Introduction
Adenoid cystic carcinoma (ACC) is one of the most
common subtypes of malignant tumors occurring in the salivary
glands. It is characterized by a high rate of recurrence, a strong
tendency of perineural invasion and an early development of
hematogenous metastasis. ACC patients possessing prognostic
factors, including lymph node positivity, a solid histological
subtype or perineural invasion with involvement of a named nerve,
suffer poor prognostic survival propsects (1). The rates of perineural invasion and
distant metastases after 10 years, regardless of aggressive
surgery, have been demonstrated to be 50 and 39%, respectively
(2). Postoperative radiotherapy is
thus recommended for its additive improvement in the control of
local and regional recurrence compared with surgery alone (1,3).
However, considering the relatively low radiosensitivity and the
aggressive growth pattern of ACC, methods to improve the
therapeutic efficiency have potential for further
investigation.
Nuclear factor κB (NF-κB) was first discovered in
1986 as a eukaryotic transcription factor that bound to the κ
immunoglobulin light chain enhancer in the nuclei of the B lymphoid
cell lineage, and is presently considered to exist in the majority
of cell types (4,5). Homo- and heterodimeric complexes of
its five members, p50, p52, p65/RelA, c-Rel and Rel-B, are
sequestered inactive in the cytoplasm preformed and bound to the
inhibitory subunits of the IκB family (IκBα, IκBβ, IκBε, Bcl-3,
p100 and p105) (6). In the
canonical NF-κB activation pathway, the activation of NF-κB depends
on stimuli including bacterial and viral products, proinflammatory
cytokines and ionizing radiation, all of which intrigue
phosphorylation of IκBα by IκB kinase (IκK)-induced ubiquitination
and subsequent proteolysis by the 26 S proteasome. The
phosphorylation of IκBα at specific serine (Ser) residues liberates
the captive cytoplasmic NF-κB for translocation to the nucleus and
initiation of target genetic transcription (7). The active NF-κB transcription factor
regulates the expression of >150 downstream genes. The majority
of proteins encoded by NF-κB target genes participate in a wide
variety of physiological processes, including embryonic
development, lymphoid differentiation, immune and inflammatory
responses and apoptotic resistance to radio- and chemotherapy, as
well as being involved in oncogenesis and tumor proliferation
(8).
The abarrent activation of NF-κB is characteristic
of numerous solid tumor types, including breast epithelial tumors,
pancreatic adenocarcinoma and bladder, prostate and non-small cell
lung cancer (9). High levels of
NF-κB activity contribute to a negative prognosis by playing a key
role in the induction of anti-apoptosis, the acceleration of cell
cycle progression, increased resistance to radiation and
chemotherapy, as well as in elevated aggressive growth and
metastatic frequency in malignant cancer (10). It is widely accepted that
constitutively activating NF-κB exerts a negative impact on the
radiosensitivity of different cancer cell lines. Sandur et
al demonstrated that suppression of the NF-κB pathway via
curcumin-induced inhibtion sensitized colorectal cancer cells to
radiation by downregulating the phosphorylation and degradation of
IκBα, inhibition of IκK activity and inhibition of Akt
phosphorylation (10). By
transfecting a gene encoding mutated IκBα that is not able to be
phosphorylated and thereby inhibits the activation of the NF-κB
pathway, similar findings have been demonstrated in that
suppressive NF-κB radiosensitizes prostate cancer cells (6). However, the role of the NF-κB pathway
in regulating the radiosensitivity remains controversial in
different diseases. Jung et al demonstrated that by
transfecting a phosphorylation-defective IκBα gene into cells
derived from a patient with ataxia telangiectasia (AT) group D, the
constitutive activation of NF-κB was suppressed and the previous
radiation hypersensitivity in AT was restored to the normal level
(11). Additionally, to the best of
our knowledge, the impact of this mutant IκBα gene on the
expression of NF-κB and subsequent changes in radiosensitivity in
ACC cells had not yet been studied.
In the present study, we investigated the role of
NF-κB in regulating the radiosensitivity of adenoid cystic
carcinoma cells (ACC-M) in vitro. To achieve a conclusion,
we transiently transfected a plasmid encoding a Flag-tagged
phosphorylation defective mutant of IκBα (S32, 36A) (pBαbe-SR-IκBα)
into ACC-M cells, thereby suppressing the activity of the NF-κB
pathway. While analyzing the results, we observed that suppressing
the NF-κB pathway by transfecting ACC-M cells with mutant IκBα, as
compared with cells with a control pBαbe plasmid transfection,
leads to increased radiosensitivity. Meanwhile, intragroup data
analysis of the SR-IκBα group demonstrated that different doses of
irradiation induced the expression of NF-κB in a dose- and
time-dependent manner, with corresponding changes in the
radiosensitivity of ACC-M cells.
Materials and methods
Chemicals and reagents
The pBαbe-SR-IκBα and control pBαbe plasmids were
provided by Professor Wantao Chen (Department of Oral and
Maxillofacial Surgery, Ninth People’s Hospital, College of
Stomatology, Shanghai Jiao Tong University, Shanghai, China).
Lipofectamine was purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA).
An MTT and an Annexin V-FLUOS staining kit were
purchased from Roche Diagnostics (Indianapolis, IN, USA). Rabbit
anti-human NF-κB, goat anti-rabbit secondary antibodies,
anti-β-actin and horseradish peroxidase (HRP)-conjugated goat
anti-rabbit immunoglobulin were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-IκBα antibody was
purchased from Cell Signaling Technology, Inc. (Denvers, MA,
USA).
Cell culture
The human salivary adenoid cystic carcinoma cell
line (ACC-M) was provided by Professor Wantao Chen. ACC-M cells
were cultured in RPMI-1640 medium supplemented with 10% filtered
fetal bovine serum (FBS; Hyclone, Logan, UT, USA) and 100 U/ml
penicillin/streptomycin. The cultures were incubated at 37°C in a
humidified 5% CO2 incubator.
Plasmid transfection
The site-specific, signal-induced degradation of
IκBα depends on phosphorylation at Ser 32 and 36. Therefore, the
pBαbe-SR-IκBα plasmid that consisted of a double point mutation
(Ser to Lactamine) was thus resistant to phosphorylation (12). The mutant and control plasmids were
transiently transfected into ACC-M cells with the use of
Lipofectamine, according to the manufacturer’s instructions. In
brief, ACC-M cells were removed by trypsin/EDTA treatment and
seeded at a density of 2×105 cells/ml in 6-cm culture
dishes. Cells were grown to 90% confluence and subjected to 24-h
synchronization in serum-free medium. ACC-M cells were transfected
with 4 μg of the pBαbe-SR-IκBα or control pBαbe plasmid per
dish with the use of Lipofectamine. Following incubation for 6 h,
the transfection medium was replaced by fresh medium for an
additional 48-h incubation to allow for gene expression to
occur.
MTT
The effect of transfection on cellular proliferation
was assessed using MTT. In brief, the non-transfected, transfected
pBαbe-SR-IκBα or control plasmid cells were seeded in 96-well
plates in fresh medium (5000 cells/well) and incubation was
continued for an additional 24, 48 or 72 h following transfection.
Thereafter, MTT solution (5 mg/ml) was added to each well.
Following incubation for 4 h at 37°C, the blue dye taken up by the
cells was dissolved in dimethyl sulfodide (100 μg/ml), and
then the optical density was measured at 570 nm using a 96-well
multiscanner. All assays were run in triplicate.
Cells in the different groups mentioned previously
were irradiated at room temperature with a medical linear
accelerator (Primus-H; Siemens, Munich, Germany) at different
doses. Cells in the transfected pBαbe-SR-IκBα plasmid group were
harvested at different time points (1, 3, 6, 10, 24 and 48 h)
following exposure to graded irradiation doses (0, 2, 4, 6, 8 and
10 Gy). Cells in the pBαbe and non-transfected groups were
harvested 3 h following exposure to graded irradiation doses. The 3
h time point was selected due to the fact that, as described by
Sandur et al, the maximum expression of NF-κB occurrs 3 h
following irradiation (10).
Treated cells in the indicated groups were prepared for sequential
immunocytochemistry, as well as western blot and flow cytometric
analyses.
Immunocytochemistry
Changes in NF-κB nuclear translocation were examined
by immunocytochemistry. In brief, cells were plated onto 10×10-mm
glass slides, and subjected to transfection and irradiation
treatment. Thereafter, cells were fixed with 4% paraformaldehyde
and permeabilized with 0.2% Triton X-100. Following washing in
phosphate-buffered saline (PBS), slides were blocked with 5% normal
goat serum for 1 h and then incubated with rabbit anti-NF-κB
antibody at a dilution of 1:100. Following overnight incubation at
4°C and rewarming to 37°C, the slides were washed with PBS and
incubated for 20 min with goat anti-rabbit secondary antibody at a
dilution of 1:100. The slides were then washed in PBS and
visualized using diaminobenzidine. Negative controls for each group
were processed in the same manner, using a non-immunized rabbit IgG
(at a dilution of 1:100) in place of the primary antibody.
Immunocytochemical staining for NF-κB in the nucleus was
quantitatively analyzed using the Image-Pro Plus image analytical
system (Media Cybernetics; Silver Spring, MD, USA) with the gray
scale method.
Western blot
The changes in the expression of NF-κB and mutant
IκBα following transfection and irradiation treatment were
evaluated by western blot analysis. In brief, whole cell extracts
were prepared in RIPA buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl;
0.5 sodium deoxycholate; 0.1% SDS; 1% NP40 and 1 mM
phenylmethylsulfonyl fluoride; Shenneng Bocai Biotechnology Co.,
Ltd.; Shanghai, China) from the treated cells grown in 6-cm dishes.
The protein concentration was quantified using the BCA protein
measurement kit (Shenneng Bocai Biotechnology Co., Ltd.). Extracts
(40 μg) were subjected to SDS-PAGE and then
electrophoretically transferred to nitrocellulose membranes, which
were then blocked with 5% (w/v) dried skimmed milk-TBST (10 mm
Tris-HCl, pH 8.0; 150 mm NaCl; 0.05% Tween-20) for 1 h at room
temperature. Membranes were probed with anti-NF-κB antibody (at a
dilution of 1:1000) or anti-IκBα antibody (at a dilution of
1:1000), incubated overnight in 5% milk-TBST, washed three times
with 1X TBST and exposed to HRP-conjugated secondary antibody (at
1:10000 dilution) in 5% dried skimmed milk for 1 h. All blots were
reprobed with anti-β-actin (at 1:1000 dilution) in 5% dried skimmed
milk, followed by HRP-conjugated secondary antibody (at a dilution
of 1:1000) in 5% dried skimmed milk. Protein bands were visualized
by the Alpha Imager 2200 system (Alpha Innotech, San Leandro, CA,
USA).
Flow cytometric analysis
The effects on apoptosis in cells following the
treatment indicated previously were determined by flow cytometric
analysis. In brief, cells were trypsinized, then harvested by
centrifugation at 1000 × g for 3 min. Thereafter, the cell
collections were washed twice with cold PBS, resuspended in Annexin
V and propidium iodide (PI), and incubated for 15 min at 4°C.
Fluorescence was measured at 488 nm in a flow cytometer (Coulter,
Luton, UK). Early apoptotic cells are indicated by Annexin
V-positive and PI-negative staining, whereas late apoptotic cells
demonstrate both Annexin V- and PI-positive staining.
Statistical analysis
SPSS/PC (version 17.0; SPSS, Inc., Chicago, IL,USA)
was used for statistical analysis. Data are expressed as means ±
standard deviation and were analyzed by a one-way ANOVA with an LSD
test. The Pearson’s correlation coefficient analysis was used to
assess the correlation between radiation doses, NF-κB expression
and apoptotic rate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of IκBα protein is affected
following transfection in ACC-M cells
To examine the changes in NF-κB following
irradiation in ACC-M cells, we transiently transfected the cells by
use of a pBαbe-SR-IκBα plasmid prior to irradiation, and thus
suppressed the activity of the NF-κB pathway. The expression of the
Flag-tagged IκBα following transfection was confirmed by western
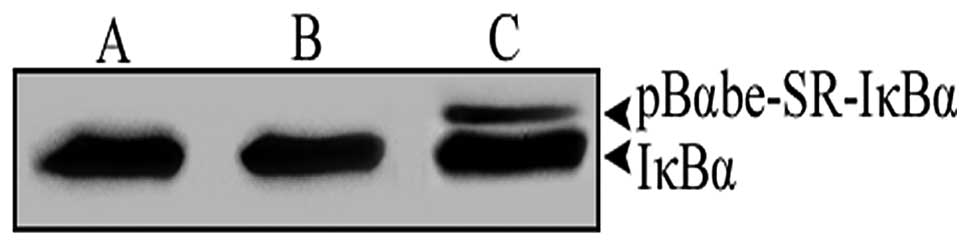
blot analysis. As demonstrated in Fig.
1, in the pBαbe and non-transfected groups, cells exhibited a
major band corresponding to endogenous IκBα protein. However, the
ACC-M cells that had been transfected with the pBαbe-SR-IκBα
plasmid expressed endogenous IκBα protein, as well as the mutant
IκBα protein with a higher molecular weight.
In vitro growth properties of transfected
ACC-M cells
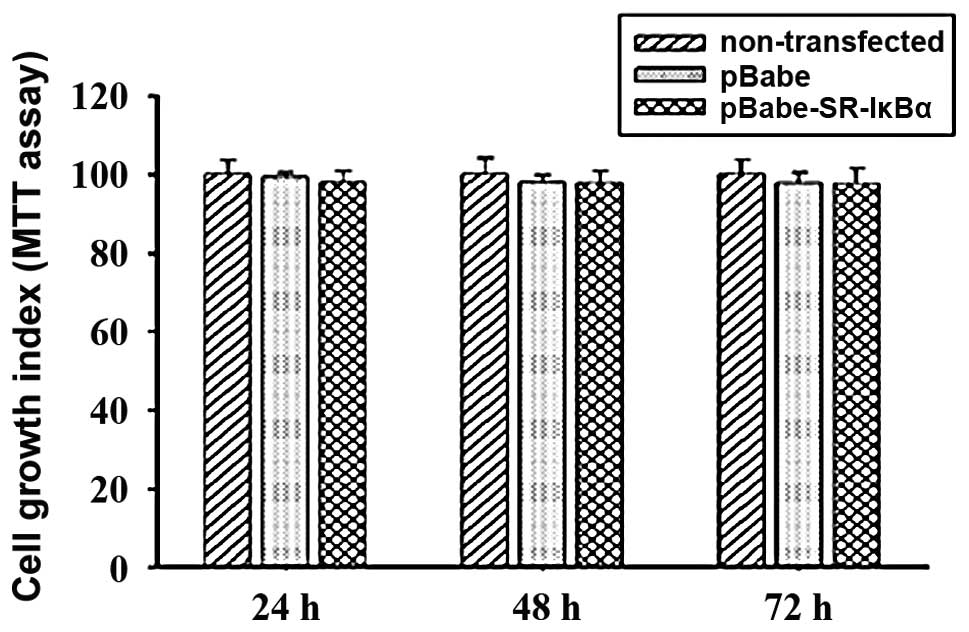
To determine whether the mutant IκBα gene exerted
its antiproliferative activity on the transfected cells, cells were
subjected to an MTT analysis. As demonstrated in Fig. 2, no visible transfection-related
inhibition was observed between the pBαbe and non-transfected
groups, while the growth property of cells in the pBαbe-SR-IκBα
group was slightly reduced to 98.07, 97.79 and 97.5% after 24, 48
and 72 h, respectively. However, no significant difference was
observed between the pBαbe-SR-IκBα group and the pBαbe or
non-transfected groups at the 95% probability level. Accordingly,
it is likely that the expression of mutant IκBα protein does not
necessarily lead to the in vitro growth inhibition of
non-stimulated ACC-M cells.
Translocation of NF-κB following
irradiation in ACC-M cells
NF-κB staining was located within the cell cytoplasm
in resting cells. Following exposure to different doses of
irradiation, the previously suppressed NF-κB pathway (achieved by
transfecting the ACC-M cells with mutant IκBα) was activated and
thereafter liberated NF-κB translocated to the nucleus. The
staining of NF-κB in the nucleus was categorized as positive
expression. Compared with the pBαbe and nontransfected groups at 3
h following exposure to equal doses of irradiation, the NF-κB
pathway was notably inhibited in the pBαbe-SR-IκBα group, as
demonstrated by a relatively lightly stained nucleus and the fact
that the immunoprecipitates were mainly distrubuted in the
cytoplasm. The majority of the NF-κB protein in the cells of the
pBαbe-SR-IκBα group was found to be clustered in the cytoplasm
around the nuclear envelope. With exposure to graded doses of
irradiation, the NF-κB protein was observed to pass through the
nuclear envelope and gradually accumulate in the nucleus. The NF-κB
protein was first observed in the center of the nucleus at 6 h
(data not shown).
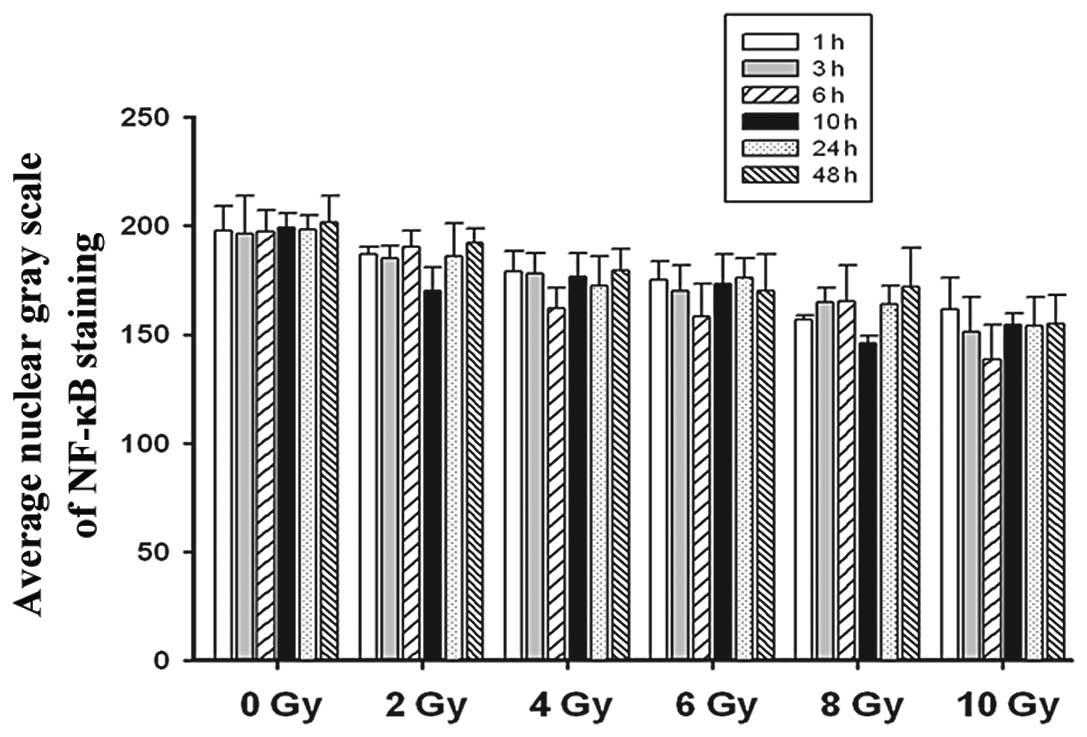
The quantitative analysis of the immunocytochemistry
images supported our findings. Compared with the pBαbe and
non-transfected groups at 3 h following exposure to equal doses of
irradiation, the average nuclear gray scale of cells in the
pBαbe-SR-IκBα plasmid group was significantly increased (P<0.05;
data not shown). As demonstrated in Fig. 3, further analysis of the SR-IκBα
group revealed that the average nuclear gray scale of the various
dose groups was lower than that of the 0 Gy group with only
marginally detectable expression of NF-κB protein, and the data
reached the deepest valley 6–10 h following irradiation. At the
same time point, we disclosed that at 3 h following irradiation,
the average nuclear gray scale of the ACC-M cells decreased as the
doses of irradiation increased (P<0.05).
Expression of NF-κB protein is affected
following irradiation in ACC-M cells
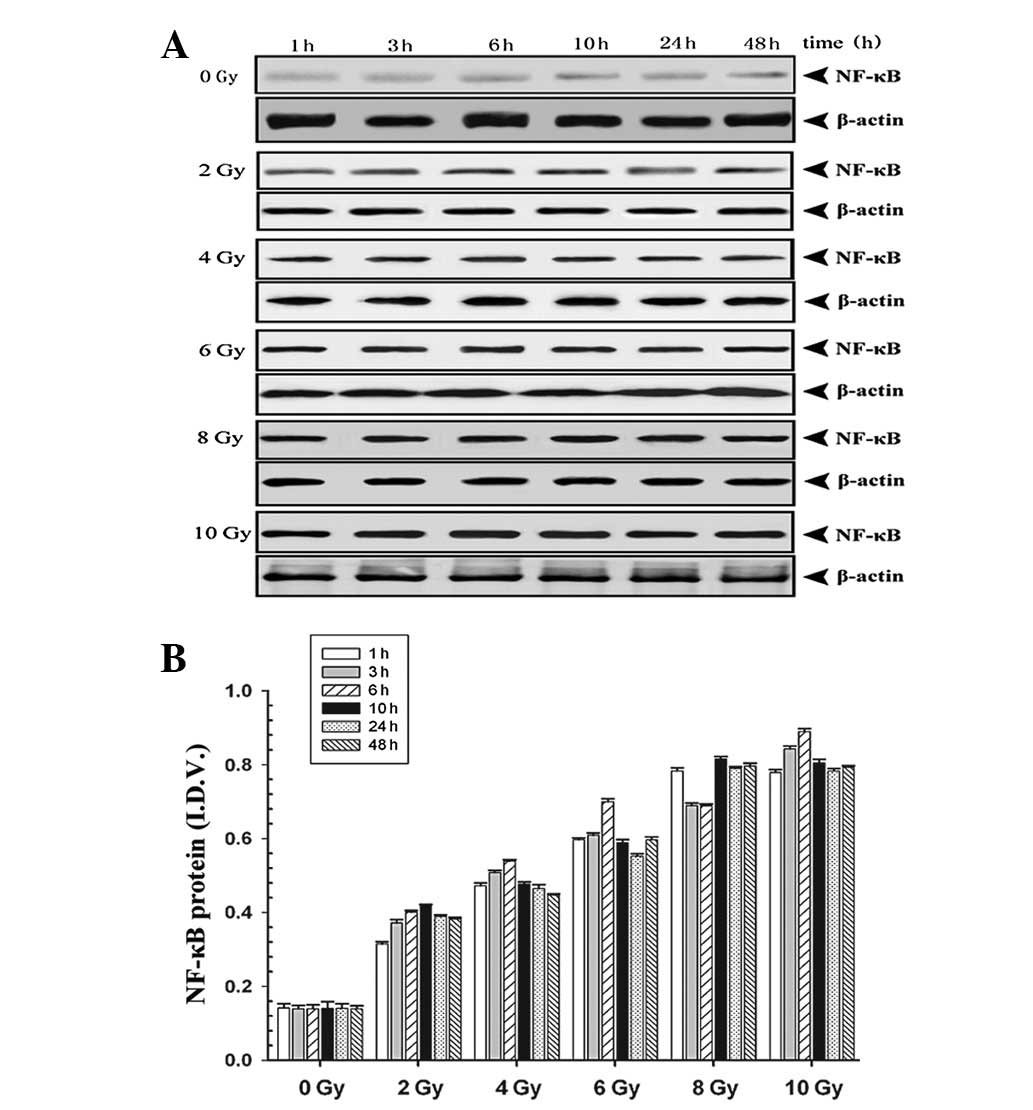
To explore the quantitative changes in NF-κB protein
levels in ACC-M cells, cell lysates were subjected to western blot
analysis following transfection and subsequent irradiation. Cells
in the pBαbe-SR-IκBα plasmid group demonstrated downregulated
levels of NF-κB protein (P<0.05), which suggested transfection
of mutant IκBα simultaneously blocked the synthesis and
translocation of NF-κB protein (data not shown). Further analysis
on the time course and dose-response characteristics of NF-κB
protein in the pBαbe-SR-IκBα plasmid group are demonstrated in
Fig. 4. The levels of NF-κB protein
in the various dosage groups were significantly higher than that of
the 0 Gy group. The NF-κB protein levels peaked at 6–10 h and began
gradually declining at 24 h in each group with equal irradiation
exposure. At the 3 h time point, the levels of NF-κB protein
increased as the dose of irradiation increased (P<0.05).
Overall, the western blot analysis results are consistent with our
immunocytochemistry data.
Changes in the apoptotic rate following
irradiation in ACC-M cells
It has been demonstrated that irradiation results in
deleterious DNA damages and consequentially launches cell
apoptosis. However, the simultaneously triggered cellular defense
mechanism prevents impaired cells from the radiation-induced
apoptosis (13,14). NF-κB has been demonstrated to be
activated by irradiation, and to therefore elucidate the elevated
irradiation resistance (6). As
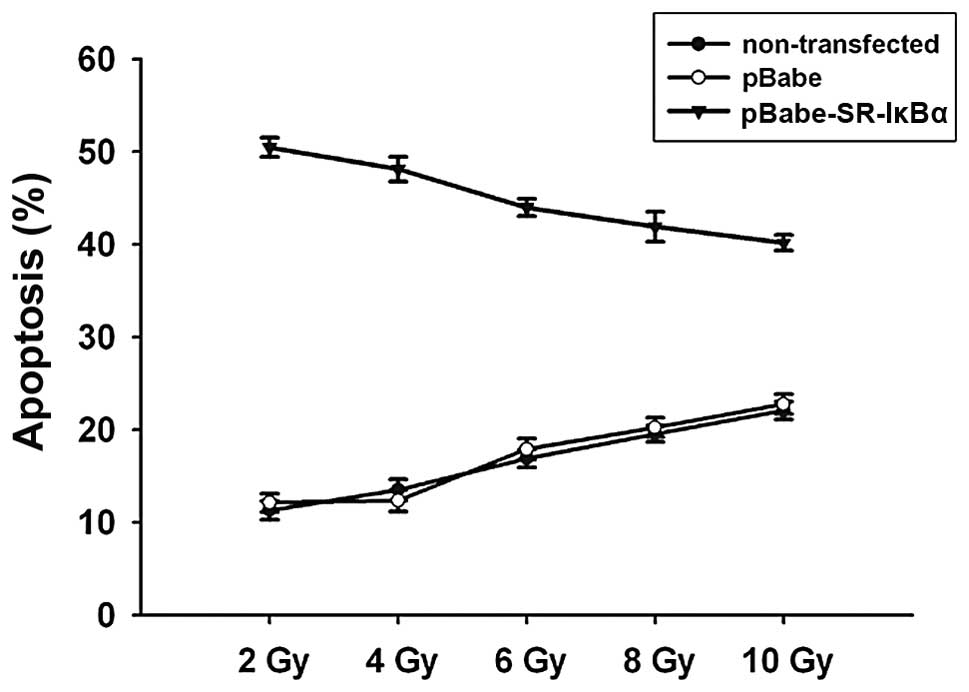
demonstrated in Fig. 5, treatment
with mutant IκBα transfection significantly enhanced the
radiosensitivity of ACC-M cells, as assessed by flow cytometric
analysis (P<0.05). A significant increase in the number of both
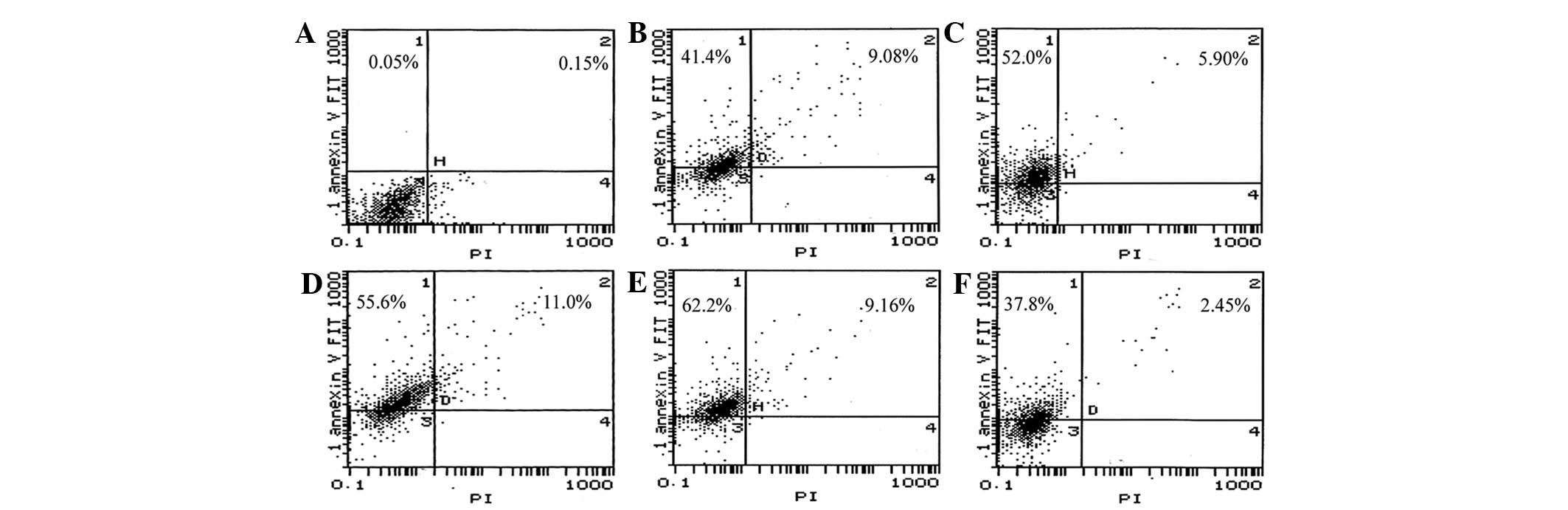
Annexin V-positive and PI-negative (early apoptosis) and Annexin V-
and PI-positive (late apoptosis) ACC-M cells was detected in the
pBαbe-SR-IκBα plasmid group with graded doses of irradiation,
compared with the 0 Gy group (Fig.
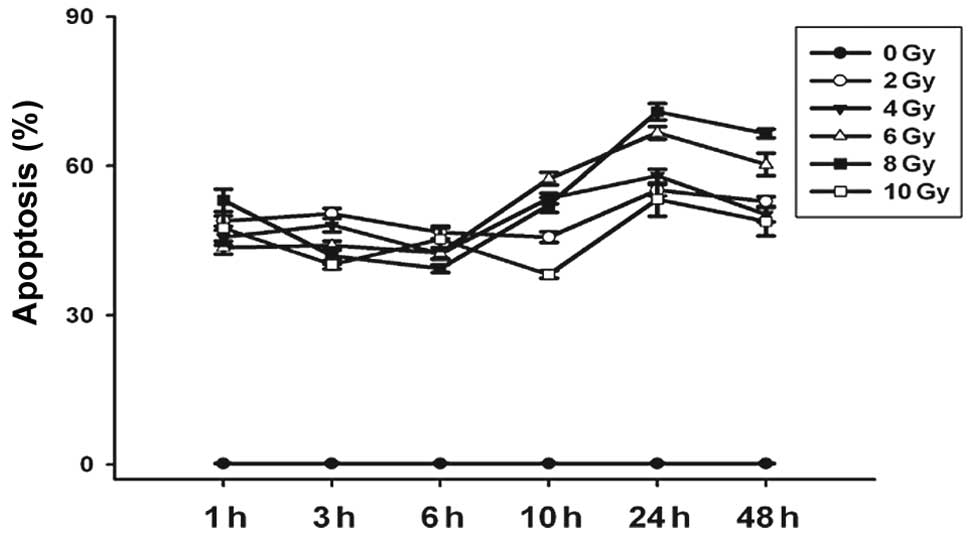
6). Furthermore, cells with equal irradiation exposure
exhibited a similar survival tendency in that the apoptotic rate
reached the minimal value at 6–10 h, and the maximal value at 24 h.
The apoptotic rates of cells with mutant IκBα transfection, 3 h
following irradiation treatment, were 50.83, 48.61, 44.32, 42.53
and 40.42%, between the different dosage groups (2, 4, 6, 8 and 10
Gy, respectively). These results suggested that the increasing
expression of NF-κB was attributed to the decreasing number of
total (early and late) apoptotic cells at 3 h (Fig. 7).
The linear correlation estimation was applied to
demonstrate that the apoptotic rates correlated well with the
quantitative changes in NF-κB protein expression. The apoptotic
rate of the cells was significantly negatively correlated with the
expression of NF-κB protein. The overexpression of NF-κB protein
6–10 h following irradiation in the pBαbe-SR-IκBα plasmid group led
to the lowest rate of apoptosis. Meanwhile, graded doses of
irradiation amplified the activation of the NF-κB pathway, which in
reverse decreased the apoptotic rates at 3 h, and the increased
expression of NF-κB at 3 h decreased the apoptotic rates.
Discussion
The primary aim of our study was to determine
whether the transfected mutant IκBα gene was able to inhibit the
activity of the NF-κB pathway, and to investigate the negative
contribution of the radio-activated NF-κB pathway on
radio-sensitivity. We transiently transfected a mutant IκBα gene
into ACC-M cells, obtaining simultaneous blockage of the synthesis
and translocation of the NF-κB protein. The mutant IκBα gene
promoted radiosensitivity via suppression of the NF-κB pathway.
Additionally, exposure to radiation invoked the activity of the
NF-κB pathway that was initially inhibited by transfection, in a
dose- and time-dependent manner. The highly active NF-κB protein in
reverse exhibited a radio-resistant effect in the ACC-M cells and
improved survival following irradiation.
NF-κB plays a pivotal role in various physiological
processes, including immune and inflammatory responses, and is also
considered to regulate apoptosis, cell proliferation and resistance
to chemotherapy- and radiotherapy-induced cytotoxicity (15). NF-κB is sequestered inactive in the
cytoplasm by interaction with an inhibitor of IκBα in resting
cells. It is now widely accepted that the degradation of IκBα plays
a crucial role in the activation of the NF-κB pathway (12). When cells were stimulated by
different exogenous stimuli, the Ser 32 and 36 residues of IκBα
were phosphorylated by IKK and IκBα underwent subsequent
ubiquitylation and degradation, thus releasing the NF-κB protein
for nuclear translocation (16).
Fujioka et al transfected a retroviral vector encoding
phosphorylation-defective IκBα by inducing point mutations at the
potential phosphoacceptor sites of Ser 32 and 36, and observed
decreased NF-κB DNA binding activity in the non-metastatic human
pancreatic tumor cell line (17).
However, to the best of our knowledge, applying a mutant form of
IκBα to specifically inhibit the NF-κB pathway in ACC-M cells has
not been previously studied. As we have demonstrated in ACC-M
cells, the synthesis and translocation of the NF-κB protein were
highly specifically suppressed by transfecting the cells with the
use of a pBαbe-SR-IκBα plasmid, and this blocking effect persisted
following irradiation and hence when the NF-κB pathway was
activated.
Given the involvement of the NF-κB pathway in
oncogenesis and induction of resistance to conventional therapy,
such as chemotherapy and irradiation, blockage of the pathway by
specific inhibitors is beneficial and may be a promising antitumor
therapy (18). NF-κB is suppressed
by multiple strategies, including the application of a
phosphorylation-defective form of IκBα, proteasome inhibitors or
pharmacological agents (10,17,18).
van Hogerlinden et al applied a super-repressor form of IκBα
with mutant phosphorylation sites that selectively inhibited the
NF-κB pathway, yielding elevated apoptosis in the basal and
suprabasal keratinocytes in vivo(19). In addition, the super-repressor
considerably sensitized the keratinocytes to UV-induced apoptosis.
Duffey et al also demonstrated that stable expression of a
mutant form of IκBα in squamous carcinoma cells resulted in an
augmented apoptotic rate, and this blocking effect was not relieved
by TNF-α induced activation (20).
However, in the present study, no significant effects on cell
proliferation were observed in ACC-M cells with pBαbe-SR-IκBα
plasmid transfection. By contrast, cells with prior mutant IκBα
plasmid transfection were susceptible to radio-induced apoptosis.
Accordingly, we estimate that inhibition of the NF-κB pathway in
non-stimulated ACC-M cells does not necessarily result in increased
apoptosis, whereas inhibition of the NF-κB pathway combined with
irradiation yielded a significant radiosensitizing outcome.
Cytoplasmic NF-κB is activated by various stimuli,
including IL-1, TNF-α and viral infection, as well as irradiation
and chemotherapeutic agents (8). Li
et al demonstrated that maslinic acid, a triterpene
derivative obtained from olive pomace, inhibited endogenous NF-κB
activity in a time-dependent manner and the TNFα-induced NF-κB
activation in a dose-dependent manner in pancreatic cancer cells,
and thus prevented the expression of downstream target genes, such
as Bcl-2, Survivin, COX-2 and IAP-1 (21). Sandur et al also demonstrated
that maximal expression of NF-κB occurred 3 h following exposure to
10 Gy irradiation in colorectal cancer cells (10). However, there are a limited number
of studies concerning the time-dose expression pattern of
radio-induced activation in previously inhibited NF-κB pathway. In
our study, a similar expression tendency was observed in the
pBαbe-SR-IκBα plasmid group; the maximal expression appeared at
6–10 h following irradiation. At 3 h, graded doses of irradiation
corresponded well with the increased activity of the NF-κB
pathway.
Accumulating studies indicate that the constitutive
activation of NF-κB is closely correlated with oncogensis and tumor
invasion. Aberrant activation of the NF-κB pathway has been
identified in various types of cancer, including both solid and
hematopoietic malignancies (22).
Zhang et al revealed that an activated NF-κB pathway was
significantly correlated with advanced clinical stage, high
frequencies of tumor angiogenesis and perineural invasion,
aggressive locoregional recurrence and distant metastasis in ACC,
all of which predict an adverse prognostic prospect (23). Sustained activation of the NF-κB
pathway has been demonstrated to promote survival in tumor cells
with radiotherapy by permitting cells to repair DNA damage and to
escape elimination by apoptosis, which is induced by the
destruction of DNA double-strand (24). In our study, the peak expression
levels of NF-κB protein at 6–10 h following irradiation were
paralleled by minimal apoptotic rates in the pBαbe-SR-IκBα plasmid
group. Amplified expression levels of NF-κB activated by graded
doses of irradiation at 3 h reflected attenuated apoptosis. These
findings to an extent explain the radio-resistant role of NF-κB in
stimulated ACC-M cells.
In the present study, we demonstrated that ACC-M
cells exhibited markedly increased radiosensitivity following
selective inhibition of the NF-κB pathway by transfection with a
mutant IκBα plasmid. However, the NF-κB pathway also plays a
crucial role in various physiological processes, such as regulation
of innate immunity by activating downstream pro-inflammatory
cytokines, including IL-1, TNF-α and RANTES. One of the pitfalls
that may be neglected is the likelihood of immunodeficiency and
great susceptibility to infections following specific inhibition of
the NF-κB pathway. The correlation between downregulation of the
NF-κB pathway and human diseases is reported in Incontinentia
pigmenti and anhidrotic ectodermal dysplasia (25). van Hogerlinden et al observed
interferences in normal epidermal homeostasis and hair follicle
development in selectively inhibited genetic mice (19). The transgenic mouse model exhibited
dysplasia of the epidermis characterized by flaky skin, hair loss
and hyperkeratotic lesions. The increased susceptibility to the
spontaneous occurrence of well-differentiated skin squamous cell
carcinoma was also demonstrated in the transgenic mice.
Accordingly, the appropriate application of NF-κB pathway
inhibitors while avoiding their side-effects requires further
investigation.
In conclusion, the results of our study demonstrated
that by transfecting a mutant form of the IκBα gene, the synthesis
and translocation of the NF-κB protein were specifically inhibited,
and a concomitant decrease in survival rate following irradiation
was observed in ACC-M cells. Furthermore, the NF-κB expression
after irradiation represents a time-dose dependent manner of the
transfected cells, followed by a corresponding change in
radiosensitivity. Accordingly, selective inhibition of the NF-κB
pathway by novel target agents reveals a promising method for
improvements in the radiosensitivity of ACC. However, the potential
side-effects that may be activated by systemic suppression of
immune system require further investigation.
References
|
1
|
Fordice J, Kershaw C, El-Naggar A and
Goepfert H: Adenoid cystic carcinoma of the head and neck:
predictors of morbidity and mortality. Arch Otolaryngol Head Neck
Surg. 125:149–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Terhaard CH, Lubsen H, Van der Tweel I, et
al: Salivary gland carcinoma: independent prognostic factors for
locoregional control, distant metastases and overall survival:
results of the Dutch head and neck oncology cooperative group. Head
Neck. 26:681–693. 2004. View Article : Google Scholar
|
|
3
|
Polat B, Fassnacht M, Pfreundner L, et al:
Radiotherapy in adrenocortical carcinoma. Cancer. 115:2816–2823.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination:the control of NF-kappa B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pajonk F, Pajonk K and McBride WH:
Inhibition of NF-κB, clonogenicity, and radiosensitivity of human
cancer cells. J Natl Cancer Inst. 91:1956–1960. 1999.
|
|
7
|
Greten FR and Karin M: The IKK/NF-κB
activation pathway-A target for prevention and treatment of cancer.
Cancer Lett. 206:193–199. 2003.
|
|
8
|
Pahl HL: Activators and target genes of
Rel/NF-κB transcription factors. Oncogene. 18:6853–6866. 1999.
|
|
9
|
Schwartz SA, Hernandez A and Mark Evers B:
The role of NF-kappaB/IkappaB proteins in cancer: implications for
novel treatment strategies. Surg Oncol. 8:143–153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sandur SK, Deorukhkar A, Pandey MK, et al:
Curcumin modulates the radiosensitivity of colorectal cancer cells
by suppressing constitutive and inducible NF-κB activity. Int J
Radiat Oncol Biol Phys. 75:534–542. 2009.PubMed/NCBI
|
|
11
|
Jung M, Zhang Y, Lee S and Dritschilo A:
Correction of radiation sensitivity in ataxia telangiectasia cells
by truncated IκB-α. Science. 268:1619–1621. 1995.
|
|
12
|
Brown K, Gerstberger S, Carlson L,
Franzoso G and Siebenlist U: Control of IκB-α proteolysis by
site-specific signal induced phosphorylation. Science.
267:1485–1488. 1995.
|
|
13
|
Cataldi A, Zauli G, Di Pietro R, Castorina
S and Rana R: Involvement of the pathway
phosphatidylinositol-3-kinase/AKT-1 in the establishment of the
survival response to ionizing radiation. Cell Signal. 13:369–375.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weichselbaum RR, Hallahan D, Fuks Z and
Kufe D: Radiation induction of immediate early genes: effectors of
the radiation-stress response. Int J Radiat Oncol Biol Phys.
30:229–234. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Magné N, Toillon RA, Bottero V, et al:
NF-κB modulation and ionizing radiation: mechanisms and future
directions for cancer treatment. Cancer Lett. 231:158–168.
2006.
|
|
16
|
Tergaonkar V, Bottero V, Ikawa M, Li Q and
Verma IM: IκB kinase-independent IκBα degradation pathway:
functional NF-κB activity and implications for cancer therapy. Mol
Cell Biol. 23:8070–8083. 2003.
|
|
17
|
Fujioka S, Sclabas GM, Schmidt C, et al:
Inhibition of constitutive NF-κB activity by IκBαM suppresses
tumorigenesis. Oncogene. 22:1365–1370. 2003.
|
|
18
|
Darnell JE Jr: Transcription factors as
targets for cancer therapy. Nat Rev Cancer. 2:740–749. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Hogerlinden M, Rozell BL,
Ahrlund-Richter L and Toftgård R: Squamous cell carcinomas and
increased apoptosis in skin with inhibited Rel/nuclear
factor-kappaB signaling. Cancer Res. 59:3299–3303. 1999.PubMed/NCBI
|
|
20
|
Duffey DC, Crowl-Bancroft CV, Chen Z, et
al: Inhibition of transcription factor nuclear factor-kappa B by a
mutant inhibitor-kappa Balpha attenuates resistance of human head
and neck squamous cell carcinoma to TNF-alpha caspase-mediated cell
death. Br J Cancer. 83:1367–1374. 2000. View Article : Google Scholar
|
|
21
|
Li C, Yang Z, Zhai C, et al: Maslinic acid
potentiates the anti-tumor activity of tumor necrosis factor alpha
by inhibiting NF-kappaB signaling pathway. Mol Cancer. 9:732010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baud V and Karin M: Is NF-kappaB a good
target for cancer therapy? Hopes and pitfalls Nat Rev Drug Discov.
8:33–40. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Peng B and Chen X: Expressions of
nuclear factor kappaB, inducible nitric oxide synthase, and
vascular endothelial growth factor in adenoid cystic carcinoma of
salivary glands: correlations with the angiogenesis and clinical
outcome. Clin Cancer Res. 11:7334–7343. 2005. View Article : Google Scholar
|
|
24
|
Magné N, Toillon RA, Bottero V, et al:
NF-nB modulation and ionizing radiation: mechanisms and future
directions for cancer treatment. Cancer Lett. 231:158–168.
2006.PubMed/NCBI
|
|
25
|
Courtois G and Gilmore TD: Mutations in
the NF-κB signaling pathway: implications for human disease.
Oncogene. 25:6831–6843. 2006.
|