Introduction
Sorafenib is an oral multikinase inhibitor that is
clinically active in advanced hepatocellular carcinoma (HCC) and is
the present standard of care in the advanced stage of this
particular malignancy. The analysis of sorafenib activity in two
phase III placebo-controlled clinical trials undertaken in patients
with advanced HCC in Western countries (the Sharp trial) and in the
Asia-Pacific area, demonstrated that sorafenib induces an overall
survival benefit that typically involves disease stabilization,
whereas objective responses are rare (1–2).
Herein, we report a case of an advanced hepatitis C virus
(HCV)-related HCC with extensive infiltration of the inferior
vena cava (BCLC stage C), which demonstrated a rapid
complete biochemical response and a long-term favorable outcome
with prolonged sorafenib treatment. Written informed consent was
obtained from the patient.
Case report
In January 2007, a 72-year-old man with a clinical
history of HCV-related hepatopathy was diagnosed with HCC. A
computed tomography (CT) scan revealed a lesion of 1.5 cm in
diameter in hepatic segment VIII with contrast enhancement in
arterial phase (BCLC stage A, Child-Pugh A). Additionally, the
patient’s α-fetoprotein (αFP) serum level was 487.3 ng/ml. Between
March and November 2007, the patient was treated with 4 courses of
percutaneous ethanol injections, which demonstrated an initial, but
transitory, clinical benefit. In February 2008, the patient was
referred to the Unit of Clinical Oncology, Department of Medical
and Surgical Sciences (Foggia, Italy) due to rapid progression of
the disease. The CT scan revealed a large mass (8.6 cm in diameter)
in hepatic segment VIII, which was formed by multiple confluent
nodules, infiltrating the inferior vena cava, altering the
liver border and exhibiting typical arterial contrast enhancement
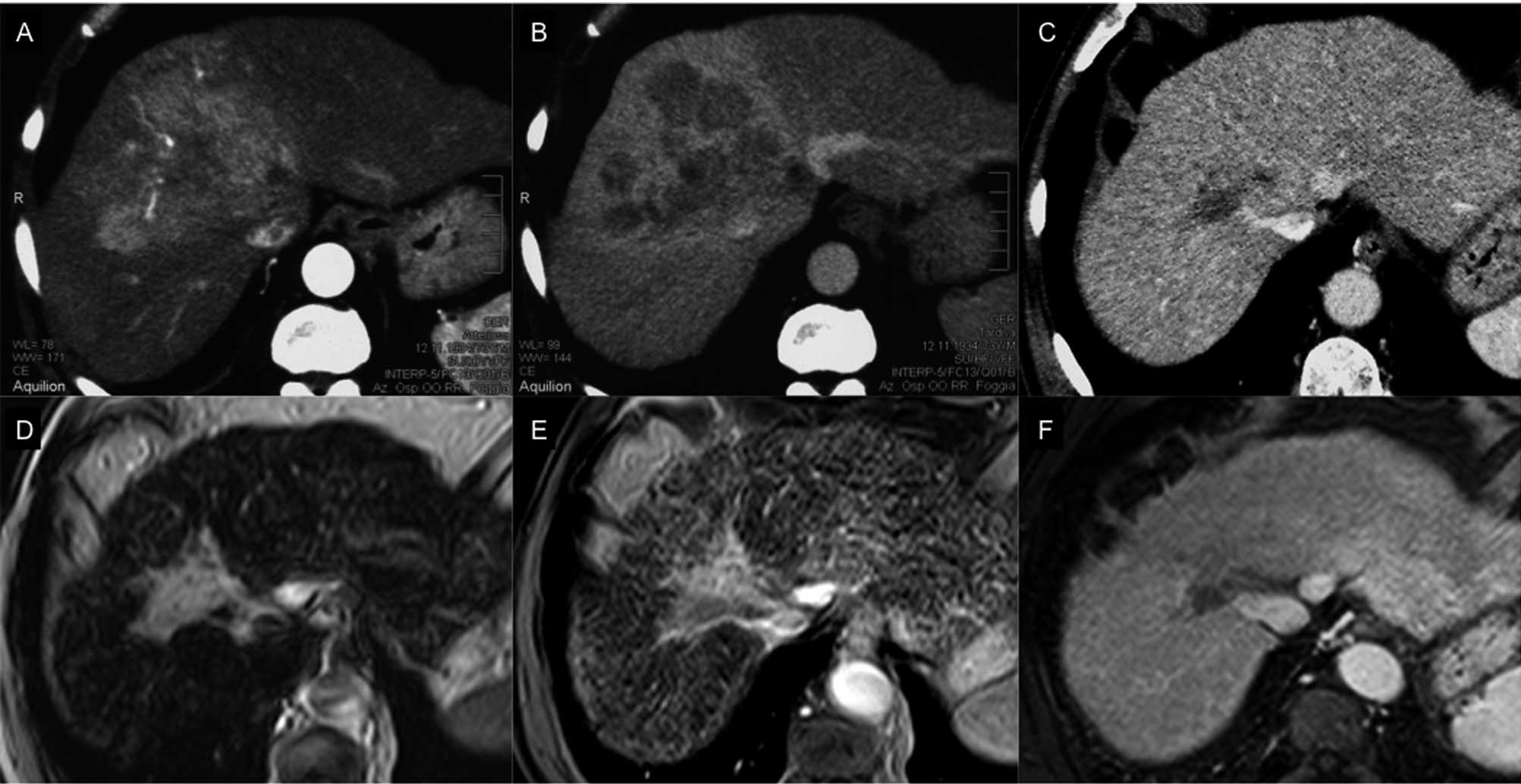
(Figs. 1A and B). A chest CT scan
did not detect extrahepatic metastasis. Based on these features,
the malignancy was classified as BCLC stage C. The hepatic function
was well-retained, with no clinical signs of liver dysfunction
(Child-Pugh A) and an αFP serum level of 179.674 ng/ml.
As it was not possible to further use locoregional
therapies, treatment with sorafenib (400 mg twice a day) was
inititated. Notably, a rapid and sustained decrease in αFP serum
level was observed with a normalization of the serum value (6.1
ng/ml) evident within 3 months of therapy. By contrast, the liver
imaging showed an initial disease stabilization and, thereafter, a
slow but progressive tumor shrinkage. Upon hepatic ultrasonography
and CT scan the neoplastic lesion measured 7.4 cm in October 2008
(following 6 months of therapy), 5.3 cm in June 2009 and 4.0 cm in
September 2010 (Fig. 1C). A
double-contrast magnetic resonance imaging (MRI) scan with
sequential use of hepato-specific superparamagnetic iron oxide
(SPIO) and paramagnetic (gadolinium) contrast agents was performed
in February 2011, revealing an area with slight signs of
vascularization in hepatic segment VIII that resembled fibrotic
tissue (Figs. 1D and E). Sorafenib
was well-tolerated and the patient only presented with a grade 2
(NCI-CTCAE v3.0) hand-foot skin reaction, which was managed with
urea cream; a grade 1–2 asthenia and occasional grade 2
hematological toxicities (leucopenia and thrombocytopenia), which
were managed by 1–2 weeks of drug interruption. After 4 years, the
patient has remained under sorafenib therapy, while αFP serum
levels have remained within the normal range and radiological
imaging has not exhibited any signs of disease progression
(Fig. 1F).
Discussion
Two issues are of particular significance in the
present case report: i) the rapid biochemical complete response,
followed by a slow but progressive and long-lasting tumor
shrinkage; and ii) the lack of major toxicities, despite 4 years of
essentially uninterrupted treatment. Sorafenib is the only
molecular-targeted agent that has demonstrated a clinical benefit
in phase III studies in advanced HCC (1–2).
However, under sorafenib therapy, the majority of patients
demonstrate disease stabilization, while tumor shrinkage is a less
commom occurrence. Complete responses have not been noted in phase
III clinical trials (1–2), while a number of recent studies have
described major responses to sorafenib (3–7).
Concordant with recent literature, the present case report suggests
that a subset of HCCs may be particularly responsive to sorafenib
and may undergo long-lasting tumor shrinkage. Thus, molecular
determinants of susceptibility to sorafenib are required, in order
to select patients that are more likely to benefit from this
molecular-targeted agent and prolonged treatment. In this regard,
we hypothesize that a rapid normalization of αFP serum levels may
represent a surrogate marker of sensitivity to sorafenib. This
hypothesis is concordant with recent reports demonstrating that the
αFP response is a statistically significant prognostic factor for
survival in HCC patients treated with sorafenib (8–9). Thus,
early evaluation of αFP may be regarded as a reliable alternative
to response evaluation criteria in solid tumors (RECIST) to capture
sorafenib activity in HCC.
Notably, no major life-threatening toxicities or
detrimental effects on quality of life were observed in the present
case report, despite the 4 years of essentially uninterrupted
treatment. This result is even more significant considering that
the median duration of treatment in the phase III Sharp trial was
5.3 months (range 0.2–16.1) (1). A
prompt initiation of sorafenib therapy, when the hepatic function
has not yet been compromised, and an optimal management of side
effects represent two prerequisites to allow a safe and, in the
case of demonstrated efficacy, prolonged treatment for HCC
patients. Consistently, recent studies have highlighted the
importance of prolonged sorafenib administration, even at reduced
doses, and personalized toxicity management to achieve tumor
responses to sorafenib therapy (10).
References
|
1
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
Schwartz M, et al SHARP Investigators Study Group: Sorafenib in
advanced hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, et al: Efficacy and
safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakazawa T, Hidaka H, Shibuya A and
Koizumi W: Rapid regression of advanced hepatocellular carcinoma
associated with elevation of des-gamma-carboxy prothrombin after
short-term treatment with sorafenib - a report of two cases. Case
Rep Oncol. 3:298–303. 2010. View Article : Google Scholar
|
|
4
|
Di Lorenzo G, Imbimbo M, Leopardo D,
Marciano R, Federico P, Buonerba C, Salvatore B, Marinelli A and
Palmieri G: A long-lasting response to sorafenib treatment in an
advanced hepatocellular carcinoma patient. Int J Immunopathol
Pharmacol. 23:951–954. 2010.PubMed/NCBI
|
|
5
|
Chelis L, Ntinos N, Souftas V, Deftereos
S, Xenidis N, Chamalidou E, Maltezos E and Kakolyris S: Complete
response after sorafenib therapy for hepatocellular carcinoma in an
HIV-HBV co-infected patient: Possible synergy with HAART? A case
report Med Oncol. 28:S165–S168. 2010.PubMed/NCBI
|
|
6
|
Inuzuka T, Nishikawa H, Sekikawa A, Takeda
H, Henmi S, Sakamoto A, Saito S, Kita R, Kimura T, Osaki Y and Kudo
M: Complete response of advanced hepatocellular carcinoma with
multiple lung metastases treated with sorafenib: a case report.
Oncology. 81:152–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sacco R, Bargellini I, Gianluigi G,
Bertini M, Bozzi E, Altomare E, Battaglia V, Romano A, Bertoni M,
Capria A, Bresci G, et al: Complete response for advanced liver
cancer during sorafenib therapy: case report. BMC Gastroenterol.
11:42011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yau T, Yao TJ, Chan P, Wong H, Pang R, Fan
ST and Poon RT: The significance of early α-fetoprotein level
changes in predicting clinical and survival benefits in advanced
hepatocellular carcinoma patients receiving sorafenib. Oncologist.
16:1270–1279. 2011.
|
|
9
|
Personeni N, Bozzarelli S, Pressiani T,
Rimassa L, Tronconi MC, Sclafani F, Carnaghi C, Pedicini V,
Giordano L and Santoro A: Usefulness of alpha-fetoprotein response
in patients treated with sorafenib for advanced hepatocellular
carcinoma. J Hepatol. 57:101–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbadessa G, Rimassa L, Pressiani T,
Carrillo-Infante C, Cucchi E and Santoro A: Optimized management of
advanced hepatocellular carcinoma: four long-lasting responses to
sorafenib. World J Gastroenterol. 17:2450–2453. 2011. View Article : Google Scholar : PubMed/NCBI
|