relative to time zero. All
analyses were performed in at least three independent replicate
experiments. Apoptosis was determined by Hoechst 33342 DNA
fragmentation assay. Briefly, cells were incubated with 10
μg/ml Hoechst 33342 for 30 min and analyzed for apoptosis by
scoring the percentage of cells with condensed chromatin and/or
fragmented nuclei by fluorescence microscopy (Olympus IX51 with
DP70, Olympus, Center Valley, PA, USA).
Matrigel invasion assay
The invasion assay was performed using Transwell
cell culture chambers (Corning Costar No. 3422; Corning, Tewksbury,
MA, USA) according to the manufacturer’s instructions with specific
modifications. Briefly, polyvinylpyrrolidone-free polycarbonate
filters (8.0-mm pore size, Nuclepore Corp., Pleasanton, CA, USA)
were pre-coated with 15 μl ice-cold Matrigel (BD
Biosciences, Bedford, MA, USA) on the upper surface for 60 min at
room temperature. Conditioned medium (500 μl medium with 10%
FBS) was added to the lower compartment of the chamber. P1, A549,
H23 and H460 cells (5×105) in 1% FBS-containing media
were added to the upper compartment of the chamber. Following 48-h
incubation, the top side of the insert membrane was scrubbed free
of cells with a cotton swab and the bottom side was fixed with
ice-cold methanol and stained with Hoechst 33342. Images were
captured and scored under a fluorescence microscope (Olympus IX51
with DP70).
Migration assay
The invasion assay was performed using Transwell
cell culture chambers. Conditioned medium (500 μl media with
10% FBS) was added to the lower compartment of the chamber. P1,
A549, H23 and H460 cells (5×105) in 1% FBS-containing
media were added to the upper compartment of the chamber. Following
12-h incubation, the top side of the insert membrane was scrubbed
with a cotton swab and the bottom side was fixed with ice-cold
methanol, stained with Hoechst 33342 and scored under a
fluorescence microscope (Olympus IX51 with DP70).
Western blot analysis
Following specific treatments, cells were incubated
in lysis buffer containing 20 mM Tris-HCl (pH 7.5), 1% Triton
X-100, 150 mM sodium chloride, 10% glycerol, 1 mM sodium
orthovanadate, 50 mM sodium fluoride, 100 mM phenylmethylsulfonyl
fluoride and a commercial protease inhibitor cocktail (Roche
Diagnostics, Basel, Switzerland) for 30 min on ice. Cell lysates
were collected and determined for protein content using the
Bradford method (Bio-Rad, Hercules, CA, USA). Equal amount of
proteins of each sample (40 μg) were denatured by heating at
95°C for 5 min with Laemmli loading buffer and subsequently loaded
onto a 10% SDS-polyacrylamide gel to undergo electrophoresis.
Following separation, proteins were transferred onto 0.45 μm
nitrocellulose membranes (Bio-Rad). The transferred membranes were
blocked for 1 h in 5% nonfat dry milk in TBST [25 mM Tris-HCl (pH
7.5), 125 mM NaCl and 0.05% Tween-20] and incubated with the
appropriate primary antibodies at 4°C overnight. Membranes were
washed twice with TBST for 10 min and incubated with horseradish
peroxidase-coupled isotype-specific secondary antibodies for 1 h at
room temperature. The immune complexes were detected by enhancement
with a chemiluminescence substrate (Supersignal West Pico; Pierce
Biotechnology, Inc., Rockford, IL, USA) and quantified using
analyst/PC densitometry software (Bio-Rad).
Statistical analysis
Data are presented as mean ± SD from three or more
independent experiments. Statistical analysis was performed by
Student’s t test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Anoikis response of P1, A549, H23 and
H460 cells
EMT is associated with the metastatic potential of
numerous types of human cancer (12). Knowledge of factors that affect
cancer metastasis may benefit the development of novel treatment
strategies as well as improve the sensitivity of methods of
diagnosis for this life-threatening disease. To investigate the
correlation between the metastatic potential of lung cancer cells
and EMT, the anoikis response was characterized in primary lung
cancer P1 and lung cancer A549, H23 and H460 cells. Cells were
detached and cultured in suspended condition over various times. At
0, 3, 6, 12 and 24 h, cell viability was evaluated using the
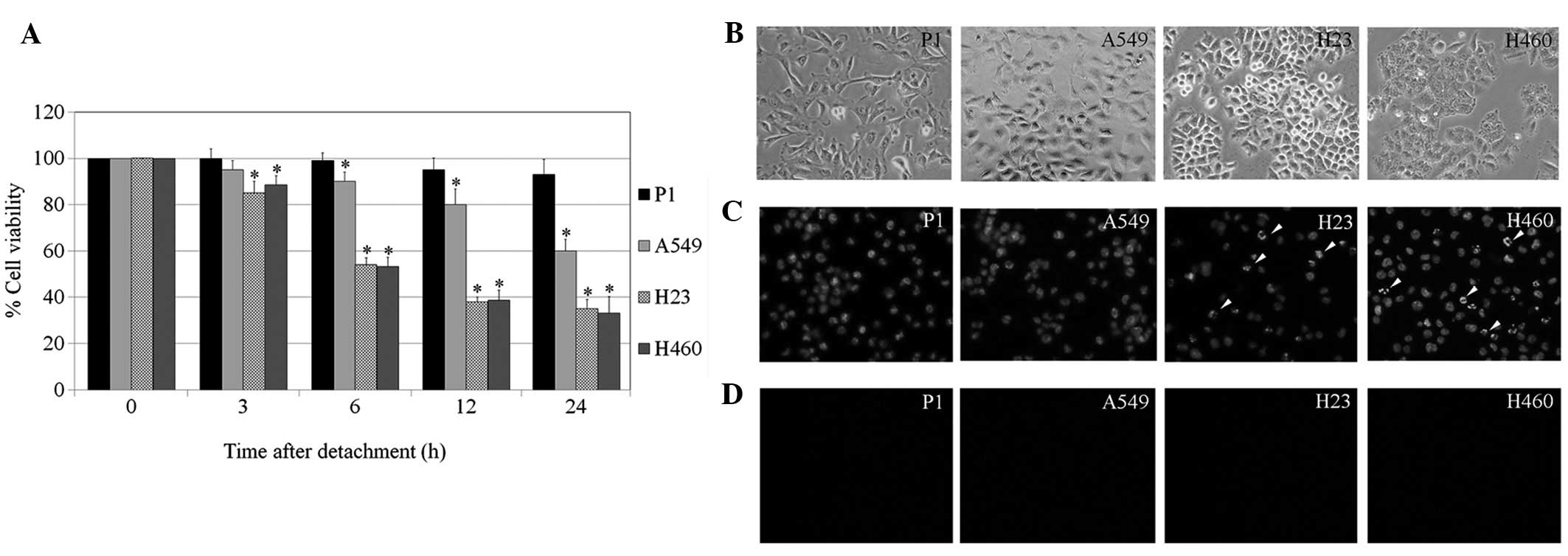
resazurin-based assay. Fig 1
indicates that the detachment-induced apoptosis was significantly
suppressed in the primary lung cancer cells which had been isolated
from a Thai patient (P1 cells), compared with standard lung cancer
cells. A significant reduction in the viability of all lung cancer
cell lines, A549, H23 and H460, was detected as early as 6 h, while
P1 exhibited non-significant reductions in viability after
detachment in the 24 h period. In addition, apoptosis and necrosis
were detected in these cells using Hoechst 33342 and PI staining.
The results indicate that cell detachment mediated cell death
largely through apoptosis, since only a limited number of
PI-positive cells were detected (data not shown). As expected, the
apoptosis found in P1 populations was extremely low compared with
that of other cells. Together, these results indicate that the
anoikis-resistance ability of P1 cells may be responsible for their
high metastatic potential.
Migration and invasion of P1, A549, H23
and H460 cells
Enhanced abilities of cancer cells to migrate and
invade are hallmarks of advanced stage cancer and aggressiveness
(13). In addition, EMT has been
linked to the increasing capacity of cancer cells to invade tissues
and migrate (2,4). The present study investigated the
migration and invasion behaviors of P1, A549, H23 and H460 cells.
The results indicate that P1 and A549 cells have significantly
enhanced invasion ability compared with H23 and H460 cells
(Fig. 2A). For invasion, A549
exhibited the highest ability to migrate in comparison with the P1,
H23 and H460 cells. These observations may be useful for
understanding the behavior of lung cancer of various origins.
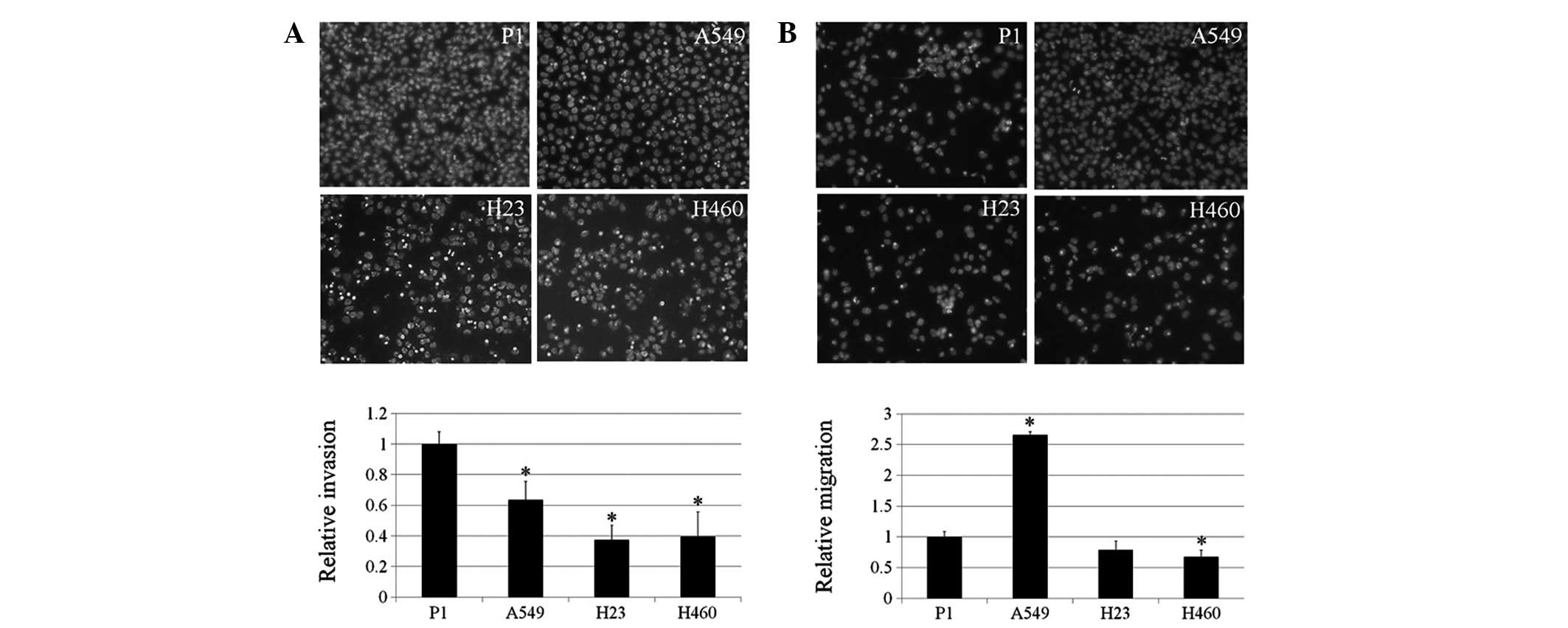 | Figure 2Invasion and migration of P1, A549,
H23 and H460 cells. (A) Lung cancer cells were assessed for their
invasive characteristics using Matrigel-coated membranes in
Transwell chambers. After 48 h, invaded cells were fixed, stained
with Hoechst 33342 and visualized under a fluorescence microscope.
Columns represent mean ± SD (n=3), *P<0.05, vs. P1
cells. (B) Cells were assessed for migratory capability. Following
12 h, migrated cells were fixed, stained with Hoechst 33342 and
visualized under a fluorescence microscope. Columns represent mean
± SD (n=3), *P<0.05, vs. P1 cells. |
Switch from E- to N-cadherin in lung
cancer cells
It is well accepted that the alteration of cell
interactions caused by the decrease of E-cadherin, concomitant with
an increase in N-cadherin, is an important indicator of EMT
(4, 12). To investigate whether EMT is the
underlying mechanism by which P1 cells exhibit an enhanced ability
to undergo metastasis, including anoikis resistance and invasion,
the present study determined the protein levels of E- and
N-cadherin in lung cancer cells.
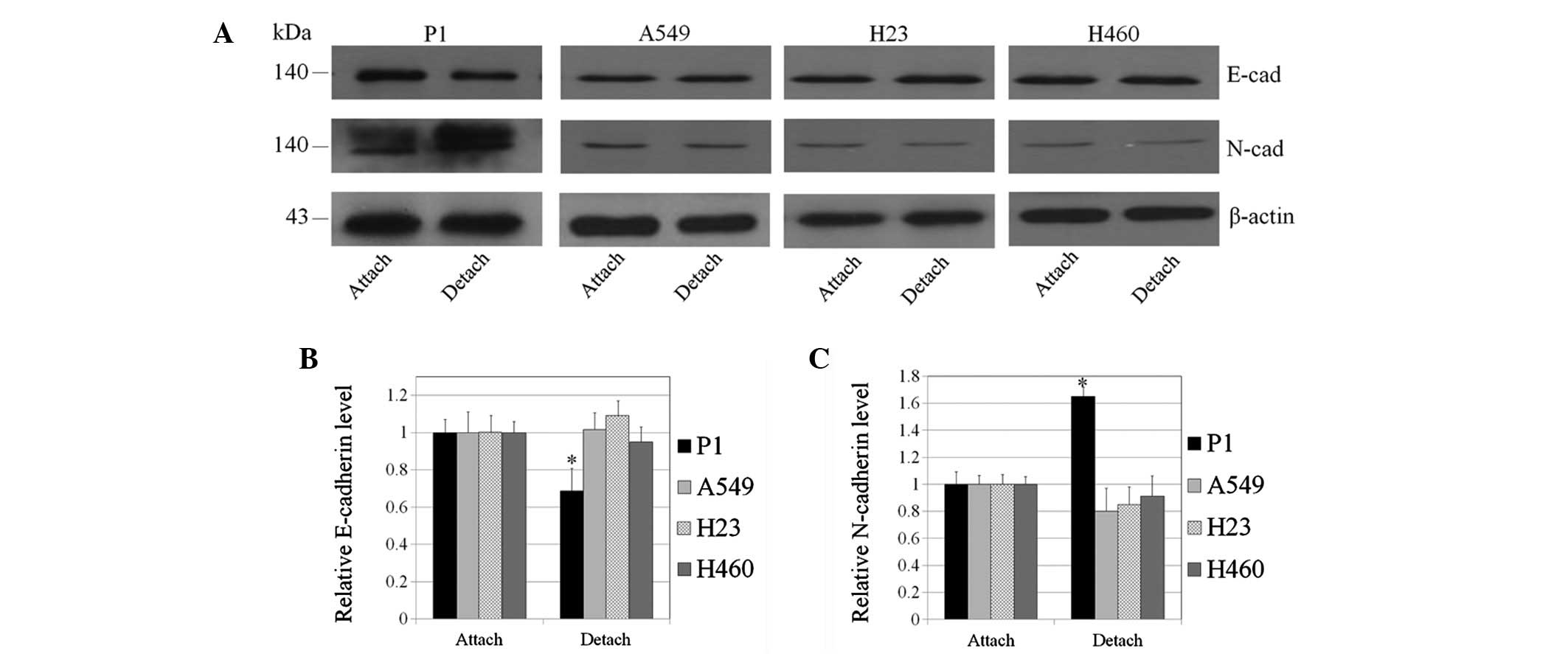
Western blot analysis revealed that E-cadherin
expression in P1, A549, H23 and H460 cells was comparable (Fig. 3A). The expression level of
N-cadherin was enhanced in P1 cells while such an expression was
barely detectable in other lung cancer cells. These results
indicate that increased EMT of P1 cells may, at least in part,
increase the metastatic potential of these cells. Induction of EMT
is hypothesized to depend on multiple signals, however, the
majority of these signals are unknown. In addition, the effect of
cell detachment on EMT was determined by comparing E- and
N-cadherin expression in the attached and detached cells. In the
present study, cell detachment has been demonstrated to
significantly enhance the cadherin switch from E- to N-cadherin in
P1 cells (Fig. 3). To a lesser
degree, this was also observed in A549, H23 and H460 cells.
Discussion
EMT is well known to have a significant impact on
cancer progression and metastasis (2,4,9,10,12).
A number of studies have demonstrated that cancer cells are able to
develop a mesenchymal phenotype which enhances their malignancy
(7,14). One characteristic of mesenchymal
cells is the ability to survive in suspended conditions which may
enable cancer cells to undergo metastasis (2,4,9,10).
In addition, reduced interaction of cells with the basement
membrane during EMT may facilitate the dissemination of cancer
cells from their tumors of origin (4).
Lung cancer in Thailand has become a significant
cause of cancer-related mortality (11), with the majority of such mortalities
due to cancer metastasis (11,
15). In our previous study of
cancer cells from various ethnicities, primary lung cancer P1 cells
were demonstrated to exhibit sufficient cisplatin resistance,
together with characteristics of cancer (16). The present study revealed that the
degree of EMT in P1 was high in comparison with that of lung cancer
cells, namely A549, H23 and H460 cells. Although the downregulation
of E-cadherin in P1 was not intense, the marked increase in levels
of N-cadherin represented EMT in the P1 cells. Consistent with
previous studies reporting that EMT enhances anoikis resistance in
numerous cells (2,4,7), the
transition of E- to N-cadherin was demonstrated in the present
study to be tightly associated with the ability to resist anoikis
in P1 cells.
A number of studies explaining the involvement of
cancer metastasis and the process of EMT have reported that EMT
enables cancer cells to migrate away from the tumour of origin
(2,4,7,9).
However, it is unclear whether the process of cell detachment
triggers EMT further. In the present study, lung cancer P1 cells
were identified to exhibit an increased expression of N-cadherin,
concomitant with decreased E-cadherin levels, following detachment.
This observation supports the hypothesis reporting a link between
EMT and the metastatic process. Not all cancer cells have been
identified to undergo EMT (17),
therefore, cells that possess the potential to carry out this
transition may have a greater probability of metastasizing
successfully.
With regard to migration and invasion, certain
studies have correlated such abilities with EMT (4,7,8,9).
However, in the current study, P1 cells only were observed to
exhibit an increased invasion capability, compared with other
cancer cells. In addition, A549 cells, which exhibited the most
significant ability to migrate and invade, revealed minimal EMT.
These results indicate that EMT in P1 cells may regulate invasion
and migration to a lesser extent compared with the anoikis
response.
Based on these observations, the ability of cancer
cells to undergo EMT may potentiate the metastasis of lung cancer
cells. In addition, the present study indicates that ethnicity may
affect EMT and may facilitate an improved understanding of cancer
cell biology.
Acknowledgements
The study was supported by the Higher
Education Research Promotion and National Research University
Project of Thailand, Office of the Higher Education Commission,
Thailand Research Fund and the Postdoctoral Fellowship
(Ratchadaphiseksompot Endowment Fund, Chulalongkorn University).
The authors thank Krich Rajprasit, a proofreader.
References
|
1
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalluri R and Neilson EG: Epithelial
mesenchymal transition and its implications for fibrosis. J Clin
Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guadamillas MC, Cerezo A and Del Pozo MA:
Overcoming anoikis - pathways to anchorage-independent growth in
cancer. J Cell Sci. 124:3189–3197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boisvert AK, Longmate W, Abel EV and Aplin
A: Mcl-1 is required for melanoma cell resistance to anoikis. Mol
Cancer Res. 7:549–556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiarugi P and Giannoni E: Anoikis: a
necessary death program for anchorage-dependent cells. Biochem
Pharmacol. 76:1352–1364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li G, Satyamoorthy K and Herlyn M:
N-cadherin-mediated intercellular interactions promote survival and
migration of melanoma cells. Cancer Res. 61:3819–3825.
2001.PubMed/NCBI
|
|
8
|
Minard ME, Ellis LM and Gallick GE: Tiam1
regulates cell adhesion, migration and apoptosis in colon tumor
cells. Clin Exp Metastasis. 23:301–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hazan RB, Qiao R, Keren R, Badano I and
Suyama K: Cadherin switch in tumor progression. Ann NY Acad Sci.
1014:155–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ko H, Kim S, Jin CH, Lee E, Ham S, Yook JI
and Kim K: Protein kinase casein kinase 2-mediated upregulation of
N-cadherin confers anoikis resistance on esophageal carcinoma
cells. Mol Cancer Res. 10:1032–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vatanasapt V, Sriamporn S and Vatanasapt
P: Cancer control in Thailand. Jpn J Clin Oncol. 32:S82–S91. 2002.
View Article : Google Scholar
|
|
12
|
Thompson EW and Newgreen DF: Carcinoma
invasion and metastasis: a role for epithelial-mesenchymal
transition? Cancer Res. 65:5991–5995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luanpitpong S, Talbott SJ, Rojanasakul Y,
Nimmannit U, Pongrakhananon V, Wang L and Chanvorachote P:
Regulation of lung cancer cell migration and invasion by reactive
oxygen species and caveolin-1. J Biol Chem. 285:38832–38840. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taweevisit M, Chirakalwasan N, Pumsuk U,
Keelawat S and Shuangshoti S: Metastatic adenocarcinoma to the
cervical lymph node: a significant proportion of cholangiocarcinoma
in Thai patients. Asian Pac J Cancer Prev. 9:39–41. 2008.PubMed/NCBI
|
|
16
|
Chanvorachote P, Luanpitpong S, Chunhacha
P, Promden W and Sriuranpong V: Expression of CA125 and cisplatin
susceptibility of pleural effusion-derived human lung cancer cells
from a Thai patient. Oncol Lett. 4:252–256. 2012.PubMed/NCBI
|
|
17
|
Tsuji T, Ibaragi S and Hu GF:
Epithelial-mesenchymal transition and cell cooperativity in
metastasis. Cancer Res. 69:7135–7139. 2009. View Article : Google Scholar : PubMed/NCBI
|