Introduction
Human clinically non-functioning pituitary adenomas
(NFPAs) are defined as adenomas lacking symptoms or signs secondary
to oversecretion of pituitary hormones by the tumor.
Non-functioning adenomas constitute 9–50% of pituitary tumors and
∼80% of pituitary macroadenomas (1,2). The
majority of patients with NFPAs seek medical attention due to the
mass effects of the tumor, which include headaches, visual
impairment and hypopituitarism (3,4).
However, ∼9% of NFPAs are subclinical adenomas which present as an
incidentally found mass in the sellar region (3). The majority of NFPAs are not
accompanied by hypersecretion of significant pituitary hormones in
serum, with the exception of mild hyperprolactinemia in certain
cases (5). However, by
immunocytochemistry, the large majority of such tumors synthesize
pituitary glycoprotein hormones (follicle-stimulating hormone, FSH;
luteinizing hormone, LH; thyroid-stimulating hormone, TSH; growth
hormone, GH and prolactin, PRL) and/or their free subunits
(α-subunit, β-FSH, β-LH and β-TSH) (4,6,7).
Pathologically, NFPAs are classified into gonadotroph, silent and
null cell adenomas, in which no immunoreactive hormone or its
corresponding subunit is found (8).
For NFPAs, surgery is the first-line treatment. Radiotherapy should
be considered for residual tumors, while asymptomatic patients
should be followed up conservatively (3,4).
Effective medical therapy has been demonstrated in functioning
pituitary adenomas (9). Medication,
such as dopamine agonists and somatostatin analogs, has been
prescribed for NFPAs; however, the efficacy has not been
satisfactory, with the exception of a small number of case studies
(9,10). Thus, there is a need for specific
and efficacious medical treatments for patients bearing NFPAs.
Notch signaling is a highly evolutionarily conserved
pathway involved in various functions during development, including
cell fate control, the maintenance of stem cells and apoptosis
(11–13). The basic components of this pathway
are Notch receptors, ligands and transcription factors. In humans,
there are four Notch receptors (Notch1, 2, 3 and 4), two Jagged
ligands (Jagged1 and Jagged2) and three δ–like ligands (Dll1, Dll3
and Dll4) (14). The activation of
Notch by its ligand frees the intracellular domain of Notch
(Notch-IC) and enables it to enter the nucleus through a cascade of
proteolytic cleavages by α- and γ-secretase. In the nucleus,
Notch-IC then activates the transcription of Notch target genes,
primarily by binding to a ubiquitous transcription factor, CSL. The
CSL pathway includes CBF-1 (also known as RBP-Jκ), Suppressor of
Hairless [Su(H)] and Lag-1 (12,15).
In addition to their function in developmental processes,
increasing evidence demonstrates that Notch-ligand interactions
also participate in the pathogenesis of a number of human diseases.
Differential expression of the Notch3 protein and its ligand
Jagged1 has been demonstrated in numerous human malignancies.
Activating mutations of Notch3 are present in human T-cell acute
lymphoblastic leukemia and a number of human solid tumors,
including ovarian, breast and colorectal cancer (16–19).
Additionally, Jagged1 is involved in the progression and
proliferation of various human tumors through its interaction with
the Notch3 receptor in human ovarian carcinoma, multiple myeloma
and colorectal cancer (20–23). These observations suggest that the
binding of Jagged1 to Notch3 contributes to the onset and
progression of human tumors by activation of the Notch signaling
pathway. Two microarray gene expression studies conducted by Moreno
et al revealed that Notch3 was overexpressed in human
NFPAs and PRL-secreting pituitary adenomas (24,25).
Another study demonstrated elevated Notch3 mRNA and protein
expression in non-functioning pituitary tumors (26). However, the role of Jagged1 in
pituitary adenomas has not yet been demonstrated, and there is no
direct evidence of the function of Notch3 in GH- and PRL-secreting
adenomas. In the current study, we investigated the role of Notch3
and its ligand Jagged1 in various types of pituitary adenoma as
well as in normal pituitary glands. We provide the first
description of the differential expression of Jagged1 in human
pituitary adenomas and its correlation with Notch3.
Materials and methods
Patients and tissues
Seventeen pituitary adenomas were obtained from
patients at the Beijing Tiantan Hospital during endoscopic
transsphenoidal surgery, and three normal human adenohypophyses
were obtained from a donation program. Informed consent was
obtained from the patients and the study was approved by the Ethics
Committee of Beijing Tiantan Hospital, Beijing, China. All samples
were rinsed in sterile saline, snap-frozen in liquid nitrogen and
then stored in liquid nitrogen until analysis. Clinical details of
the patients are summarized in Table
I. Individual adenomas were classified based on the profile of
adenohypophyseal hormone content, by histology and
immunohistochemistry prior to molecular analysis.
 | Table IClinical and pathological
characteristics of the 17 pituitary adenomas from the patients in
this study. |
Table I
Clinical and pathological
characteristics of the 17 pituitary adenomas from the patients in
this study.
| Patient ID | Gender | Age (years) | Tumor size
(cm) | Clinical
characteristics | Immunohistochemical
analysis |
|---|
| 1 | M | 32 | 2.4 |
Hyperprolactinemia |
PRL+ |
| 2 | F | 43 | 3.5 | Headache and visual
defects | NF− |
| 3 | M | 55 | 3.0 | Visual defects | NF+:
FSH+ |
| 4 | F | 48 | 2.2 | Acromegaly | GH+ |
| 5 | M | 33 | 1.7 | Acromegaly | GH+ |
| 6 | M | 52 | 3.6 | Acromegaly | GH+ |
| 7 | F | 41 | 4.9 | Acromegaly | GH+ |
| 8 | F | 55 | 2.8 | Symptomless | NF+:
LH+, FSH+ |
| 9 | M | 51 | 4.5 | Headache and
hypopituitarism | NF+:
LH+, FSH+ |
| 10 | M | 22 | 4.4 |
Hyperprolactinemia |
PRL+ |
| 11 | F | 35 | 2.0 |
Hyperprolactinemia |
PRL+ |
| 12 | F | 46 | 2.5 |
Hyperprolactinemia |
PRL+ |
| 13 | F | 26 | 1.8 | Acromegaly | GH+ |
| 14 | M | 46 | 4.6 | Headache and visual
defects | NF− |
| 15 | F | 37 | 7.1 | Headache, visual
loss and hydrocephalus | NF− |
| 16 | M | 47 | 2.6 | Headache |
NF+:LH+ |
| 17 | M | 29 | 3.0 | Visual defects | NF− |
RNA extraction and quantitative real-time
reverse transcription-polymerase chain reaction (RT-PCR) assay
Total RNA was extracted from frozen pituitary
adenomas and normal pituitaries (40–60 mg) using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and first-strand
cDNA was synthesized from total RNA using the SuperScript
First-Strand Synthesis system with SuperScript II reverse
transcriptase, according to the manufacturer’s instructions
(Invitrogen Life Technologies). RT-PCR was performed in an Applied
Biosystems 7500 Fast system using Platinum SYBR-Green/ROX qPCR
Supermix-UDG (Invitrogen Life Technologies). The qPCR reaction
system was performed in a 25-μl reaction, which comprised 2X
Master mix (12.5 μl), forward/reverse primers (0.5 μl
each, 10 μmol/l), sample cDNA (1 μl) and double
distilled water (ddH2O; 10.5 μl). The
amplification conditions were 50°C for 120 sec, 95°C for 120 sec,
as well as 40 cycles at 95°C for 15 sec and 60°C for 30 sec. The
fluorescence of the PCR products was read following completion of
the extension step. The expression of mRNA was determined from the
threshold cycle (CT), and the relative expression levels of the
tested genes were normalized relative to that of GAPDH and
calculated from the CT value using the 2−ΔΔCT method for
quantification (27). The primers
used in the RT-PCR assay are listed in Table II.
 | Table IIPrimers used for RT-PCR in this
study. |
Table II
Primers used for RT-PCR in this
study.
| Gene name | Amplification
(bp) | Forward sequence
(5′ to 3′) | Reverse sequence
(5′ to 3′) | Temp. (°C) |
|---|
| Notch3 | 141 |
TGGCGACCTCACTTACGACT |
CACTGGCAGTTATAGGTGTTGAC | 60.9 |
| Jagged1 | 77 |
GGGGCAACACCTTCAACCTC |
CCAGGCGAAACTGAAAGGC | 60.0 |
| GAPDH | 228 |
GAAGGTCGGAGTCAACGGATT |
CGCTCCTGGAAGATGGTGAT | 60.0 |
Protein preparation and western blot
analysis
Pituitary adenomas or normal pituitary gland tissue
from humans were homogenized in lysis buffer in a handheld
micro-tissue homogenizer. The homogenate was then centrifuged at
12,000 × g for 15 min at 4°C, and the supernatant was denatured for
5 min at 95°C in loading buffer. Protein concentrations were
measured using the bicinchoninic acid protein assay with bovine
serum albumin as the standard. Soluble proteins (60 μg) were
separated by electrophoresis in 8 or 10% sodium dodecyl sulfate
polyacrylamide gels, transferred to nitrocellulose membranes and
incubated with blocking buffer (5% non-fat milk in Tris-buffered
saline Tween-20 (TBST) for 1 h at room temperature. Membranes were
then probed overnight with the corresponding primary antibody at
4°C, followed by three 10 min washes with TBST. Subsequently,
membranes were incubated with secondary antibodies conjugated with
horseradish peroxidase at room temperature for 1 hour. Rat
polyclonal Notch3 antibody (1:1,000, 8G5; Cell Signaling
Technology, Inc., Boston, MA, USA) and rabbit polyclonal Jagged1
antibody (1:1,000, 28H8; Cell Signaling Technology, Inc.) were
used. Enhanced chemiluminescence, performed according to the
manufacturer’s instructions (Amersham Pharmacia Biotech,
Piscataway, NJ, USA) was used to demonstrate positive bands that
were visualized after exposure on a transparent medical X-ray film.
The final data were subjected to grayscale scanning and
semi-quantitative analysis using Quantity One software (Bio-Rad,
Hercules, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard error.
Statistical analyses of protein expression between tumor types were
performed using Student’s t-tests or non-parametric Mann-Whitney U
tests. Correlations were performed using the Pearson Rank Sum test.
P<0.05 was considered to indicate a statistically significant
difference. The Statistical Package for the Social Sciences version
17.0 (SPSS; SPSS Inc., Chicago, IL, USA) was used for statistical
analyses.
Results
Tumor classification
The clinical and pathological characteristics of the
17 adenomas used in this study are listed in Table I. There were nine male and eight
female patients. The average age of the patients was 41 years
(range, 22–55) and the average tumor diameter was 3.3 cm (range,
1.7–7.1). There were eight NFPAs, five GH-secreting adenomas and
four PRL-secreting adenomas. Four NFPAs were not positive with
anterior pituitary hormone histochemistry and were designated
immunohistochemically negative (NF−) tumors, while four
NFPA tumors were stained with LH and/or FSH and were designated
immunohistochemically positive (NF+). The four
PRL-secreting adenomas manifested as hyperprolactinemia, while the
five GH-secreting adenomas were characterized with acromegaly. For
the eight NFPAs, headache and visual defects were the main
symptoms. Three normal pituitary controls were obtained from a
donation program, and these patients did not have any
endocrinological diseases.
RT-PCR analysis
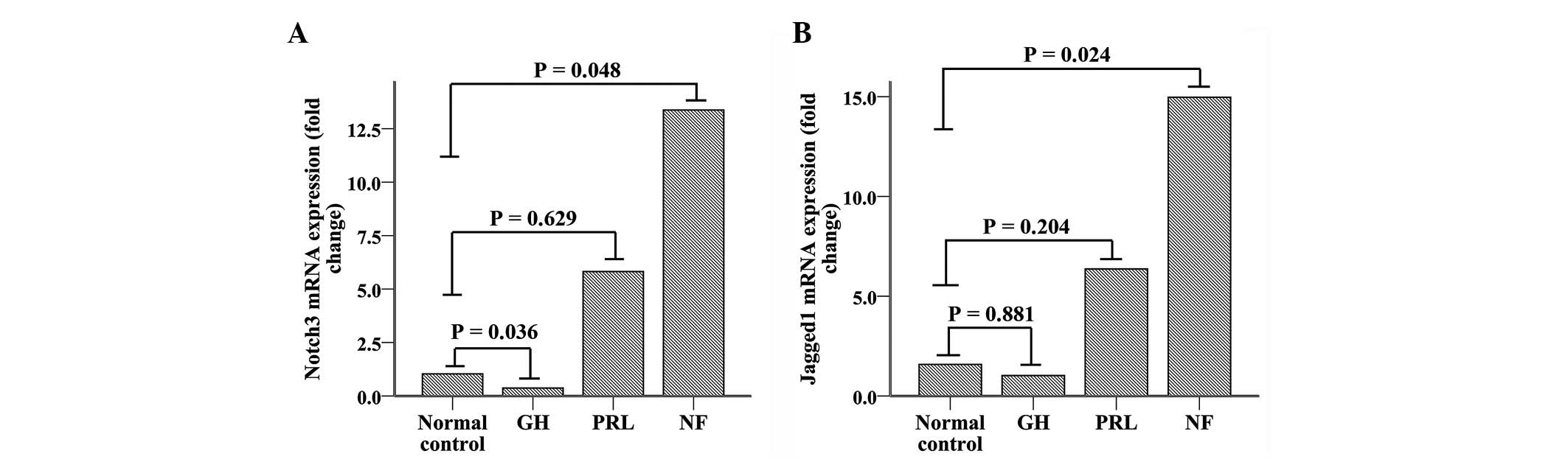
Notch3 mRNA expression (Fig. 1A) increased ∼6.5-fold in NFPAs
(n=6), compared with normal pituitary tissue controls (n=3,
P=0.048). Although PRL-secreting adenomas (n=4) demonstrated a
1.5-fold increase in Notch3 mRNA compared with normal
pituitary tissue, there was no significant difference between these
two groups (P=0.629). GH-secreting adenomas (n=5) demonstrated
significantly reduced expression (∼75% reduction) of Notch3
compared with normal tissue (P=0.036). Overall, pituitary adenomas
(n=15) demonstrated a 4-fold increase in Notch3 mRNA
expression compared with normal pituitary tissue; however, this
increase was not significantly different (P=0.100). Additionally,
nonfunctioning adenomas demonstrated increased expression of
Notch3 compared with functioning adenomas, which included
PRL- and GH-secreting adenomas (P=0.026).
Jagged1 mRNA expression (Fig. 1B) was also markedly increased
(∼11.2-fold) in NFPAs (n=6) compared with normal pituitary tissues
(n=3, P=0.024). PRL-secreting adenomas (n=4) demonstrated a
3.9-fold increase in Jagged1 mRNA expression compared with
normal pituitary tissue, although the difference was not
statistically significant (P=0.204). In contrast to its receptor
(Notch3), Jagged1 mRNA expression levels in GH-secreting
adenomas were similar to normal (P= 0.881). There was no
significant difference between all pituitary adenomas and normal
pituitary tissue (P=0.824). As with its receptor, Jagged1
mRNA expression significantly increased in NFPAs compared with
functioning adenomas (P=0.005).
Western blot analysis
Western blot analysis (Fig. 2A and C) demonstrated that Notch3
protein expression was consistent with the mRNA findings and was
significantly increased in NFPAs (n=8) compared with normal
pituitary tissue (n=3, P= 0.014). Notch3 protein expression levels
of GH- and PRL-secreting adenomas were similar to those of normal
pituitary tissue (n=5, P>0.05; n=4, P>0.05, respectively). As
a group, pituitary adenomas (n=17) did not demonstrate an elevated
expression of Notch3 protein compared with normal pituitary tissue
(n=3, P=0.335). Unlike RT-PCR analysis, Notch3 protein expression
was significantly elevated in NFPAs compared with functioning
adenomas (n=9, with 5 GH-secreting adenomas and 4 PRL-secreting
adenomas; P=0.002).
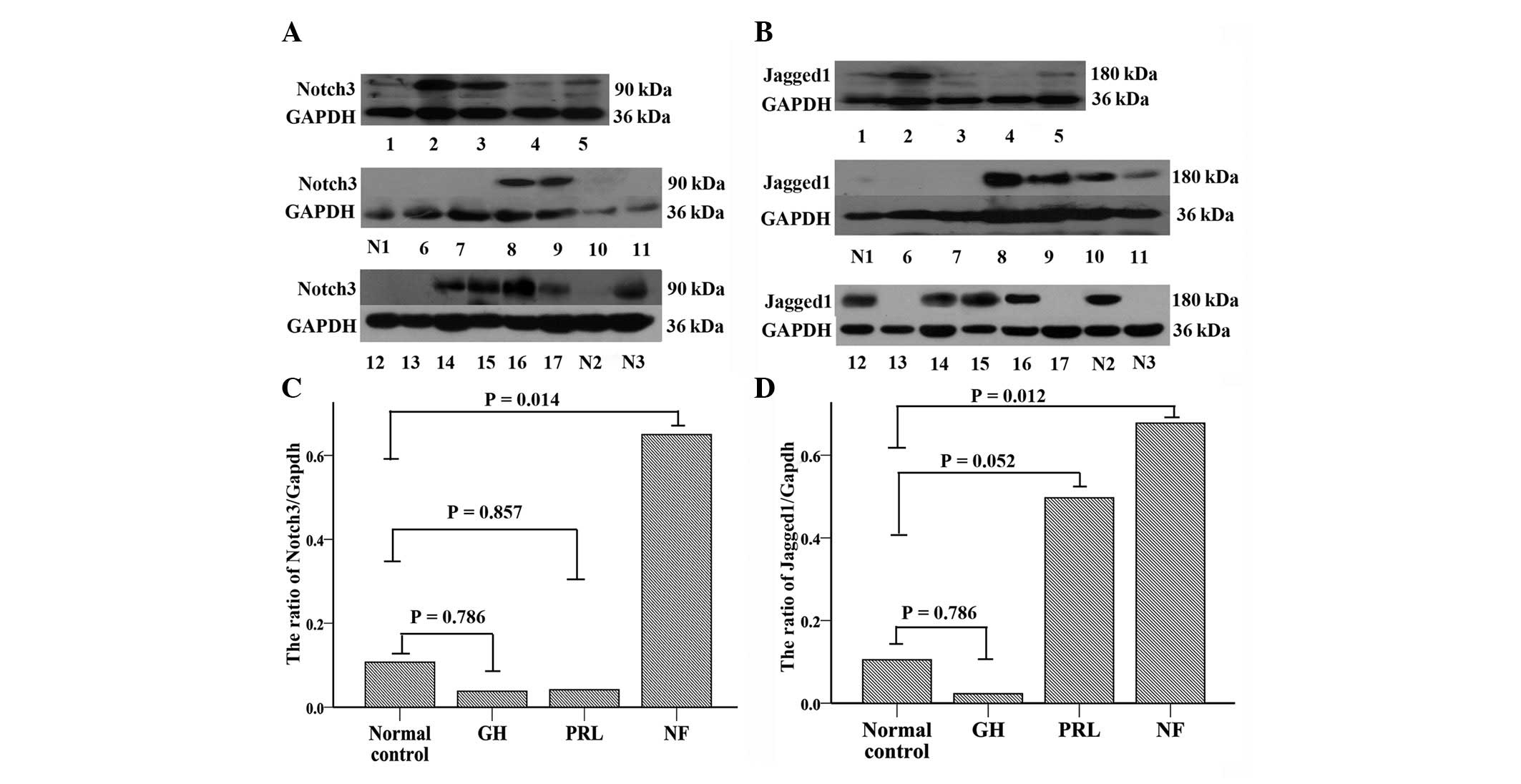 | Figure 2Western blot analysis. (A and B)
Expression of Notch3 and Jagged1 protein in NFPAs (n=8, samples 2,
3, 8, 9, 14, 15, 16 and 17), GH-secreting adenomas (n=5, samples 4,
5, 6, 7 and 13), PRL-secreting adenomas (n=4, samples 1, 10, 11, 12
and 13) and normal pituitary tissue (n=3, samples N1, N2 and N3).
GAPDH was used for normalization. (C and D) Western blot analysis
reveals a significant increase in the Notch3 and Jagged1 proteins
in NFPAs compared with the normal pituitary tissue (P<0.05). |
Consistent with the RT-PCR findings, Jagged1 protein
expression (Fig. 2B and D) in NFPAs
(n=8) was elevated significantly compared with that in normal
pituitary glands (n=3, P=0.012). As for GH-secreting (n=5) or
PRL-secreting adenomas (n=4), the Jagged1 expression levels were
not significantly different from those in normal tissue (P=0.786
and P=0.052, respectively). Additionally, pituitary adenomas (n=17)
and normal pituitary glands (n=3, P=0.18) expressed similar Jagged1
levels. Although the protein expression level of Jagged1 was not
statistically significantly different between NFPAs and functioning
adenomas (P>0.05), NFPAs exhibited a significantly increased
expression of Jagged1 compared with GH-secreting adenomas
(P=0.011).
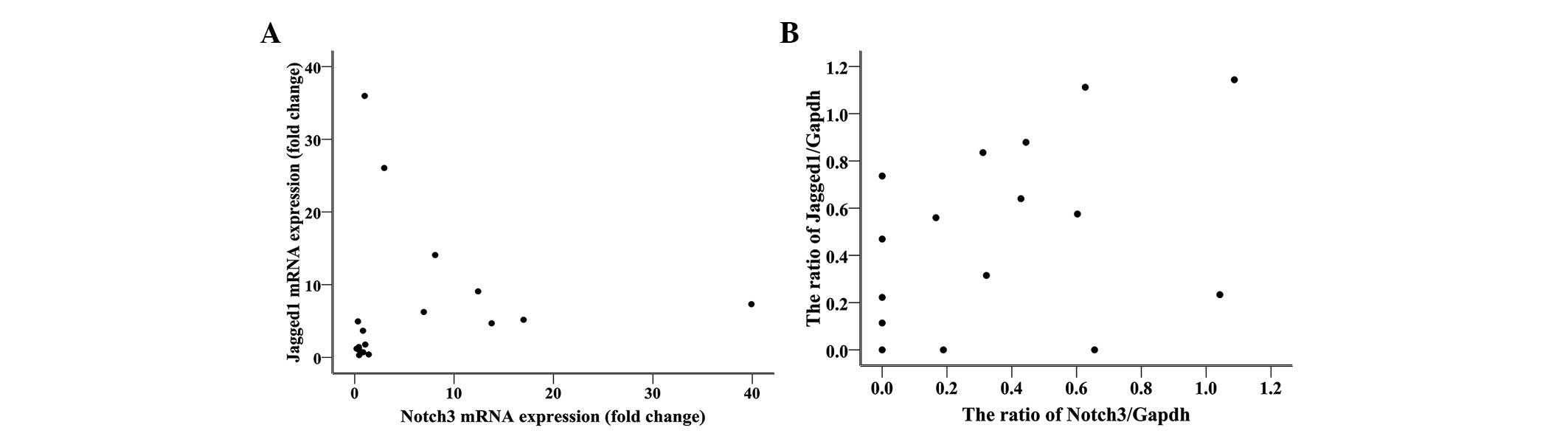
Correlation between expression of Notch3
and Jagged1
The expression of Notch3 mRNA was positively
correlated with Jagged1 mRNA expression (Fig. 3A), with a Pearson’s correlation
coefficient of 0.560 (n=20, P=0.016). Similarly, a positive
correlation was observed between the expression of Notch3
and Jagged1 proteins (Fig. 3B),
with a Pearson’s correlation coefficient of 0.532 (n=18, P=
0.012).
Discussion
As significant oncogenes in humans, Notch receptors
and their ligands have been demonstrated to be involved in the
pathogenesis of numerous neoplasms via various mechanisms. Notch3
affects apoptosis and tumor growth in lung cancer by co-operating
with the EGFR-MAPK pathway (28,29).
Additionally, Notch3 promotes proliferation and inhibits apoptosis
of ErbB2-negative breast tumor cells via activation of the CSL
(CBF-1/RBP-Jκ, Su(H) and Lag-1) pathway (18). Also, through the canonical
CSL-mediated transcriptional network, Notch3 is capable of
regulating esophageal squamous cell differentiation and
proliferation (30). In addition to
solid tumors, Notch3 is able to promote the survival of T acute
lymphoblastic leukemia cells via regulation of MKP-1, which is a
member of the MAPK pathway (31).
However, the regulatory functions of Notch3 described previously
require activation by Notch3 ligands. Abundant evidence suggests
that the interaction between Notch3 and Jagged1 plays a key role in
the tumorigenesis of numerous diverse malignancies, including
ovarian cancer, colorectal cancer and multiple myeloma cells
(20–22). Thus, Jagged1 is an important ligand
of Notch3 and is involved in the pathogenesis of neoplasms.
In the present study, we provide the first
description of the differential expression of the Notch3
receptor and its ligand Jagged1 in various types of human pituitary
adenomas at the mRNA and protein levels. The expression of Notch3
mRNA and protein was significantly elevated in the NFPAs compared
with normal pituitary tissue, whereas all pituitary adenomas do not
overexpress Notch3. Our results are consistent with the results of
previous studies. Overexpression of Notch3 had been observed in
human clinically NFPAs in the study by Miao et al(26). In this study, additional
immunohistochemical analyses were performed, demonstrating that the
Notch3 receptor is primarily expressed in the cytoplasm of NFPAs.
Gene microarrays and proteomic analyses have demonstrated that
Notch3 gene and protein expression are increased in human
clinically NFPAs (24,32). However, in the study conducted by
Moreno et al(24), all types
of non-functioning adenomas were evaluated, whereas in our study,
only two types (NF−, LH/FSH+) were analyzed.
These results suggest that Notch3 may play a significant role in
the development of human NFPAs other than GH- and PRL-secreting
adenomas. However the exact mechanism of Notch3 in the
tumorigenesis of NFPAs remains to be elucidated.
In the current study, we have provided evidence of
Notch3 expression in human functioning adenomas, a finding that has
not been previously reported. The expression of Notch3 was
moderately elevated in NFPAs compared with functional adenomas,
which included GH- and PRL-secreting adenomas. However, ACTH- and
TSH-secreting adenomas were not evaluated in our study. The
function of Notch in the development and cell specification of the
pituitary gland has been explained by several studies (33–35).
Activation of Notch in zebrafish has been reported to lead to the
loss of lactotropic cell specification, and increase the number of
gonadotropes, corticotropes and melanotropes in the anterior
pituitary (35). Another study
demonstrated that Notch regulates the specification of diverse cell
types in the pituitary of mice (34). Notch has been demonstrated to
repress Math3, which is a Pit1 target gene that is specifically
required for the maturation and proliferation of the GH-producing
somatotrope (34). Coincidently,
our data demonstrated that Notch3 receptor expression was slightly
decreased in GH-secreting adenomas compared with controls, although
those changes were not statistically significant. The
aforementioned studies, together with our experimental data, imply
that Notch may regulate the specification of cell types in
pituitary adenomas. In addition, Notch3 may promote the maturation
and proliferation of gonadotropes, which are the predominant cell
type of NFPAs. GeneChip microarrays and proteomic analyses have
demonstrated an increased expression of Notch3 in PRL-secreting
adenomas (25). Our data
demonstrated paralleled expression of Notch3 between PRL-secreting
adenomas and normal controls. Notably, the adenomas used in the
previous study were larger in size (diameters were >3 cm) and
patients presented with markedly elevated serum prolactin levels
(>1000 ng/ml), compared with those in our study. These
differences imply that Notch3 may stimulate the growth and hormone
production of PRL-secreting adenomas, although the molecular
mechanism involved requires clarification.
Ligands of the Notch3 receptor also play a role in
the pathogenesis of pituitary adenomas. Previous studies have
demonstrated that Dll1, a potential ligand of Notch3, was strongly
downregulated in non-functional tumors and in PRL-secreting
adenomas (24,25). In the present study, we have
provided the first evidence of Jagged1 overexpression in NFPAs. Our
data demonstrated that Jagged1 expression, similar to the Notch3
receptor but opposite to Dll1, was increased in NFPAs compared with
the control pituitary tissue. A positive correlation was also
observed between Notch3 and Jagged1 expression at the mRNA and
protein levels. These results imply that there is a link between
Jagged1 and Notch3 in the pathogenesis of NFPAs. Abundant evidence
describes the participation of Jagged1 in the angiogenesis and
tumorigenesis of various types of malignancies, including ovarian
and colorectal cancer, squamous cell carcinoma and multiple myeloma
(20–23,36,37).
The majority of these studies revealed that the interaction between
Notch3 and Jagged1 participates in the growth, proliferation and
angiogenesis of the tumors (20–23,36).
The present study also demonstrated that Jagged1, like the Notch3
receptor, has a higher expression level in NFPAs than in
functioning adenomas. Thus, we speculate that Jagged1 may play an
important role in the specification, initiation and/or
proliferation of NFPAs via the activation of the Notch3 pathway.
However, the exact mechanism of the interaction between Notch3 and
Jagged1 in pituitary adenomas remains to be elucidated.
The Notch signaling pathway regulates the
initiation, specification and proliferation of neoplasms, primarily
through two different mechanisms. The first mechanism is the
canonical CSL-mediated transcriptional network. Ligand binding
leads to the release of the Notch-IC, which then translocates into
the nucleus and interacts with the CSL DNA-binding protein to
generate a transcriptional activator complex. The latter induces
expression of target genes, including Hes/Hey family genes, cyclin
D and NF-κB. These genes participate in cell specification, growth,
progression and survival (11).
Notch3-induced activation of NF-κB induces
differentiation or neoplastic transformation of T-cells (38,39).
Notch3 promotes tumor cell growth and proliferation via the
Hes gene in a CSL-dependent fashion (22,30).
The Notch3 and Hes genes regulate the differentiation and
specification of progenitor cells in pituitary development
(40,41). Cyclin D1, one of the Notch target
genes, has been demonstrated to be overexpressed in NFPAs (42). These data suggest that the Notch
signaling pathway may regulate the pathogenesis of NFPAs via the
canonical CSL-mediated transcriptional network. Until now, there
was no direct evidence to support this hypothesis. The second
mechanism of the Notch3 promotion of tumorigenesis is through
co-operation with other signaling pathways. Interaction between
Notch3 and other signaling pathways, including the Wnt, MAPK, and
EFGR pathways, plays a key role in the growth and proliferation of
various neoplasms (21,28,36).
Further study is required to elucidate the exact mechanism of
Notch3 signaling in pituitary tumor development and
tumorigenesis.
As the Notch signaling pathway is involved in the
pathogenesis of numerous diverse malignancies, it is plausible that
inhibition of this pathway may have antitumor effects. Following
activation of the Notch receptor, the proteolytic processing of
Notch by the γ-secretase protein complex is an essential step that
leads to the release of the Notch-IC and transcription of target
genes (43). Therefore, γ-secretase
inhibitors are capable of specially blocking Notch signaling
pathway activation. It has been demonstrated that γ-secretase
inhibitors suppress proliferation and induce apoptosis in T-cell
leukemia, and in lung and breast cancer (44–46).
Furthermore, γ-secretase inhibitors have been used to treat
lymphoma in pre-clinical trials (47). However, the anti-oncogenic potential
of γ-secretase inhibitors in NFPAs requires further study.
In conclusion, the present study demonstrated
increased Notch3 and Jagged1 expression, as well as a positive
correlation between Notch3 and Jagged1, in human NFPAs. Further
studies are required to elucidate the exact mechanism whereby the
Notch signaling pathway participates in the pathogenesis of NFPAs.
The present study implies that the Notch signaling pathway may be a
potential therapeutic target in the treatment of NFPAs.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China, No. 81072075.
References
|
1
|
Buurman H and Saeger W: Subclinical
adenomas in postmortem pituitaries: classification and correlations
to clinical data. Eur J Endocrinol. 154:753–758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saeger W, Lüdecke DK, Buchfelder M,
Fahlbusch R, Quabbe HJ and Petersenn S: Pathohistological
classification of pituitary tumors: 10 years of experience with the
German Pituitary Tumor Registry. Eur J Endocrinol. 156:203–216.
2007.PubMed/NCBI
|
|
3
|
Greenman Y and Stern N: Non-functioning
pituitary adenomas. Best Pract Res Clin Endocrinol Metab.
23:625–638. 2009. View Article : Google Scholar
|
|
4
|
Jaffe CA: Clinically non-functioning
pituitary adenoma. Pituitary. 9:317–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Black PM, Hsu DW, Klibanski A, et al:
Hormone production in clinically nonfunctioning pituitary adenomas.
J Neurosurg. 66:244–250. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gittoes NJ: Current perspectives on the
pathogenesis of clinically non-functioning pituitary tumours. J
Endocrinol. 157:177–186. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katznelson L, Alexander JM and Klibanski
A: Clinical review 45: Clinically nonfunctioning pituitary
adenomas. J Clin Endocrinol Metab. 76:1089–1094. 1993.PubMed/NCBI
|
|
8
|
Korbonits M and Carlsen E: Recent clinical
and pathophysiological advances in non-functioning pituitary
adenomas. Horm Res. 71(Suppl 2): 123–130. 2009. View Article : Google Scholar
|
|
9
|
Colao A, Pivonello R, Di Somma C,
Savastano S, Grasso LF and Lombardi G: Medical therapy of pituitary
adenomas: effects on tumor shrinkage. Rev Endocr Metab Disord.
10:111–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Colao A, Di Somma C, Pivonello R, Faggiano
A, Lombardi G and Savastano S: Medical therapy for clinically
non-functioning pituitary adenomas. Endocr Relat Cancer.
15:905–915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bianchi S, Dotti MT and Federico A:
Physiology and pathology of notch signalling system. J Cell
Physiol. 207:300–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai EC: Notch signaling: control of cell
communication and cell fate. Development. 131:965–973. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: cell fate control and signal integration in
development. Science. 284:7701999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greenwald I: LIN-12/Notch signaling:
lessons from worms and flies. Genes Dev. 12:1751–1762. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miele L, Miao H and Nickoloff BJ: NOTCH
signaling as a novel cancer therapeutic target. Curr Cancer Drug
Targets. 6:313–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Indraccolo S, Minuzzo S, Masiero M and
Amadori A: Ligand-driven activation of the notch pathway in T-ALL
and solid tumors: why Not(ch)? Cell Cycle. 9:80–85. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JT, Li M, Nakayama K, et al: Notch3
gene amplification in ovarian cancer. Cancer Res. 66:6312–6318.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaguchi N, Oyama T, Ito E, et al: NOTCH3
signaling pathway plays crucial roles in the proliferation of
ErbB2-negative human breast cancer cells. Cancer Res. 68:1881–1888.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Serafin V, Persano L, Moserle L, et al:
Notch3 signalling promotes tumour growth in colorectal cancer. J
Pathol. 224:448–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Stoeck A, Lee SJ, Shih Ie M, Wang
MM and Wang TL: Jagged1 expression regulated by Notch3 and
Wnt/beta-catenin signaling pathways in ovarian cancer. Oncotarget.
1:210–218. 2010.PubMed/NCBI
|
|
21
|
Rodilla V, Villanueva A, Obrador-Hevia A,
et al: Jagged1 is the pathological link between Wnt and Notch
pathways in colorectal cancer. Proc Natl Acad Sci USA.
106:6315–6320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jundt F, Probsting KS, Anagnostopoulos I,
et al: Jagged1-induced Notch signaling drives proliferation of
multiple myeloma cells. Blood. 103:3511–3515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi JH, Park JT, Davidson B, Morin PJ,
Shih Ie M and Wang TL: Jagged-1 and Notch3 juxtacrine loop
regulates ovarian tumor growth and adhesion. Cancer Res.
68:5716–5723. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moreno CS, Evans CO, Zhan X, Okor M,
Desiderio DM and Oyesiku NM: Novel molecular signaling and
classification of human clinically nonfunctional pituitary adenomas
identified by gene expression profiling and proteomic analyses.
Cancer Res. 65:10214–10222. 2005. View Article : Google Scholar
|
|
25
|
Evans CO, Moreno CS, Zhan X, et al:
Molecular pathogenesis of human prolactinomas identified by gene
expression profiling, RT-qPCR, and proteomic analyses. Pituitary.
11:231–245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miao Z, Miao Y, Lin Y and Lu X:
Overexpression of the Notch3 receptor in non-functioning pituitary
tumours. J Clin Neurosci. 19:107–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
28
|
Konishi J, Yi F, Chen X, Vo H, Carbone DP
and Dang TP: Notch3 cooperates with the EGFR pathway to modulate
apoptosis through the induction of Bim. Oncogene. 29:589–596. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haruki N, Kawaguchi KS, Eichenberger S, et
al: Dominant-negative Notch3 receptor inhibits mitogen-activated
protein kinase pathway and the growth of human lung cancers. Cancer
Res. 65:3555–3561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohashi S, Natsuizaka M, Yashiro-Ohtani Y,
et al: NOTCH1 and NOTCH3 coordinate esophageal squamous
differentiation through a CSL-dependent transcriptional network.
Gastroenterology. 139:2113–2123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Masieron M, Minuzzo S, Pusceddu I, et al:
Notch3-mediated regulation of MKP-1 levels promotes survival of T
acute lymphoblastic leukemia cells. Leukemia. 25:588–598. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Desiderio DM and Zhan X: The human
pituitary proteome: the characterization of differentially
expressed proteins in an adenoma compared to a control. Cell Mol
Biol (Noisy-le-grand). 49:689–712. 2003.PubMed/NCBI
|
|
33
|
Goldberg LB, Aujla PK and Raetzman LT:
Persistent expression of activated Notch inhibits corticotrope and
melanotrope differentiation and results in dysfunction of the HPA
axis. Dev Biol. 358:23–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu X, Zhang J, Tollkuhn J, et al:
Sustained Notch signaling in progenitors is required for sequential
emergence of distinct cell lineages during organogenesis. Genes
Dev. 20:2739–2753. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dutta S, Dietrich JE, Westerfield M and
Varga ZM: Notch signaling regulates endocrine cell specification in
the zebrafish anterior pituitary. Dev Biol. 319:248–257. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng Q, Li S, Chepeha DB, et al: Crosstalk
between tumor and endothelial cells promotes tumor angiogenesis by
MAPK activation of Notch signaling. Cancer Cell. 8:13–23. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Steg AD, Katre AA, Goodman B, et al:
Targeting the notch ligand JAGGED1 in both tumor cells and stroma
in ovarian cancer. Clin Cancer Res. 17:5674–5685. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bellavia D, Campese AF, Alesse E, et al:
Constitutive activation of NF-kappaB and T-cell leukemia/lymphoma
in Notch3 transgenic mice. EMBO J. 19:3337–3348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vacca A, Felli MP, Palermo R, et al:
Notch3 and pre-TCR interaction unveils distinct NF-kappaB pathways
in T-cell development and leukemia. EMBO J. 25:1000–1008. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Monahan P, Rybak S and Raetzman LT: The
notch target gene HES1 regulates cell cycle inhibitor expression in
the developing pituitary. Endocrinology. 150:4386–4394. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kita A, Imayoshi I, Hojo M, et al: Hes1
and Hes5 control the progenitor pool, intermediate lobe
specification, and posterior lobe formation in the pituitary
development. Mol Endocrinol. 21:1458–1466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jordan S, Lidhar K, Korbonits M, Lowe DG
and Grossman AB: Cyclin D and cyclin E expression in normal and
adenomatous pituitary. Eur J Endocrinol. 143:R1–R6. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shih IeM and Wang TL: Notch signaling,
gamma-secretase inhibitors, and cancer therapy. Cancer Res.
67:1879–1882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tammam J, Ware C, Efferson C, et al:
Down-regulation of the Notch pathway mediated by a gamma-secretase
inhibitor induces anti-tumour effects in mouse models of T-cell
leukaemia. Br J Pharmacol. 158:1183–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Konishi J, Kawaguchi KS, Vo H, et al:
Gamma-secretase inhibitor prevents Notch3 activation and reduces
proliferation in human lung cancers. Cancer Res. 67:8051–8057.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kondratyev M, Kreso A, Hallett RM, et al:
Gamma-secretase inhibitors target tumor-initiating cells in a mouse
model of ERBB2 breast cancer. Oncogene. 31:93–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ramakrishnan V, Ansell S, Haug J, et al:
MRK003, a γ-secretase inhibitor exhibits promising in vitro
pre-clinical activity in multiple myeloma and non-Hodgkin’s
lymphoma. Leukemia. 26:340–348. 2012.
|