Introduction
Advances in resection techniques and adjuvant
therapies continue to improve the outcome of patients with
malignant primary brain tumors. However, substantial gains have
been slow and the prognosis of patients with glioblastoma
multi-forme (GBM), the most common type of primary malignant brain
tumor in adults, remains poor. The median survival time following
diagnosis is 14 months. A greater understanding of the
epidemiology, in particular with regard to immunological factors,
may increase insights into more effective therapeutic
strategies.
According to the 2000–2004 data of the Central Brain
Tumor Registry of the United States (1), GBM accounts for 19% of all primary
intraparenchymal brain tumors. Although GBM is relatively rare in
younger patients, its incidence increases with age; individuals
between 75 and 84 years of age have the highest incidence rates.
The median affected age is 64 years and the overall annual
incidence rate is 3.1/100,000 individuals. The prognosis remains
poor, with a 5-year survival rate of <4% from the time of
diagnosis.
The highest incidence of primary brain tumors is due
to GBM, second only to meningioma. Similar to with GBM, the
incidence of meningioma increases with age. The total number of
meningioma cases in the USA between 2004 and 2006 was 53,455,
compared with 27,040 cases of GBM. The occurrence of meningioma is
7-fold more common in Caucasians compared with African-Americans,
and its frequency of occurrence in females is 2.7-fold higher that
in males (1). In contrast to GBM,
the overall 5-year survival rate of patients with benign menigiomas
is 70%; skull base meningioma patients and younger patients have an
even higher survival rate (2).
Given the poor prognosis associated with malignant
brain tumors, intense research of the epidemiological and molecular
profile of this disease is underway. Established risk factors for
the development of primary brain tumors include exposure to
ionizing radiation, increasing age, male gender, Caucasian
ethnicity and familial tumor syndromes.
The hepatitis B (HBV) and C (HCV) viruses are well
characterized. A correlation between hepatitis infection and
neoplasia has also been identified, and is reflected in the
increased risk of hepatocellular carcinoma observed in patients
with HBV/HCV (3–6). It is estimated that ∼4% of individuals
in the USA are affected by HBV. The annual incidence represents
200–300,000 new cases in the USA, or an incidence rate of 1/1,359
(0.07%). By comparison, 2–5 million people in the USA are proposed
to be affected by HCV, often as asymptomatic carriers. The annual
incidence is 150,000 new cases, with a rate of 1/1,813 (0.06%)
(7).
A correlation between brain tumors and hepatitis
infection is yet to be reported. An impact of co-morbidities,
including the effect of HBV/HCV infection on the occurrence and/or
progression of GBM, has not yet been described. One line of
evidence supporting the hypothesis that a chronic hepatitis
infection may decrease the risk of tumorigenesis is epidemiological
data suggesting a correlation between immune system status and the
risk of gliomagenesis. Population-based studies have focused on the
presence of allergic and inflammatory states in patients diagnosed
with glioma. Certain studies indicate that the risk of developing
glioma is decreased by heightened immune states. Schoemaker et
al identified a negative correlation between the risk of
developing glioma and a history of asthma and hay fever (8). Likewise, Wiemels et al
demonstrated that patients with allergies were 50% less likely to
develop gliomas (9). Additionally,
certain infections have been demonstrated to induce a similar
effect. For example, patients with gliomas are less likely to have
had a herpes virus infection (10).
Furthermore, it is also possible that hepatitis infection, by way
of immunomodulation, decreases the risk of glioblastoma
tumorigenesis independently of the shortened lifespan associated
with the viral illness.
The mechanisms underlying these epidemiological
observations remain unclear. However, animal studies have clearly
indicated that natural killer cell activity, macrophage activation
and interferon production following viral infection are increased
in the setting of chronic irritation of the immune system, as
observed in murine hepatitis infections (11–14).
Notably, mice previously infected with murine hepatitis virus
demonstrated augmented responses to antitumor chemotherapy,
resulting in longer survival times (15). Human studies have also revealed that
interferon levels are significantly higher in patients with HCV
compared with healthy controls (16). It is well-known that macrophage
activity and endogenous interferon levels are key elements in the
cancer immunosurveillance response (17). Thus, it is reasonable to expect that
a heightened immune state is able to alter glioma formation and/or
progression.
Given the epidemiological findings relevant to other
viruses and the molecular basis of the immune response, we
hypothesize that patients with GBM have a lower incidence of prior
hepatitis B/C virus infection compared with the overall population.
Conversely, this finding may be interpreted as evidence that
patients with a history of HBV/HCV are less likely to be diagnosed
with GBM.
Materials and methods
Patients
Patient data were obtained by performing a
retrospective chart review at two different institutions in
Southern California, following IRB approval. The first institution
was the City of Hope National Medical Center (COH), a comprehensive
cancer center located in Duarte (CA, USA), and the second was the
Arrowhead Regional Medical Center (ARMC) in Colton (CA, USA). At
both institutions, a search of the electronic medical record was
performed for all patients diagnosed with GBM. Data were available
from 1998–2009 at the COH and 2001–2010 at the ARMC. Only patients
with a tissue diagnosis of GBM were considered in the study. When a
patient was identified as having GBM, the remainder of the
patient’s medical record was explored, including past laboratory
studies, serological testing and the patient’s response to medical
history questionnaires included within the medical record. As our
comparison group, HBV/HCV infection was also measured in patients
with meningiomas, in the same manner that patients with GBM were
evaluated. Given the mostly benign pathology of meningiomas,
patients may elect not to undergo biopsy or resection. Thus,
patients with a radiographic diagnosis of meningioma in addition to
tissue diagnosis were included in the study.
Statistical analysis
Given the study design, which included cases and
controls, we deemed an odds ratio to be an appropriate measure of
relative risk. The incidence of meningiomas with or without a
history of HBV/HCV served as a control in order to compare and
calculate an odds ratio, and to estimate the relative risk of GBM
with a prior hepatitis infection. The odds ratio was calculated by
dividing the number of positive HBV/HCV patients in the GBM
population (GP) by the number of negative HBV/HCV patients (GN);
this number was then divided by the number of positive HBV/HCV
patients in the meningioma population (MP) divided by the number of
negative HBV/HCV patients (MN). Thus, odds ratio = (GP/GN)/(MP/MN).
An odds ratio of 1 implies there is an equal probability of HBV/HCV
in GBM and meningioma. An odds ratio of >1 suggests a higher
probability of having HBV/HCV and GBM compared with meningioma.
Conversely, an odds ratio of <1 suggests a lower probability of
having HBV/HCV and GBM versus meningioma. P<0.05 was considered
to indicate a statistically significant result.
Results
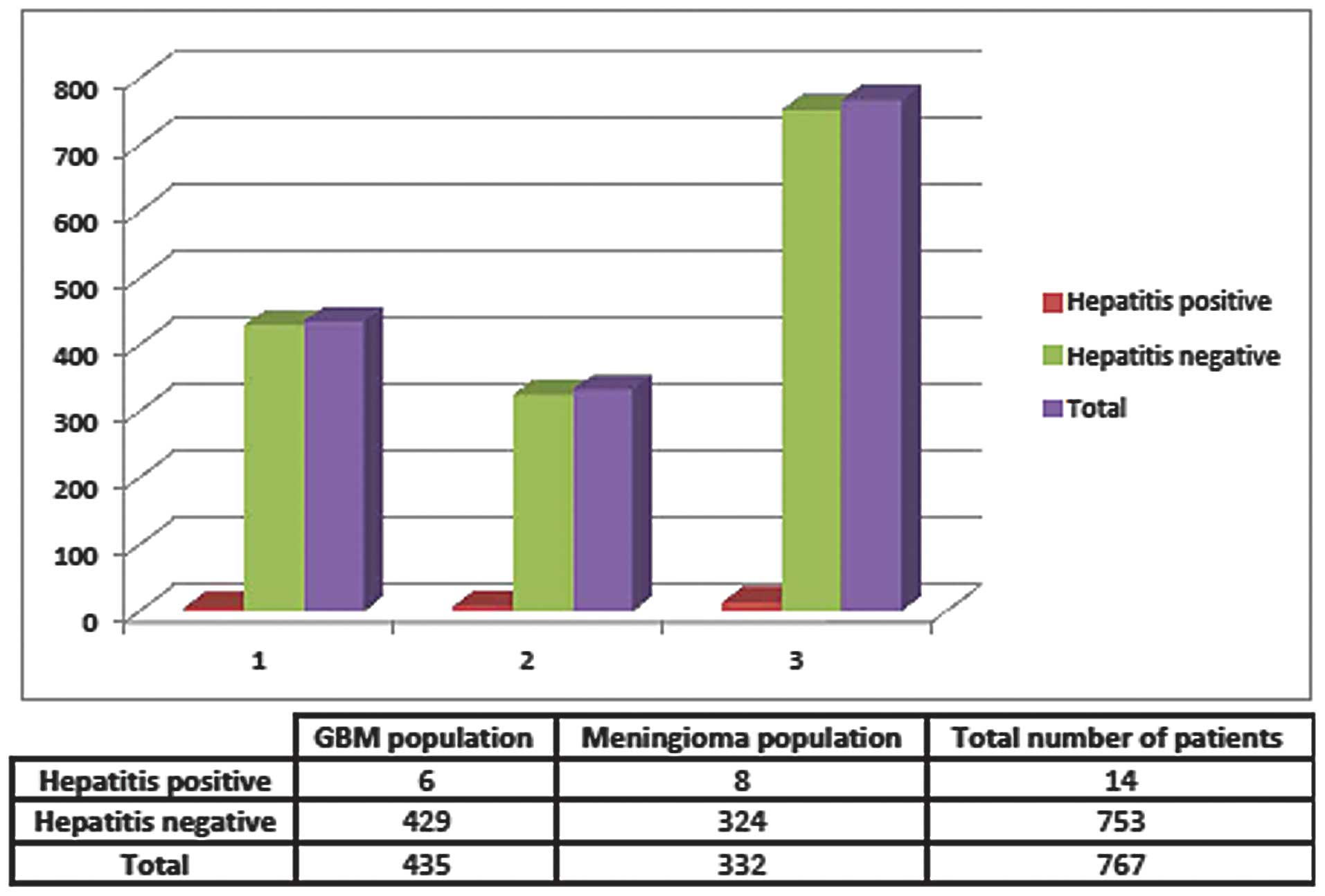
Between 2001 and 2010, 76 ARMC patients were
identified as having GBM, 3 of whom also had positive anti-HCV
antibody serologies and no positive HBV serologies. At the COH,
between 1998 and 2010, 359 patients had GBM and again only 3 were
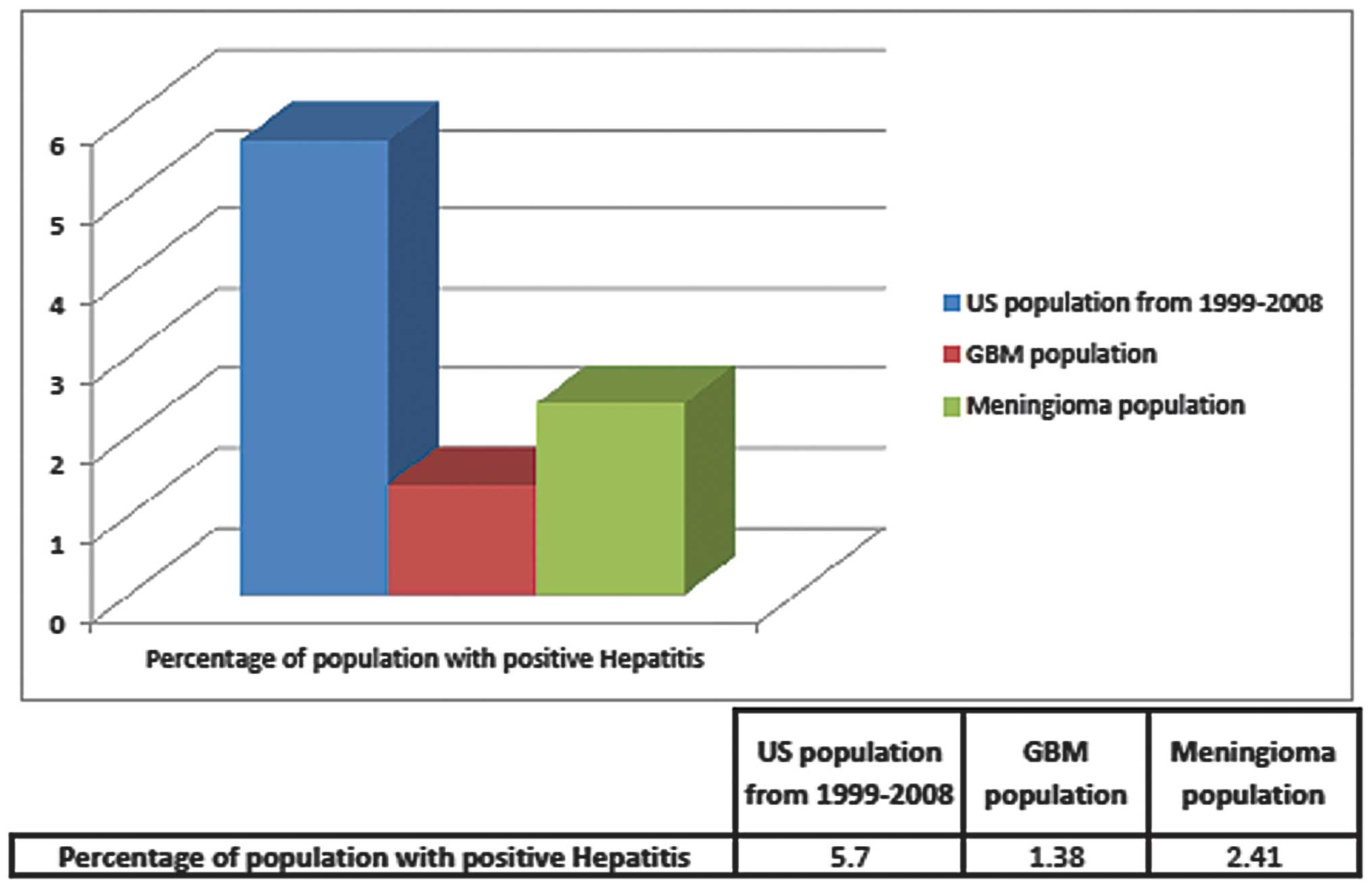
found to have HBV or HCV. Retrospectively, the overall prevalence
of any type of hepatitis virus within this combined GBM population
was 1.38%, or a rate of 0.0138. As a control, the rates of
hepatitis infection in patients with meningioma were also examined
at both institutions. Of the 332 meningioma patients, 8 had either
active infection or a history of hepatitis infection, producing a
rate of 0.0241, or 2.4%, of the meningioma population (Figs. 1 and 2). The odds ratio of having HBV or HCV
with GBM compared with HBV or HCV with meningiomas was 0.566 with a
95% confidence interval of 0.194–1.648 (Table I). A χ2 test of
association revealed a Pearson’s value of 1.115 (P=0.29) and a
one-tailed Fisher’s exact probability of P=0.21.
 | Table IStatistical analysis. |
Table I
Statistical analysis.
| Condition and
population | Rate (%) | Test | Result | 95% confidence
interval |
|---|
| Hepatitis in GBM
population | 1.38 | Odds ratio | 0.566 | 0.195–1.649 |
| Hepatitis in
meningioma population (control) | 2.41 | Fisher’s exact
probability | P=0.21 | |
Discussion
To the best of our knowledge, this is the first
investigation of the correlation between GBM and hepatitis virus
infection. The main limitations of our study are two-fold. The
first limitation is that this was a retrospective analysis.
Therefore, patients in this study were not prospectively tested or
specifically asked about hepatitis status at the time of diagnosis
of GBM or meningioma, and thus the incidence of infection may be
underreported. The second limitation was the small sample size
relative to the number of patients who were expected to have GBM
and hepatitis B/C if the two diseases did not preclude each other.
The discordance between the co-diagnosis of GBM and hepatitis is
that patients with hepatitis often have associated medical or
social factors that predispose them to shorter life spans and thus
are less likely to be diagnosed with GBM, a condition associated
with increasing age. This may further explain the
lower-than-expected prevalence of HBV/ HCV in our study
populations.
Our study limitations allows us to only calculate an
odds ratio, which is not necessarily a relative risk, which is the
most accurate value for correlational statistics. However, given
our sample size, our limitation is that we are assuming odds ratio
is approaching a relative risk if it was able to be determined. In
other words, we are assuming the two values are similar for this
study. Our original hypothesis was that a prior hepatitis B or C
infection would confer resistance to GBM, and thus patients with
hepatitis B and C would have a lower relative risk of contracting
the disease. However, calculation of the relative risk is flawed by
the retrospective nature of the study in which we evaluated GBM
populations rather than undertaking a prospective analysis of
hepatitis B and C populations. Also, it is noteworthy that relative
risk in this instance is only applicable if hepatitis infection
occurred prior to the diagnosis of GBM or meningioma. Although not
all patient information relevant to the timing of hepatitis
infection was available, it is almost certain that hepatitis
infection occurred prior to GBM, due to the rapid progression and
lethality of the disease. However, this assumption may be invalid
for meningioma patients whose disease typically occurs over a
chronic course. Nonetheless, although our findings are not
statistically significant, we propose that further, statistically
powerful prospective studies are required to investigate the
correlation between hepatitis infection status and GBM.
It may be speculated that currently unidentified
factors underlie the lower incidence of HBV/HCV observed in GBM
patients and that infection with one of these two viruses provides
a form of immunoprotection against the development of GBM. One
possible mechanism to support this idea is that patients with a
chronic hepatitis infection demonstrated elevated levels of
interleukin 13 (IL13). IL13, a potent mediator of apoptosis in
tumor cells, has been demonstrated to be significantly increased in
patients with chronic hepatitis infection and allergies (18,19).
High grade gliomas frequently overexpress an IL13 receptor subtype,
designated IL13Rα2. IL13Rα2 functions as a ‘decoy receptor’ and is
ineffective in activating the apoptotic cascade upon ligand
binding. We speculate that hepatitis infections may increase IL13
levels, dampening the competitive effects of IL13Rα2 and thereby
inhibiting gliomagenesis.
Glioma progression is also associated with
immunosuppression in both murine models and patients. This state of
immunosuppression is characterized by a diminished capacity of
macrophages and lymphocytes to secrete interleukins and tumor
necrosis factor α (TNFα) (20,21).
Therefore, increased circulating levels of TNFα released as a
consequence of hepatitis-induced stimulation of the immune system
may diminish tumor viability.
In summary, there is limited epidemiological
evidence in support of a role for HBV/HCV in GBM tumorigenesis
and/or progression. In our study, we observed a lower incidence of
HBV/HCV in GBM patients compared with the general population,
suggesting that HCV/HBV may interfere with GBM development.
However, there was no statistically significant difference when
compared with the incidence of hepatitis in meningioma patients
that served as a control group.
Identifying factors that are able to influence
gliomagenesis may substantially increase our understanding of GBM
biology and enhance the treatment of patients affected by this
invariably life-threatening disease. We suggest that a future
prospective study following patients with HCV and HBV to assess
their rates of acquired GBM compared with non-hepatitis patients,
in addition to prospectively screening patients with GBM for HCV
and HBV serological markers, is required.
Acknowledgements
The authors would like to thank Dr
Rebecca Nelson of the COH for her assistance with this study, in
addition to the staff and administration of the COH and the
ARMC.
References
|
1
|
Dolecek TA, Propp JM, Stroup NE, et al:
CBTRUS Statistical Report: Primary Brain and central nervous system
tumors diagnosed in the United States in 2005–2009. Neuro Oncol.
14(suppl 5)2012.PubMed/NCBI
|
|
2
|
McCarthy BJ, Davis FG, Freels S, et al:
Factors associated with survival in patients with meningioma. J
Neurosurg. 88:831–839. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kasai Y, Takeda S and Takagi H:
Pathogenesis of hepatocellular carcinoma: a review from the
viewpoint of molecular analysis. Semin Surg Oncol. 12:155–159.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zondervan PE, Wink J, Alers JC, et al:
Molecular cytogenetic evaluation of virus-associated and non-viral
hepatocellular carcinoma: analysis of 26 carcinomas and 12
concurrent dysplasias. J Pathol. 192:207–215. 2000. View Article : Google Scholar
|
|
5
|
Hsia CC, Scudamore CH, Di Bisceglie AM, et
al: Molecular and serological aspects of HBsAg-negative hepatitis B
virus infections in North America. J Med Virol. 70:20–26. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bréchot C: Pathogenesis of hepatitis B
virus-related hepatocellular carcinoma: old and new paradigms.
Gastroenterology. 127:S56–S61. 2004.PubMed/NCBI
|
|
7
|
Everhart JE: Digestive Diseases in the
United States: Epidemiology and Impact. US Department of Health and
Human Services, Public Health Service, National Institutes of
Health, National Institute of Diabetes and Digestive and Kidney
Diseases. US Government Printing Office; Washington, DC: 1994
|
|
8
|
Schoemaker MJ, Swerdlow AJ, Hepworth SJ,
et al: History of allergies and risk of glioma in adults. Int J
Cancer. 119:2165–2172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wiemels JL, Wiencke JK, Sison JD, et al:
History of allergies among adults with glioma and controls. Int J
Cancer. 98:609–615. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wrensch M, Weinberg A, Wiencke J, et al:
Does prior infection with varicella-zoster virus influence risk of
adult glioma? Am J Epidemiol. 145:594–597. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dempsey WL, Smith AL and Morahan PS:
Effect of inapparent murine hepatitis virus infections on
macrophages and host resistance. J Leukoc Biol. 39:559–565.
1986.PubMed/NCBI
|
|
12
|
Schindler L, Engler H and Kirchner H:
Activation of natural killer cells and induction of interferon
after injection of mouse hepatitis virus type 3 in mice. Infect
Immun. 35:869–873. 1982.PubMed/NCBI
|
|
13
|
Tamura T, Sakaguchi A, Kai C, et al:
Enhanced phagocytic activity of macrohpages in mouse hepatitis
virus-infected nude mice. Microbiol Immunol. 24:243–247. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Virelizier JL, Virelizier AM and Allison
AC: The role of circulating interferon in the modifications of
immune responsiveness by mouse hepatitis virus (MHV-3). J Immunol.
117:748–753. 1976.PubMed/NCBI
|
|
15
|
Li LH, DeKoning TF, Nicholas JA, et al:
Effect of mouse hepatitis virus infection on combination therapy of
P388 leukemia with cyclophosphamide and pyrimidinones. Lab Anim
Sci. 37:41–44. 1987.PubMed/NCBI
|
|
16
|
Kawakami Y, Nabeshima S, Furusyo N, et al:
Increased frequency of interferon-gamma-producing peripheral blood
CD4+ T cells in chronic hepatitis C virus infection. Am J
Gastroenterol. 95:227–232. 2000.
|
|
17
|
Morahan PS, Edelson PJ and Gass K: Changes
in macrophage ectoenzymes associated with anti-tumor activity. J
Immunol. 125:1312–1317. 1980.PubMed/NCBI
|
|
18
|
Inoue M, Kanto T, Miyatake H, et al:
Enhanced ability of peripheral invariant natural killer T cells to
produce IL-13 in chronic hepatitis C virus infection. J Hepatol.
45:190–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Xia Y, Nguyen A, et al: Effects of
Th2 cytokines on chemokine expression in the lung: IL-13 potently
induces eotaxin expression by airway epithelial cells. J Immunol.
162:2477–2487. 1999.PubMed/NCBI
|
|
20
|
Kennedy BC, Maier LM, D’Amico R, et al:
Dynamics of central and peripheral immunomodulation in a murine
glioma model. BMC Immunol. 10:112009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao C, Parney IF, Roa WH, et al: Cytokine
and cytokine receptor mRNA expression in human glioblastomas:
evidence of Th1, Th2 and Th3 cytokine dysregulation. Acta
Neuropathol. 103:171–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McQuillian GM, Kruszon-Moran D, Denniston
MM, et al: Viral hepatitis. NCHS Data Brief 1–8. 2010
|