Introduction
Renal cancer is the 15th most common type of cancer
in the world and causes the deaths of more than 91,000 individuals
every year (1). Wilms' tumor
(nephroblastoma) accounts for almost 6% of all pediatric cancers
and more than 95% of all kidney tumors in children (2). It is an embryonal malignancy that
afflicts 1 in 10,000 children. The principle risk factors for renal
cancer include inherited germline mutations. A number of loci
involved in the development of Wilms' tumor have been
characterized, and the key locus is WT1, a tumor suppressor
gene located on chromosome 11p (1).
N-methyl-N-nitrosourea (MNU), a
direct-acting alkylating agent that interacts with DNA, is toxic
and carcinogenic to the breast and pancreas as well as the immune,
hematopoietic, reproductive, dental, gastrointestinal, nervous and
sensory systems (3–5). Nitrosourea compounds including MNU
have carcinogenic potency in the kidney of rats (6–8), and
MNU induces mesenchymal tumors and nephroblastomas in rats
(9,10). The formation and persistence of DNA
adducts such as O6-methylguanine in renal cortical
tubular cells and mesenchymal interstitial cells are related to the
tumor development induced by alkylating agents (8).
Arachidonic acid (AA; 20:4n-6) is a polyunsaturated
fatty acid present in the phospholipids of cell membranes (11). AA in the human body comes from
dietary sources such as egg yolk, or it is synthesized from
linoleic acid (12). AA is
naturally found in human breast milk. AA, together with
docosahexaenoic acid, is commonly added as a functional food
ingredient to commercial infant formula worldwide, in accordance
with the international standards of Codex Alimentarius (13). Omega-3 fatty acids, such as
docosahexaenoic acid, affect the growth of several cancers
(14) and AA has been reported to
affect carcinogenesis in certain organs. AA promotes the growth of
tumors from an orthotopically transplanted breast cancer cell line
(KPL-1) in female athymic BALB/c mice, urinary bladder tumors in a
medium-term multi-organ rat carcinogenesis study (15), and preneoplastic lesions of the
exocrine pancreas in an MNU-treated rat model (5). AA metabolites and enzymes are
associated with renal tumors and related disease; cyclooxygennase-2
expression is associated with renal carcinoma (16,17),
and prostaglandin E2 is associated with paraneoplastic
hypercalcemia in nephroblastoma (18,19).
The aim of the present study was to elucidate the effect of
prenatal and postnatal dietary AA on MNU-induced renal
carcinogenesis in young Lewis rats.
Materials and methods
Animal procedures
The study protocol and all animal procedures were
approved by the Animal Care and Use Committee of Kansai Medical
University and were in accordance with the guidelines for animal
experimentation at Kansai Medical University. Sixteen female
SPF/VAF rats (LEW/CrlCrlj) that were 10 weeks old and one-week
pregnant were purchased from Charles River Japan (Yokohama, Japan).
Rats were maintained in specific pathogen-free conditions and had
free access to water and CE-2-modified diets containing different
doses of AA. Animals were housed in plastic cages with paper-chip
bedding (Paper Clean, SLC, Hamamatsu, Japan) in an air-conditioned
room at 22±2°C and 60±10% relative humidity with a 12 h light/dark
cycle. The illumination intensity in the cages was less than 60
lux. Offspring were culled to a maximum of 10 per dam, and the dams
were maintained on their respective diets during the 21-day
lactation period. During a post-weaning period of up to 60 days,
the offspring were maintained on a CE-2 diet. A total of 115 male
and female pups were used in this study. Four to ten rats were
sacrificed at each time point (7, 14, 21, 28 and 60 days), and
there were similar numbers of males and females in each dietary
group.
Chemical and dose formulation
MNU was obtained from Sigma-Aldrich (St. Louis, MO,
USA) and was kept at −80°C in the dark. The MNU solution was
dissolved in physiologic saline containing 0.05% acetic acid
immediately prior to use. MNU (35 mg/kg) or vehicle (physiological
saline containing 0.05% acetic acid) was administered by
intraperitoneal (i.p.) injection. In our preliminary experiment,
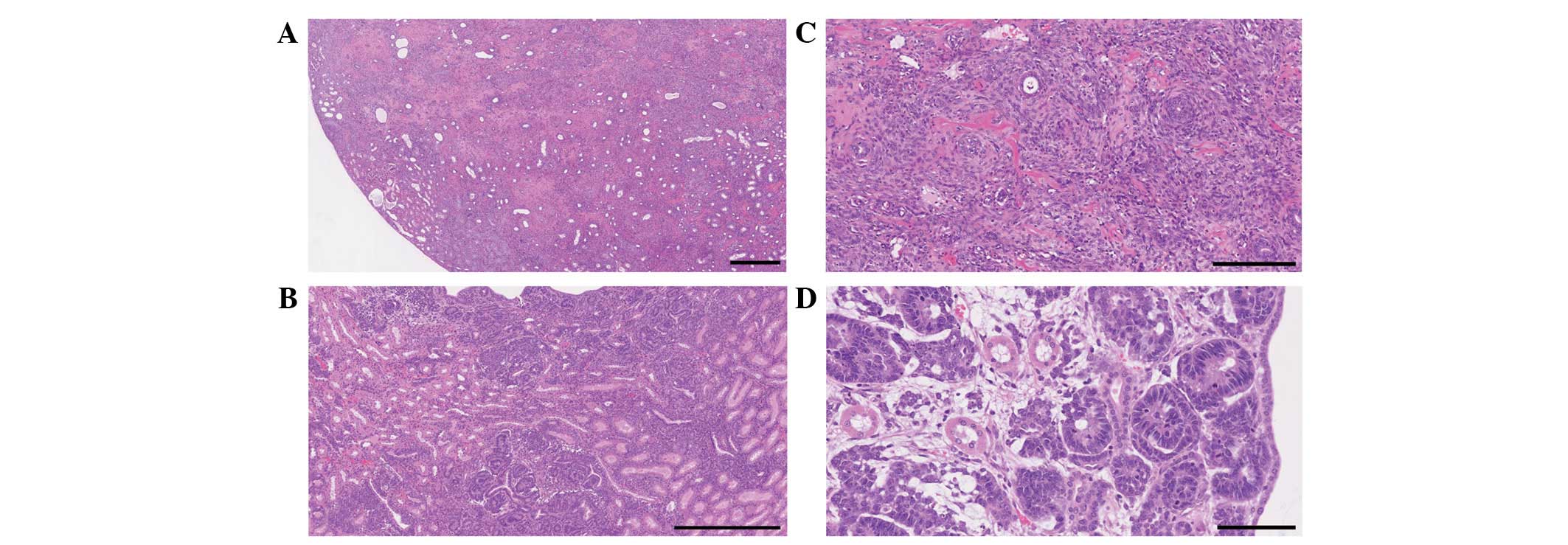
mesenchymal tumors and nephroblastoma developed in 10 and 3 rats,
respectively, of the 14 surviving rats that were treated with 50
mg/kg MNU at birth, respectively (Fig.
1). However, almost 50% of the rats died, and all surviving
female rats developed mammary cancers with severe hematotoxicity.
Therefore, 35 mg/kg MNU was selected as a non-lethal lower dose
without the incidence of mammary cancers in the present short-term
study.
Arachidonic acid-supplemented diet
As in the previous study, the AA-supplemented diet
was formulated by CLEA Japan (5,15). AA
was purchased from Cargill Alking Bioengineering (Wuhan and Hubei,
China). The diet with 2.0 w/w% AA was semi-purified based on the
modified CE-2 formulation (CLEA Japan, Tokyo, Japan). The basal
diet consisted of modified CE-2. Gas chromatographic analyses of
the fatty acid composition of the diets are described in a previous
study (5). The total fatty acid
volumes were 47.20, 86.75 and 126.63 mg/mg of diet for the CE-2
diet (0.006 w/w% AA), basal diet (0.008 w/w% AA), and 2.0% AA diet,
respectively. The diets were stored at 4°C to prevent lipid
oxidation before use.
Experimental procedures
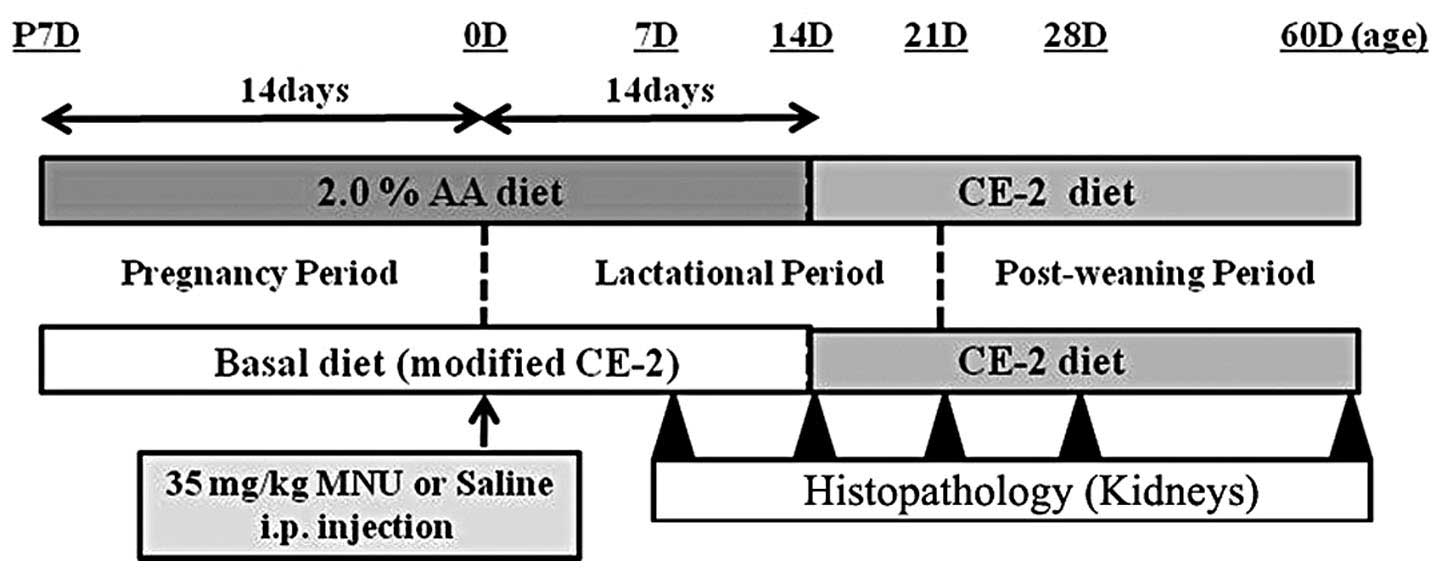
Male and female Lewis rats were exposed to the basal
or experimental diet (2.0% AA) from fertilization to sacrifice. At
birth (0 days of age), the rats received an i.p. injection of
vehicle (physiological saline) or 35 mg/kg MNU (Fig. 2). At 7, 14, 21, 28 and 60 days after
MNU or vehicle treatment, rats were anesthetized with isoflurane
(Forane®; Abbot Japan, Tokyo, Japan) and sacrificed by
exsanguination via aortic transection. During the experiment, all
pups were observed daily for clinical signs of toxicity and were
weighed at the time of MNU treatment and on the day of sacrifice.
Both kidneys were removed at the time of sacrifice and complete
necropsies were conducted on all animals to check for systemic
toxicities induced by AA supplementation. Food consumption and body
weight of the dams were measured once per week to estimate the
actual dosage of AA during the pregnancy and lactation periods.
Histopathological examination
Renal tissues were fixed overnight in 10% neutral
buffered formalin, embedded in paraffin, sectioned at a thickness
of 4 mm and stained with hematoxylin and eosin (HE).
Histopathological evaluation was performed by a toxicologic
pathologist certified by the Japanese Society of Toxicologic
Pathology and/or the International Academy of Toxicologic Pathology
(K.Y. and A.T.). Histopathological terminology and diagnostic
criteria of rodent renal neoplastic lesions were in accordance with
the guidelines of the International Harmonization Nomenclature and
Diagnostic Criteria for Lesions in Rats and Mice Project (20). Nephroblastoma, known as Wilms' tumor
in humans, originates from the metanephric blastema. It is
characterized by discrete clusters of highly basophilic blast cells
surrounding mature ducts and organoid differentiation as epithelial
rosettes, primitive basophilic tubules, attempted glomerulus
formation or mature epithelial ducts (20,21).
Nephroblastomatosis is a small, solitary, basophilic cell mass
consisting of densely crowded blast cells with ill-defined
cytoplasm and basophilic nuclei and a few signs of early organoid
differentiation into epithelial rosettes (20,22,23).
Nephroblastomatosis has the potential to develop into a
nephroblastoma as it grows, and is regarded as a preneoplastic
lesion of nephroblastoma (24).
Mesenchymal tumors originate from foci of atypical fibroblast-like
cells in the interstitium of the outer stripe of outer medulla,
similar to a renal tumor of infancy described as congenital
mesoblastic nephroma (22,25,26).
Mesenchymal tumors are characterized by heterogeneous connective
tissue cell composition with a predominance of spindle cells,
primitive mesenchyme, and smooth muscle fibers and occasional
rhabdomyoblasts, striated muscle, cartilage, osteoid or
hemangiosarcoma-like areas (20,21).
Renal mesenchymal tumors are frequently misdiagnosed as
nephroblastomas due to the pre-existing tubules that survive within
the tumor tissue and which can become hyperplastic and/or
metaplastic (9,24). Mesenchymal cell proliferation is
defined as small and solitary foci of atypical fibroblast-like
cells, regarded as preneoplastic lesions and early stage
mesenchymal tumors (6).
Statistical analysis
The incidence of renal preneoplastic lesions was
analyzed using the χ2 test. The results presented
include comparisons between rats fed a basal diet and rats fed an
AA-supplemented diet in the MNU-treated groups. P<0.05 was
considered to indicate a statistically significant result.
Results and Discussion
No mortalities occurred and no clinical signs or
symptoms related to any treatment were evident in any of the pups
or dams during the experimental period. None of the pups developed
mammary tumors. The 2.0% AA diet did not influence food consumption
in dams during the experimental period. During the pregnancy and
lactation periods, the AA intake of dams was 6.3 and 8.5 mg/kg/day
in the basal diet group and 1,477 and 1,876 mg/kg/day in the 2.0%
AA group, respectively. The 2.0% AA diet did not influence body
weight gain (the growth rate) in pups or cause weight changes in
dams with or without MNU treatment. The growth rate in MNU-treated
pups tended to be lower than that in vehicle-treated pups, however
(data not shown).
The incidence of preneoplastic renal lesions is
shown in Table I. Macroscopic
nodular lesions were not detected in any group. In the
vehicle-treated rats with or without the AA-rich diet, no
proliferative lesions were observed at any time point. In contrast,
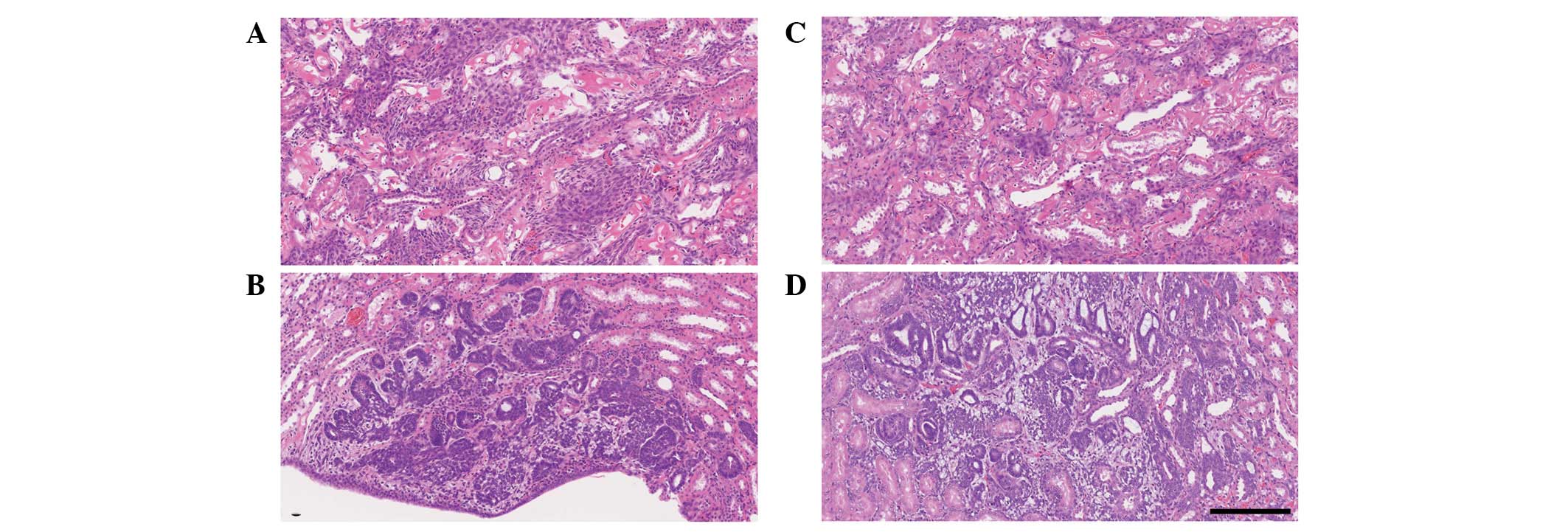
single or multifocal preneoplastic lesions occurred in the basal
diet-fed rats 60 days after MNU treatment (38% incidence, 3 lesions
in 8 kidneys); mesenchymal cell proliferation was detected
histopathologically in 2 kidneys (Fig.
3A) and nephroblastomatosis in 1 kidney (Fig. 3C). The AA-rich diet-fed rats also
developed preneoplastic lesions 60 days after MNU treatment (31%
incidence, 5 lesions in 16 kidneys); mesenchymal cell proliferation
was detected in 2 kidneys (Fig. 3B)
and nephroblastomatosis in 3 kidneys (Fig. 3D). There was no significant
difference in lesion incidence between the MNU-treated rats fed a
basal diet and the MNU-treated rats fed an AA-rich diet. The
multiplicity of these lesions was not different between MNU-treated
rats with or without AA supplementation. In these lesions, the
normal tubules were entrapped and there was no evidence of
capsulation. Tubular tumors were not observed in any group at any
time point. Pelvic dilation was detected in some rats of each group
at any time point, which is characteristic of spontaneously
occurring lesions in this strain (data not shown).
 | Table IIncidence of renal preneoplastic
lesions induced by MNU. |
Table I
Incidence of renal preneoplastic
lesions induced by MNU.
| Treatment | Food | Days after MNU
treatment
|
|---|
| 7 | 14 | 21 | 28 | 60 |
|---|
| Vehicle | Basalb | 0 (0/12)d | 0 (0/12) | 0 (0/10) | 0 (0/10) | 0 (0/20) |
| AAc | 0 (0/12) | 0 (0/12) | 0 (0/10) | 0 (0/10) | 0 (0/10) |
| MNUa | Basal | 0 (0/12) | 0 (0/12) | 0 (0/10) | 0 (0/10) | 38 (3/8e) |
| AA | 0 (0/12) | 0 (0/12) | 0 (0/10) | 0 (0/10) | 31 (5/16f)g |
The known risk factors include therapeutic doses of
ionizing radiation and inherited and genetic alterations. Together
they are estimated to account for 5–10% of childhood cancers
(27). Nephroblastoma accounts for
95% of kidney malignancies during childhood (27). Children are exposed to potentially
carcinogenic chemicals, such as pesticides, from use in homes,
schools and gardens and through contaminated food and drinking
water. Parent exposure during the child's gestation or even
preconception may also be important. The household or occupational
use of pesticides increases the risk of renal tumors in children
(28). Tea or coffee consumption
and certain parental occupations have been consistently associated
with this type of tumor (27). The
main purpose of the present study was to determine whether
increased levels of AA during gestation and lactation
proportionally enhance the development of renal preneoplastic
lesions in MNU-treated rat pups. Renal morphology in rats treated
with 35 mg/kg MNU showed nephroblastomatosis and mesenchymal cell
proliferation in those fed a basal or AA-rich diet (2.0% AA). These
results suggest that the increased incidence of these lesions is an
early indicator of renal carcinogenesis induced by chemicals
(6,24). The results demonstrate that 2.0% AA
did not have any morphological effect on renal preneoplastic
lesions. Recently, AA showed no promoting effects on kidneys in a
rat medium-term multi-organ carcinogenesis model with 5 carcinogens
including MNU (29).
In a study by Sharma et al, 50 mg/kg MNU i.p.
injections 2 and 4 days after birth induced renal tumors at between
4 and 8 months of age. Renal tumors occurred in 63 of 140 kidneys
(45%); there were 29 mesenchymal tumors, 18 nephroblastomas and 16
other tumors. The incidence of mortality, mammary tumors and
hematotoxicity in this model was not reported, however (10). In this preliminary study, a single
injection of 50 mg/kg MNU given to Lewis rats at birth induced a
high incidence of mortality and mammary cancers with severe
hematotoxicity. Rats are most sensitive to the induction of renal
tumors when alkylating agents are administered at the early weeks
after birth, when the rate of cell division in the immature kidney
is the highest (10,22). Like this experimental protocol, a
short-term study (60 days) with 35 mg/kg MNU as a non-lethal lower
dose without the incidence of mammary cancers, may therefore be
extremely useful for testing the promotion, progression or
inhibitory effects of chemical and physical agents on cell
proliferation and transformation in rat kidneys.
The AA intake by Japanese infants via breast milk is
∼14.3 mg/kg/day (29). The 2.0% AA
diets in this present study provide an AA dose of 1,477 mg/kg/day
during pregnancy and 1,876 mg/kg/day during lactation, representing
∼103- and 131-fold, respectively, the amount consumed by human
infants. Taken together, the results indicate that an AA-enriched
diet in the prenatal and postnatal periods is unlikely to cause
renal carcinogenesis in human infants. In conclusion, an AA-rich
diet in dams during gestation and lactation does not modify
MNU-induced renal preneoplastic lesions in young rats. Further
studies with other animal models are necessary to fully elucidate
the effects of AA on renal carcinogenesis.
Acknowledgements
This research was supported in part by
Health and Labour Sciences Research Grants
(H22-Shokuhin-Ippan-002). The authors thank Ms. T. Akamatsu for her
technical assistance and Dr T. Sasaki, Maruho Co. Ltd., for her
excellent scientific advice. All authors read and approved the
final manuscript.
References
|
1
|
World Health Organization, International
Agency for Research on Cancer (WHO IARC): Kidney cancer. World
Cancer Report. Stewart BW and Kleihues P: IARC Press; Lyon: pp.
261–264. 2003
|
|
2
|
Davidoff AM: Wilms tumor. Curr Opin
Pediatr. 21:357–364. 2009. View Article : Google Scholar
|
|
3
|
Kimura A, Yoshizawa K, Sasaki T, Uehara N,
Kinoshita Y, Miki H, Yuri T, Uchida T and Tsubura A:
N-methyl-N-nitrosourea-induced changes in epithelial
rests of Malassez and the development of odontomas in rats. Exp
Ther Med. 4:15–20. 2012.
|
|
4
|
Tsubura A, Lai YC, Miki H, Sasaki T,
Uehara N, Yuri T and Yoshizawa K: Animal models of
N-methyl-N-nitrosourea-induced mammary cancer and
retinal degeneration with special emphasis on therapeutic trials.
In Vivo. 25:11–22. 2011.
|
|
5
|
Yoshizawa K, Uehara N, Kimura A, Emoto Y,
Kinoshita Y, Yuri T, Takada H, Moriguchi T, Hamazaki T and Tsubura
A: Promoting effect of arachidonic acid supplementation on
N-methyl-N-nitrosourea-induced pancreatic acinar cell
hyper-plasia in young Lewis rats. Oncol Lett. 5:76–82.
2013.PubMed/NCBI
|
|
6
|
Warzok R, Schreiber D and Blaufuss EM:
Tumors of the rat kidney induced by nitrosourea compounds. Exp
Path. 17:394–402. 1979.PubMed/NCBI
|
|
7
|
Hard GC: Experimental models for the
sequential analysis of chemically-induced renal carcinogenesis.
Toxicol Pathol. 14:112–122. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hard GC: Mechanisms of chemically induced
renal carcinogenesis in the laboratory rodent. Toxicol Pathol.
26:104–112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turusov VS, Alexandrov VA and Timoshenko
IV: Nephroblastoma and renal mesenchymal tumor induced in rats by
N-nitrosoethyl- and N-nitrosomethylurea. Neoplasma.
27:229–235. 1980.PubMed/NCBI
|
|
10
|
Sharma PM, Bowman M, Yu BF and Sukumar S:
A rodent model for Wilms tumors: embryonal kidney neoplasms induced
by N-nitroso-N-methylurea. Proc Natl Acad Sci, USA.
91:9931–9935. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Davis-Bruno K and Tassinari MS: Essential
fatty acid supplementation of DHA and ARA and effects on
neurodevelopment across animal species: a review of the literature.
Birth Defects Res (Part B). 92:240–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le HD, Meisel JA, de Meijer VE, Gura KM
and Puder M: The essentiality of arachidonic acid and
docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids.
81:165–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Codex Alimentarius Commission, Joint
FAO/WHO Food Standards Programme: Report of the 28th Session of the
Codex Committee on Nutrition and Foods for Special Dietary Uses.
2007.
|
|
14
|
Gleissman H, Johnsen JI and Kogner P:
Omega-3 fatty acids in cancer, the protectors of good and the
killers of evil? Exp Cell Res. 316:1365–1373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamazaki T: Reports on Research on the
toxicity of arachidonic acid supplementation. The Health and Labor
Sciences Research Grants, Japan (H22-Shokuhin-Ippan-002),. 2012.(in
Japanese).
|
|
16
|
Hashimoto Y, Kondo Y, Kimura G, Matsuzawa
I, Sato S, Ishizaki M, Imura N, Akimoto M and Hara S:
Cyclooxygenase-2 expression and relationship to tumour progression
in human renal cell carcinoma. Histopathology. 44:353–359. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsuyama M and Yoshimura R: Relationship
between arachidonic acid pathway and human renal carcinoma. Onco
Targets and Therapy. 1:41–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calo L, Cantaro S, Bertazzo L, Vianello A,
Vido L and Borsatti A: Synthesis and catabolism of PGE2 by a
nephroblastoma associated with hypercalcemia without bone
metastases. Cancer. 54:635–637. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calo L, Cantaro S, Vianello A, Vido L,
Rizzoni G and Borsatti A: Arachidonic acid metabolites in a
nephroblastoma associated with paraneoplastic hypercalcemia.
Prostaglandins. 32:116–120. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frazier KS, Seely JC, Hard GC, Betton G,
Burnett R, Nakatsuji S, Nishikawa A, Durchfeld-Meyer B and Bube A:
Proliferative and nonproliferative lesions of rat and mouse urinary
system. Toxicol Pathol. 40:14S–86S. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mitsumori K, Yoshida M, Iwata H, Katsuda
O, Kouchi M and Tsuda H: Classification of renal proliferative
lesions in rats and/or mice and their diagnostic problems: report
from the working group of the Japanese Society of Toxicologic
Pathology. J Toxicol Pathol. 15:175–190. 2002. View Article : Google Scholar
|
|
22
|
Frank AA, Heidel JR, Thompson DJ, Carlton
WW and Beckwith JB: Renal transplacental carcinogenicity of
3,3-dimethyl-phenyltriazene in rats: relationship of renal
mesenchymal tumor to congenital mesoblastic nephroma and intralobar
nephrogenic rests. Toxicol Pathol. 20:313–322. 1992.
|
|
23
|
Klalaiselvan P, Mathur KY, Pande VV,
Madheswaran R, Bhelonde JJ, Shelar PD, Udupa V and Shingatgeri VM:
Intralobar nephroblastomatosis in a nine-week-old Wistar rat.
Toxicol Pathol. 37:819–825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seely JC: Renal mesenchymal tumor vs
nephroblastoma: revised. J Toxicol Pathol. 17:131–136. 2004.
View Article : Google Scholar
|
|
25
|
Deshpande RB and Hasgekar NN: Rat renal
mesenchymal tumor as an experimental model for human congenital
mesoblastic nephroma: II. Comparative pathology. Pediatr Pathol.
9:141–151. 1989. View Article : Google Scholar
|
|
26
|
Hasgekar NN, Pendse AM and Lalitha VS: Rat
renal mesenchymal tumor as an experimental model for human
congenital mesoblastic nephroma: I. Induction. Pediatr Pathol.
9:131–139. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bunin GR: Nongenetic causes of childhood
cancers: evidence from international variation, time trends, and
risk factor studies. Toxicol Appl Pharmacol. 199:91–103. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zahm SH and Ward MH: Pesticides and
childhood cancer. Environ Health Perspect. 106:893–908. 1998.
View Article : Google Scholar
|
|
29
|
Imai N, Kawabe M, Tamano S, Doi Y,
Nakashima H, Suguro M, Numano T, Hara T, Hagiwara A, Furukawa F,
Kaneda Y, Tateishi N, Fujii W, Kawashima H, Shibata H and
Sakakibara Y: Arachidonate-enriched triglyceride oil does not
promote tumor development in a rat medium-term multi-organ
carcinogenesis model. Food Chem Toxicol. 50:2780–2791. 2012.
View Article : Google Scholar : PubMed/NCBI
|