Introduction
Upper urinary tract urothelial carcinomas (UUTUCs)
are relatively uncommon tumors, accounting for ∼5% of all
urothelial and 5–10% of all renal tumors. However, UUTUCs are
associated with severe morbidity and mortality (1,2). These
carcinomas are located more commonly in the renal pelvis than in
the ureter, at a ratio of 3:1 (2,3). The
incidence of bilateral UUTUCs is 2–8% (2,4). The
development of UUTUC following primary diagnosis of bladder cancer
is a rare event, occurring in only 2–4% of patients with bladder
cancer (5); however, the
development of secondary bladder cancer following primary UUTUC is
more frequent with a risk of 20–50% (3,6–8). In
addition, UUTUCs are more invasive and poorly differentiated
compared with bladder cancer (9).
Furthermore, microsatellite alterations in UUTUCs differ from
bladder cancer (10). These
observations indicate that UUTUCs and bladder cancer may exhibit
specific variations with respect to cancer initiation and
progression.
In a previous study, the 5-year disease-specific
survival rates of the patients by primary tumor stage were
identified as 100% for Ta/cis, 91.7% for T1, 72.6% for T2 and 40.5%
for T3. Patients with primary stage T4 tumors had a median survival
of 6 months (6). Radical
nephroureterectomy with excision of an ipsilateral bladder cuff
remains the gold standard for treatment of invasive UUTUCs
(2,11,12).
Since the development of metastatic disease leads to treatment
failure in patients with locally advanced upper tract urothelial
carcinoma and the high risk of disease relapse and cancer mortality
for patients with stages III and IV UUTUCs, it is critical to
prevent relapse following initial aggressive surgical therapy.
Systemic adjuvant and neoadjuvant chemotherapy may prevent
progression to metastatic disease and prolong survival (1,13).
Furthermore, with increasing use of renal-sparing therapy by
endoscopic means, chemotherapeutic agents are likely to become more
important for the treatment of cancer, including urothelial
carcinomas (14). Therefore, the
development of a more effective adjuvant systemic therapy may
provide significant benefit to patients with invasive UUTUCs.
UUTUC affects more males than females with a
male-to-female ratio of 3:2 for tumors in the renal pelvis and 2:1
for those in a ureteral location (12). Disease-specific annual mortality is
greater in females than in males (11). However, the mechanism by which
gender difference affects the progression and prognosis is not
clearly understood.
The role of androgen receptor (AR) in urothelial
carcinoma of the ureter and renal pelvis remains unclear; however,
AR has been demonstrated to affect urothelial carcinoma of the
bladder (15). AR was identified in
patients with localized and advanced transitional cell carcinoma of
the bladder and kidney (16). AR
protein expression has been found to decrease in tumors at higher
pathological stages and grades, indicating that the loss of AR
expression is associated with higher grade urothelial carcinomas
(UCs) and invasive UCs, but has limited effect on the prognosis for
survival (17,18). In a rat model of bladder
carcinogenesis, testosterone was found to increase carcinogenesis
(19). In addition, in a mouse
model of bladder cancer and bladder cancer cells, androgens/AR were
demonstrated to promote bladder cancer development and increase
bladder cancer cell proliferation in vitro and xenograft
tumor growth in vivo(15).
Furthermore, in human transitional carcinoma AR-positive cell
lines, knockdown of AR expression increased cell death and
decreased proliferation and migration of bladder cancer cells. In
xenograft models, AR knockdown in implanted bladder cancer cells
suppressed AR-positive bladder tumor growth, indicating that AR is
a potential therapeutic target for the treatment of bladder cancer
(20). These observations indicate
a role for AR in enhancing bladder cancer development. However, for
other forms of urothelial carcinomas, the role of AR in UUTUC
development and progression is not clear. We recently revealed that
there is a positive correlation with higher AR expression found in
superficial or low-grade UUTUCs of the ureter (21). However, the effect of AR on the
therapeutic efficacy of chemotherapeutic drugs has not been
previously investigated.
Materials and methods
Cell lines and chemicals
UUTUC cell line, BFTC 909 (from a UUTUC of a renal
pelvis patient), was kindly provided by Dr Tzeng (Cheng Kung
University, Tainan, Taiwan) and cultured in Dulbecco’s modified
Eagle’s medium, containing 10% heat-inactivated fetal bovine serum
(FBS) at 37°C in an atmosphere of 5% CO2(22).
To establish BFTC 909 cell lines overexpressing AR,
cells were stably transfected with a pBabe-hAR plasmid using
Lipofectamine (Invitrogen Life Technologies, Carlsbad, CA, USA)
following the manufacturer’s instructions. Cells were selected
using 1 μg/ml puromycin and AR-overexpressing clones (as
verified by western blot analysis) were named BFTC 909 pBabeAR1 and
BFTC 909 pBabeAR2.
To exogenously express AR, a recombinant lentiviral
vector containing wild-type AR (pWPI hAR) and a control lentiviral
vector expressing the enhanced green fluorescent protein (pWPI)
were used to overexpress AR. Lentiviral PWPI-AR/PWPI-control with
pMD2.G packaging and psPAX2 envelope plasmids
(lentivirus:packaging:envelopeZ, 2:1:1) were co-transfected into
293T cells. Following 48 h transfection, the target cells were
cultured in the presence of viral supernatant containing 8 mg/ml
polybrene (Millipore, Billerica, MA, USA) for 6 h. Flow cytometry
was used to analyze cells overexpressing AR and dead cells were
evaluated by propidium iodine (PI) under chemotherapeutic drug
treatment.
Cisplatin (P4394) was purchased from Sigma-Aldrich
(St. Louis, MO, USA). Doxorubicin hydrochloride and mitomycin C
were obtained from the Hospital Pharmacy at China Medical
University Hospital, Taiwan, China. ASC-J9®
(5-hydroxy-1,7-bis(3,4-dimethoxyphenyl)-1,4,6-heptatrien-3-one) was
donated by AndroScience Corp. (San Diego, CA, USA).
Cell viability assay
Viability of BFTC 909 cells was evaluated in 96-well
plates 48 h after treatment by measuring cellular viability using
the Cell Viability kit (XTT) (Roche Diagnostics, Indianapolis, IN,
USA). The cytotoxic effects of chemotherapeutic drugs were
determined by the colorimetric XTT assay based on the activities of
mitochondrial enzymes in viable cells. Cells were seeded into
96-well culture plates at a seeding density of 1x104
cells/well. After 24 h, complete medium was removed and changed to
DMEM containing 10% FBS and cisplatin, doxorubicin or mitomycin C
at designated concentrations. After 48 h, fresh DMEM containing 50
μl XTT solution was added directly to the medium and cells
were incubated for an additional 3 h at 37°C. A test wavelength
between 450–500 nm and a reference wavelength of 650 nm were used
in the assay.
Protein analysis
For western blot analysis, protein extracts of each
sample (100 μg/lane) were electrophoretically separated and
transferred onto nitrocellulose. Membranes were incubated with
antibodies against AR or ABCG2 (Santa Cruz Biotechnology, Santa
Cruz, CA, USA), followed by horseradish peroxidase-conjugated
secondary antibody. Protein-antibody complexes were detected by an
enhanced chemiluminescence system (Millipore, Bedford, MA, USA)
using the Bio-Rad imaging system (Hercules, CA, USA).
Quantitative real-time (qRT)-PCR
Total RNA was isolated using the TRIzol method
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. Total RNA was reverse transcribed into cDNA using
BluePrint RT Reagent Kit (Takara Bio, Inc., Shiga, Japan). The
primer sequences were as follows: human AR, forward 5′-TGT CCA TCT
TGT CGT CTT C-3′ and reverse 5′-CTC TCC TTC CTC CTG TAG-3′; human
β-actin, forward 5′-TCA CCC ACA CTG TGC CCA TCT ACG A-3′ and
reverse 5′-CAG CGG AAC CGC TCAT TGC CAA TGG-3′; and human ABCG2,
forward 5′-GGG TTC TCT TCT TCC TGA CGA CC-3′ and reverse 5′-TGG TTG
TGA GAT TGA CCA ACA GAC C-3′. qRT-PCR was performed using the
Bio-Rad CFX96 real-time thermal cycler and SYBR Premix. Relative
mRNA expression levels were normalized against β-actin (as an
internal control) and determined by the 2−ΔΔCt
method.
Cell death assay
PI exclusion was used to measure cell death. Cells
were treated with various drugs for 48 h at 37°C. After 48 h, cells
were trypsinized and resuspended in 1 ml PBS with 20 μl PI
(0.5 mg/ml) for 10 min at room temperature. Cell death was analyzed
by flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA).
Statistical analysis
Statistical analysis was performed using Microsoft
Excel using a two-sided Student’s t-test. Data are presented as
mean ± SD. P<0.05 was considered to indicate a statistically
significant difference.
Results
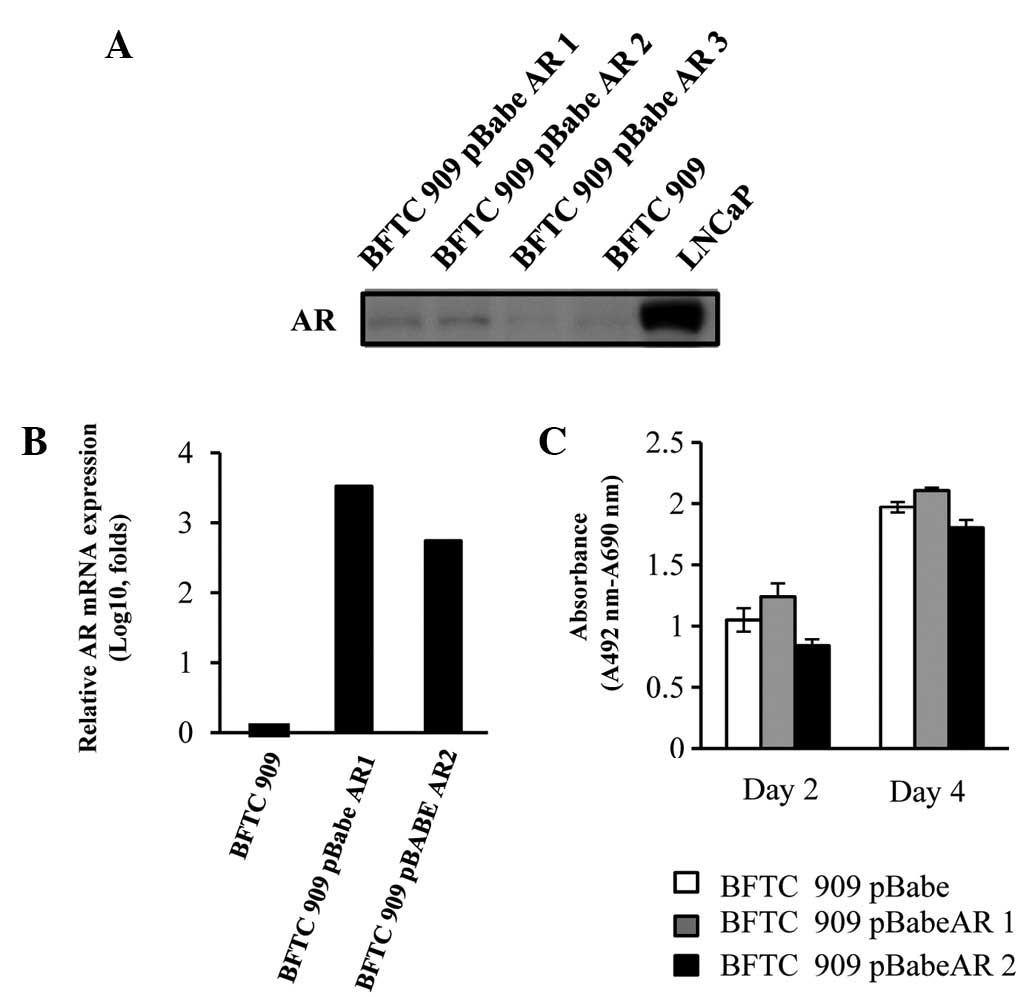
AR overexpression in BFTC 909 cells
To determine the role of AR in the progression of
UUTUCs, AR was overexpressed by stably transfecting AR cDNA into
the UUTUC cell line, BFTC 909 (22). Two cell lines were generated (BFTC
909 pBabeAR1 and BFTC 909 pBabeAR2) by stable transfection. As
demonstrated in Fig. 1, AR protein
was increased in BFTC 909 pBabeAR1 and BFTC 909 pBabeAR2 and when
comparing their relative AR mRNA expression with BFTC 909 control
cells, the AR mRNA levels were increased 100-fold as compared with
BFTC 909 control cells. In addition, expression of AR in BFTC cells
did not affect cell growth (Fig.
1C).
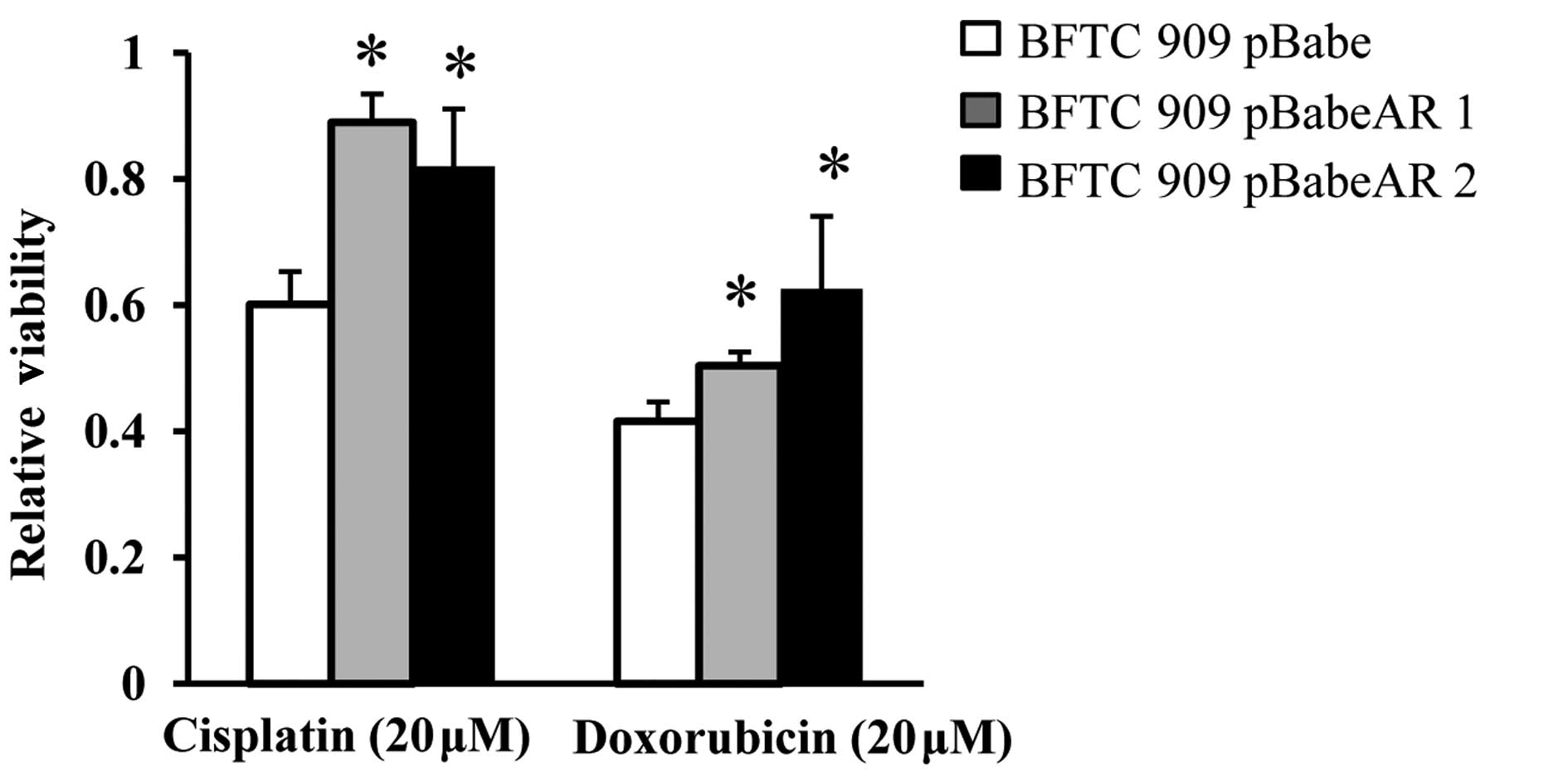
AR overexpression on viability of UUTUC
cells treated with chemotherapeutic agents
To explore the effect of AR on cancer cells, the
effect of AR on the cytotoxic effect of chemotherapeutic agents was
determined. Adjuvant systemic chemotherapy is known to provide
therapeutic benefit in patients and prevent recurrent bladder
tumors with invasive UUTUCs (23,24).
Cisplatin and doxorubicin have been used as anticancer drugs in the
adjuvant chemotherapy of UUTUCs to improve the overall mortality
rate associated with UUTUCs by targeting the remaining cancer cells
(2,13). To investigate the potential role of
AR during chemotherapy of UUTUCs, the effect of AR on the the
cytotoxic effect of cisplatin and doxorubicin was analyzed. BFTC
909 pBabe, BFTC 909 pBabeAR1 and BFTC 909 pBabeAR2 cells were
treated with cisplatin and doxorubicin for 2 days and the cytotoxic
effect was measured by XTT assay to determine the viability. As
revealed in Fig. 2, BFTC 909
pBabeAR1 and BFTC 909 pBabeAR2 cells exhibited higher viability
compared with BFTC 909 cells stably transfected with vector
control.
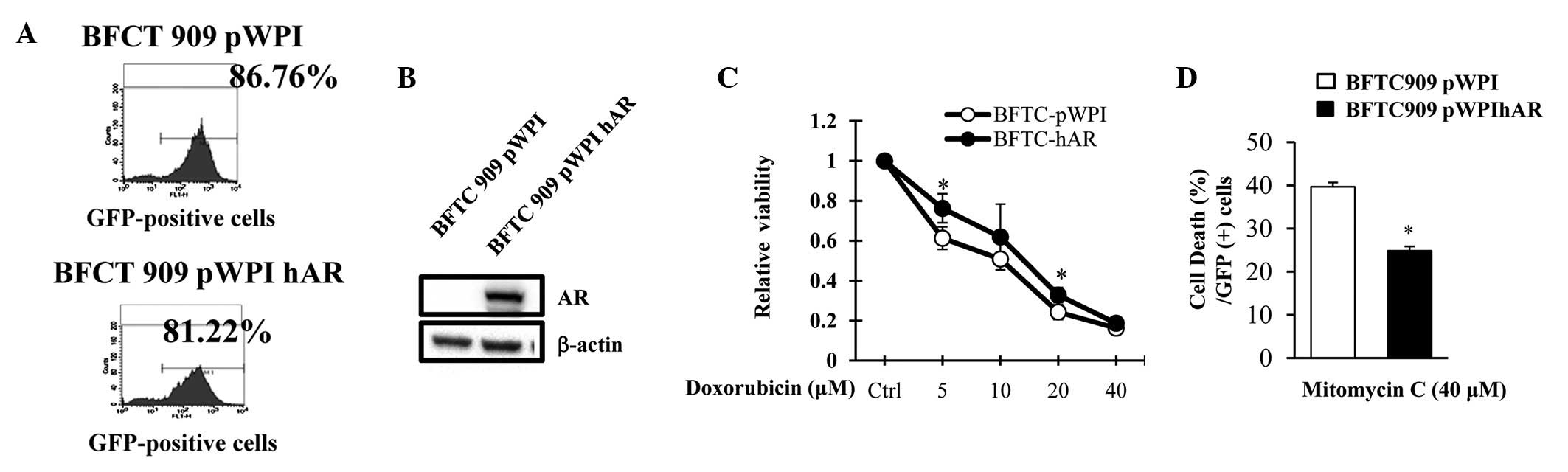
Addition of AR via lentiviral vector on
the viability and cell death of UUTUC cells treated with
chemotherapeutic agents
To further determine the effect of AR on UUTUC cells
treated with chemotherapeutic drugs and reduce the potential
artificial effects via overexpression AR using plasmids, BFTC 909
cells were transfected with a lentiviral system carrying pWPI hAR
or control pWPI parental vectors expressing green fluorescent
protein (GFP) and treated with doxorubicin (Fig. 3C). With similar infection efficiency
(Fig. 3A), exogenous addition of AR
via a lentiviral vector in BFTC cells (GFP-positive cells)
increased the viability of cells under various concentrations of
doxorubicin treatment compared with cells transfected with pWPI
control vector. Mitomycin C is an additional chemotherapeutic drug
used in UUTUC therapy (14,25). Therefore, the effect of AR status on
cell resistance to mitomycin C was analyzed and mitomycin C was
identified to induce more cell death in BFTC cells infected with
lentiviral vector than in BTFC 909 infected with lentiviral vector
expressing AR (Fig. 3D). These
results demonstrate that the status of AR expression in BFTC cells
plays a role in increasing the resistance to mitomycin C-induced
cell death
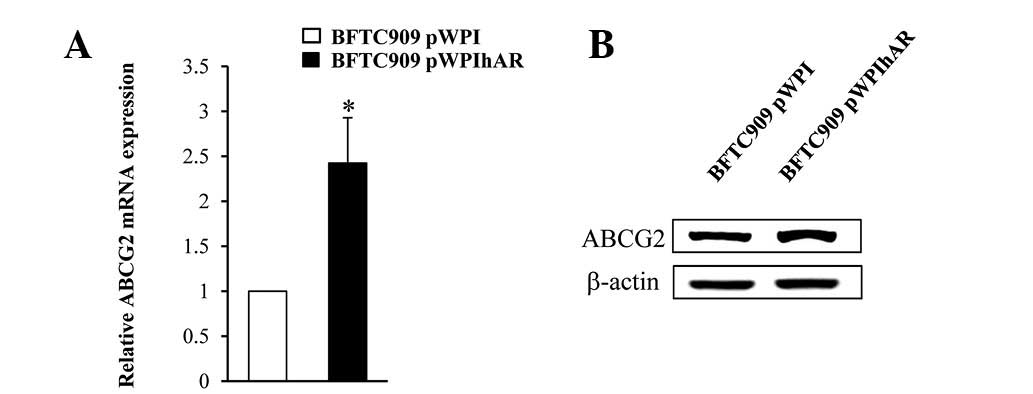
Expression of ABCG2 was increased in BFTC
909 cells over-expressing AR
The membrane transporter protein ABCG2 is linked to
multi-drug chemoresistance in cancer cells due to its ability to
reduce the intracellular concentrations of anticancer drugs using
the energy of ATP hydrolysis to transport drugs across the cell
membrane (26). To determine the
mechanism by which AR overexpression by stable transfection or
lentiviral infection results in increased viability of BFTC 909
cells, mRNA expression of ABCG2 was determined by qRT-PCR and
protein expression of ABCG2 by western blot analysis. As
demonstrated in Fig. 4A, BFTC
pWPI-hAR cells expressed higher levels of ABCG2 compared with BFTC
cells without AR overexpression. ABCG2 protein expression was also
increased in BFTC pWPI-hAR cells compared with their counterpart
controls (Fig. 4B).
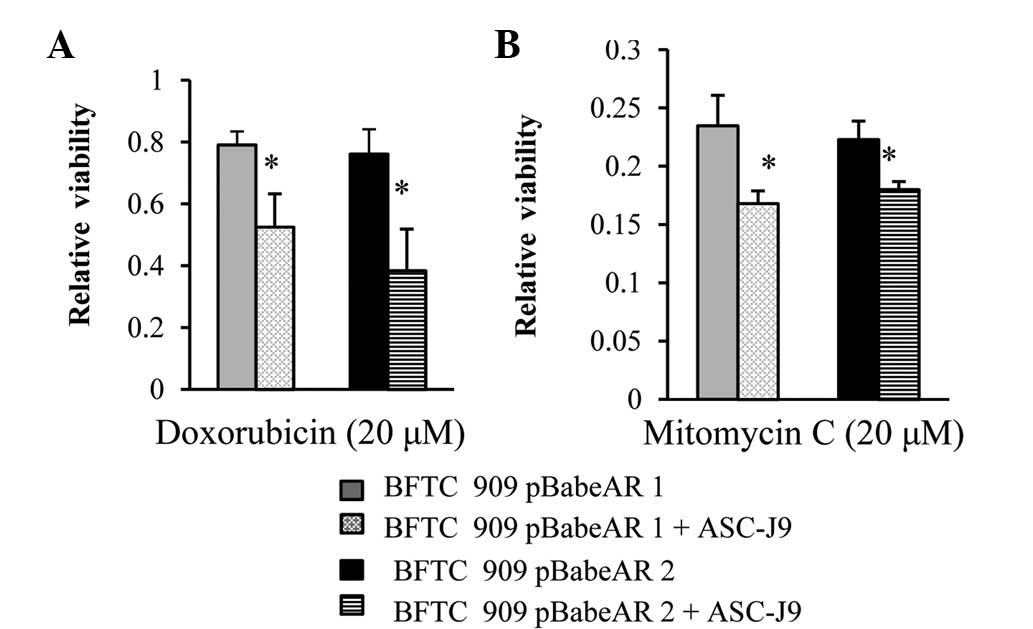
AR degradation enhancer, ASC-J9, blocked
the effect of AR on the cytotoxity of chemotherapeutic drugs
Observations of the present study indicate that it
may be possible to alter the response of UUTUC cells to the
cytotoxic effect of chemotherapeutic drugs via alteration of AR
expression in the cells to enhance their destruction. ASC-J9, the
first AR degradation enhancer to be identified that degraded AR in
selective cells with few side-effects (15,27–30)
was applied to chemotherapeutic drug-treated cells to determine
whether degradation of AR affects cell response to drugs. In the
presence of ASC-J9, BFTC 909 cells overexpressing AR became more
sensitive to doxorubicin treatment (Fig. 5A) or mitomycin C treatment (Fig. 5B).
Discussion
Previous studies have hypothesized that gender is a
factor in the incidence and progression of UUTUCs due to the
observation that males have a substantially higher risk of
developing UUTUCs. In addition, it has been identified that females
tend to have more aggressive tumors (2,3,11).
Although sex hormones and their receptors are involved in
gender-specific differences, the exact roles of sex hormone
receptors in UUTUCs remain unclear. AR has been previously
demonstrated to promote urothelium carcinoma of bladder development
(15,19), indicating that AR may be involved in
UUTUC progression. In the present study, addition AR was revealed
to lead to a decreased cytotoxic effect of chemotherapeutic agents,
including cisplatin, doxorubicin and mitomycin C, on UUTUC cells,
indicating that the status of AR expression affects the treatment
and progression of UUTUCs during chemotherapy.
Although several studies examined the AR expression
pattern in human bladder cancer, the prognostic significance of AR
expression is inconsistent with studies demonstrating that loss of
AR expression is associated with invasive bladder cancer (17,18) or
has no association (31). Miyamoto
et al demonstrated the role of androgen and AR in chemically
induced mouse bladder cancer and identified that androgen and AR
are involved in the carcinogenesis of bladder carcinoma, indicating
that AR may be an androgen-independent carcinogenesis factor for
bladder cancer development (15).
It is likely that AR in the urothelium of the upper urinary tract
may have a similar role in carcinogenesis. Accordingly, AR was
proposed as a potential therapeutic target for the treatment of
bladder cancer since the silencing of AR expression inhibits cell
growth in vitro and in vivo(20).
Chemotherapy is used to increase lifespan and
prevent disease recurrence following surgical resection of
localized cancer. Chemotherapy is also utilized as part of the
multimodal treatment of locally advanced cancer, allowing for more
limited surgery with organ sparing and even cure. Therefore, more
effective and less toxic chemotherapy regimens are likely to
significantly benefit cancer patients. Cisplatin and doxorubicin
have been previously used in adjuvant chemotherapy regimens for
patients with upper tract transitional cell carcinoma and a
positive outcome was reported (23,32).
Mitomycin C was also applied in the chemotherapy of UUTUCs of the
renal pelvis or ureter to prevent the recurrence following surgery
with promising results (33,34).
In the present study, BFTC 909 cells overexpressing AR were
demonstrated to reduce the cytotoxicity of doxorubicin, cisplatin
and mitomycin C in UUTUC cells, indicating that the presence of AR
in BFTC 909 cells increases their drug resistance. These
observations were confirmed further by expressing AR using a viral
vector, revealing that following infection with pWPI hAR, BFTC 909
cells underwent less cell death with mitomycin C treatment than
parent lentiviral vector-infected cells. Resistance to chemotherapy
affects the drug efficacy and the factors affecting the
chemoresistance of cells to chemotherapeutic drugs are largely
associated with the multidrug-resistance-1 gene which encodes
P-glycoprotein, whose function is to pump chemotherapeutic drugs
from the inside of cells and from membranes to the outside
(35). Other factors include drug
inactivation, alterations in drug target, processing of
drug-induced damage and evasion of apoptosis, all contributing to
altered chemoresistance of cells (36). Whether AR affects these factors to
alter chemoresistance requires further investigation. In the
present study, however, the presence of AR was found to increase
cancer cell chemoresistance and ABCG2 expression (Fig. 4). ABCG2 is an ABC transporter which
belongs to a superfamily of transmembrane proteins that transport
substrates across extra- and intracellular membranes and is
associated with drug resistance (37). The increased level of ABCG2 in BFTC
cells overexpressing AR may explain their higher viability under
anti-cancer drug treatment.
Furthermore, the role of AR in promoting cancer is
well characterized in prostate cancer with multiple mechanisms
involved, including cell cycle regulation, apoptosis and kinase
signals (38). However, the role of
AR in UUTUCs is not well characterized. The results of the current
study indicate that AR has the capability to increase the cell
ability to resist cytotoxic agents and the mechanism may be
associated with one of the multiple pathways of AR signaling. A
previous study demonstrated that androgen, mediated by AR, induces
Akt activation and causes the nuclear localization of a
serine-threonine kinase, Akt/PKB (39). Akt/PKB is linked to cell
proliferation, cell survival and anti-apoptotic pathways (40,41).
It is possible that activation of Akt/PKB by AR may provide a
survival advantage under the drug treatment.
To explore the clinical application of AR signaling
on the treatment of UUTUC, ASC-J9, an AR degradation enhancer,
which selectively targets AR without affecting libido, fertility
and sexual behavior, was used. Our previous study demonstrated that
ASC-J9 treatment led to degradation of AR in a number of cell lines
and the effect was AR-specific. ASC-J9 was revealed to have
therapeutic effects on spinal bulbar muscular atrophy mice via
degradation of AR without affecting serum testosterone levels
(30). ASC-J9 was also identified
to improve wound healing (27). In
addition, ASC-J9 was demonstrated to reduce AR-promoted tumor
growth in liver (28) and bladder
cancer (15). In the present study,
ASC-J9 was combined with specific chemotherapeutic drugs to treat
UUTUC cells, indicating that in AR-overexpressing cells,
BFTC-pBabeAR1 and BFTC-pBabeAR2, ASC-J9 restored the cyototoxic
effect of anti-cancer drugs, revealing that AR plays a significant
role in the chemoresistance of UUTUC cells. In addition, the
combination therapy of anti-AR and chemotherapeutic agents may
exhibit an advantage for enhancement of the efficacy of
chemotherapeutic agents in UUTUC cells with higher AR
expression.
In the present study, AR was demonstrated to play a
role in the suppression of the cytotoxic effect of doxorubicin,
cisplatin and mitomycin C treatment to UUTUC cells, indicating that
the AR expression status of UUTUCs affects chemotherapeutic
efficacy. In addition, AR was identified as a novel therapeutic
target in UUTUCs to increase the efficacy of chemotherapeutic
agents. Therapies targeting the androgen-receptor signaling axis
have been used for the treatment of prostate cancer with various
strategies (42,43) and may be suitable for application in
the treatment of UUTUCs. Thus, the combination of AR targeted
therapy with chemotherapy may increase the efficacy of therapy to
cure invasive and metastatic forms of cancer previously considered
to be difficult to treat. Results of this study may aid
understanding of the mechanism by which AR affects the progression
of UUTUCs. More importantly, understanding the actions of AR in
UUTUCs may lead to further understanding of the mechanisms of UUTUC
progression and may identify novel targets for therapy.
Acknowledgements
The authors thank Karen Wolf for
assistance with manuscript preparation. The study was supported by
grants from the NIH George Whipple Professorship Endowment (no.
CA155477) and Taiwan Department of Health Clinical Trial and
Research Center of Excellence (no. DOH99-TD-B-111-004; China
Medical University). ASC-J9 was patented by the University of
Rochester, University of North Carolina and AndroScience Corp. and
licensed to AndroScience Corp. The University of Rochester and
Chawnshang Chang own royalties and equity in AndroScience Corp.
References
|
1
|
Kirkali Z and Tuzel E: Transitional cell
carcinoma of the ureter and renal pelvis. Crit Rev Oncol Hematol.
47:155–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oosterlinck W, Solsona E, van der Meijden
AP, et al: EAU guidelines on diagnosis and treatment of upper
urinary tract transitional cell carcinoma. Eur Urol. 46:147–154.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krogh J, Kvist E and Rye B: Transitional
cell carcinoma of the upper urinary tract: prognostic variables and
post-operative recurrences. Br J Urol. 67:32–36. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lehmann J, Suttmann H, Kovac I, et al:
Transitional cell carcinoma of the ureter: prognostic factors
influencing progression and survival. Eur Urol. 51:1281–1288. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanderson KM, Cai J, Miranda G, Skinner DG
and Stein JP: Upper tract urothelial recurrence following radical
cystectomy for transitional cell carcinoma of the bladder: an
analysis of 1,069 patients with 10-year followup. J Urol.
177:2088–2094. 2007.PubMed/NCBI
|
|
6
|
Hall MC, Womack S, Sagalowsky AI, Carmody
T, Erickstad MD and Roehrborn CG: Prognostic factors, recurrence
and survival in transitional cell carcinoma of the upper urinary
tract: a 30-year experience in 252 patients. Urology. 52:594–601.
1998.PubMed/NCBI
|
|
7
|
Kang CH, Yu TJ, Hsieh HH, et al: The
development of bladder tumors and contralateral upper urinary tract
tumors after primary transitional cell carcinoma of the upper
urinary tract. Cancer. 98:1620–1626. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zigeuner RE, Hutterer G, Chromecki T,
Rehak P and Langner C: Bladder tumour development after urothelial
carcinoma of the upper urinary tract is related to primary tumour
location. BJU Int. 98:1181–1186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Catto JW, Yates DR, Rehman I, et al:
Behavior of urothelial carcinoma with respect to anatomical
location. J Urol. 177:1715–1720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Catto JW, Azzouzi AR, Amira N, et al:
Distinct patterns of microsatellite instability are seen in tumours
of the urinary tract. Oncogene. 22:8699–8706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Munoz JJ and Ellison LM: Upper tract
urothelial neoplasms: incidence and survival during the last 2
decades. J Urol. 164:1523–1525. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zigeuner R and Pummer K: Urothelial
carcinoma of the upper urinary tract: surgical approach and
prognostic factors. Eur Urol. 53:720–731. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turkeri LN: Neo/adjuvant therapy in upper
tract urothelial carcinoma. Eur Urol Suppl. 6:549–554. 2007.
View Article : Google Scholar
|
|
14
|
Seaman EK, Slawin KM and Benson MC:
Treatment options for upper tract transitional-cell carcinoma. Urol
Clin North Am. 20:349–354. 1993.PubMed/NCBI
|
|
15
|
Miyamoto H, Yang Z, Chen YT, et al:
Promotion of bladder cancer development and progression by androgen
receptor signals. J Natl Cancer Inst. 99:558–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noronha RF and Rao BR: Sex hormone
receptors in localized and advanced transitional cell carcinoma of
urinary tract in humans. Urology. 28:401–403. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boorjian S, Ugras S, Mongan NP, et al:
Androgen receptor expression is inversely correlated with
pathologic tumor stage in bladder cancer. Urology. 64:383–388.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tuygun C, Kankaya D, Imamoglu A, et al:
Sex-specific hormone receptors in urothelial carcinomas of the
human urinary bladder: a comparative analysis of
clinicopathological features and survival outcomes according to
receptor expression. Urol Oncol. 29:43–51. 2011. View Article : Google Scholar
|
|
19
|
Imada S, Akaza H, Ami Y, Koiso K, Ideyama
Y and Takenaka T: Promoting effects and mechanisms of action of
androgen in bladder carcinogenesis in male rats. Eur Urol.
31:360–364. 1997.PubMed/NCBI
|
|
20
|
Wu JT, Han BM, Yu SQ, Wang HP and Xia SJ:
Androgen receptor is a potential therapeutic target for bladder
cancer. Urology. 75:820–827. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shyr CR, Chen CC, Hsieh TF, et al: The
expression and actions of androgen receptor in upper urinary tract
urothelial carcinoma (UUTUC) tissues and the primary cultured
cells. Endocrine. 43:191–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tzeng CC, Liu HS, Li C, et al:
Characterization of two urothelium cancer cell lines derived from a
blackfoot disease endemic area in Taiwan. Anticancer Res.
16:1797–1804. 1996.PubMed/NCBI
|
|
23
|
Soga N, Arima K and Sugimura Y: Adjuvant
methotrexate, vinblastine, adriamycin and cisplatin chemotherapy
has potential to prevent recurrence of bladder tumors after
surgical removal of upper urinary tract transitional cell
carcinoma. Int J Urol. 15:800–803. 2008. View Article : Google Scholar
|
|
24
|
Kwak C, Lee SE, Jeong IG and Ku JH:
Adjuvant systemic chemotherapy in the treatment of patients with
invasive transitional cell carcinoma of the upper urinary tract.
Urology. 68:53–57. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O’Donoghue JP and Crew JP: Adjuvant
topical treatment of upper urinary tract urothelial tumours. BJU
Int. 94:483–485. 2004.PubMed/NCBI
|
|
26
|
Robey R, Polgar O, Deeken J, To K and
Bates S: ABCG2: determining its relevance in clinical drug
resistance. Cancer Metastasis Rev. 26:39–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lai JJ, Lai KP, Chuang KH, et al:
Monocyte/macrophage androgen receptor suppresses cutaneous wound
healing in mice by enhancing local TNF-alpha expression. J Clin
Invest. 119:3739–3751. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma WL, Hsu CL, Wu MH, et al: Androgen
receptor is a new potential therapeutic target for the treatment of
hepatocellular carcinoma. Gastroenterology. 135:947–955. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu MH, Ma WL, Hsu CL, et al: Androgen
receptor promotes hepatitis B virus-induced hepatocarcinogenesis
through modulation of hepatitis B virus RNA transcription. Sci
Transl Med. 2:32–35. 2010.PubMed/NCBI
|
|
30
|
Yang Z, Chang YJ, Yu IC, et al: ASC-J9
ameliorates spinal and bulbar muscular atrophy phenotype via
degradation of androgen receptor. Nat Med. 13:348–353. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mir C, Shariat SF, van der Kwast TH, et
al: Loss of androgen receptor expression is not associated with
pathological stage, grade, gender or outcome in bladder cancer: a
large multi-institutional study. BJU Int. 108:24–30. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
See WA: Continuous antegrade infusion of
adriamycin as adjuvant therapy for upper tract urothelial
malignancies. Urology. 56:216–222. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eastham JA and Huffman JL: Technique of
mitomycin C instillation in the treatment of upper urinary tract
urothelial tumors. J Urol. 150:324–325. 1993.PubMed/NCBI
|
|
34
|
Sakamoto N, Naito S, Kumazawa J, et al:
Prophylactic intravesical instillation of mitomycin C and cytosine
arabinoside for prevention of recurrent bladder tumors following
surgery for upper urinary tract tumors: A prospective randomized
study. Int J Urol. 8:212–216. 2001. View Article : Google Scholar
|
|
35
|
Hoffmeyer S, Burk O, von Richter O, et al:
Functional polymorphisms of the human multidrug-resistance gene:
multiple sequence variations and correlation of one allele with
P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci
USA. 97:3473–3478. 2000. View Article : Google Scholar
|
|
36
|
Longley DB and Johnston PG: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Szakacs G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heinlein CA and Chang C: Androgen receptor
in prostate cancer. Endocr Rev. 25:276–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang HY, Cho CL, Huang KL, et al:
Nongenomic androgen activation of phosphatidylinositol 3-kinase/Akt
signaling pathway in MC3T3-E1 osteoblasts. J Bone Miner Res.
19:1181–1190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kennedy SG, Wagner AJ, Conzen SD, et al:
The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic
signal. Genes Dev. 11:701–713. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scher HI and Sawyers CL: Biology of
progressive, castration-resistant prostate cancer: Directed
therapies targeting the androgen-receptor signaling axis. J Clin
Oncol. 23:8253–8261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Niu Y, Chang TM, Yeh S, Ma WL, Wang YZ and
Chang C: Differential androgen receptor signals in different cells
explain why androgen-deprivation therapy of prostate cancer fails.
Oncogene. 29:3593–3604. 2010. View Article : Google Scholar : PubMed/NCBI
|