Introduction
Although chronic lymphocytic leukemia (CLL) patients
with early-stage disease have a life expectancy of >10 years,
those with progression or who have advanced-stage disease (Binet
stages B and C) have a median survival of 2–7 years (1). A number of patients are asymptomatic
and may survive for decades without requiring treatment, whereas
others experience an aggressive form of disease and may succumb to
the disease or due to therapy-associated complications shortly
after diagnosis. However, the majority of research on CLL is from
Western countries, with few studies reporting results in Chinese
patients.
For more than 30 years, CLL has been treated with
various chemotherapeutic drugs, including the alkylating drug
chlorambucil (Ch). In the past decade, the combination of purine
analogs with alkylating drugs, particularly fludarabine (F) and
cyclophosphamide (C), has improved the rate of clinical response,
complete remission (CR) and progression-free survival (2). Additionally, the use of rituximab (R)
has improved the efficacy of CLL treatment, with complete response
rates of 40–70% (3–5). Since these trials enrolled younger
patients with a good performance status and no severe morbidities,
the results of these studies are most relevant to the treatment of
younger Western patients with CLL. The effect of anti-CD20
antibodies in Asian patients with CLL remains to be determined. The
primary objective of our study was to retrospectively analyze the
clinical characteristics of CLL patients in China and to evaluate
the outcome of R treatment.
Patients and methods
Patients
Between January 2002 and December 2011, 210 patients
with CLL were treated in our institution. The history, physical
findings and laboratory data of the patients were collected for
analysis. The diagnostic criteria for CLL were as follows: i)
Persistent lymphocytosis of >5×109/l lasting for ≥3
months; ii) morphologically small- to medium-sized lymphocytes
without nucleoli; iii) no cell membrane hair formation; iv)
CD5+, CD19+ and CD10−
immunophenotypes; and v) no cyclin D1 expression. The study methods
were reviewed and approved by Union Hospital of Fujian Medical
University Review Board.
Serum parameters
Serum and heparinized blood samples were obtained
from all patients before treatment. Serum lactate dehydrogenase
(LDH) and serum β2-microglobulin (β2-MG) levels were evaluated by
radioimmunoassay.
Immunoglobulin variable heavy-chain
(IgVH) mutation status analysis
To determine the VH gene mutation status, reverse
transcription-polymerase chain reaction (RT-PCR) was performed
using VH primers (6). Amplified PCR
products were purified and directly sequenced using a sequencing
kit (Applied Biosystems, Carlsbad, CA, USA). Mutation status was
determined by comparison with the consensus germline sequence
according to IgBlast (www.ncbi.nlm.nih.gov/igblast) and IMGT/V-QUEST
(http://www.imgt.org). We determined the gene as
‘mutated’ when the sequence deviated by ≥2% from the consensus
sequence.
Evaluation of CD38 and ZAP-70
expression
Flow cytometry analysis in this study was performed
on a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA). The expression of CD38 was analyzed by 3-color
immunofluorescence (7) and the
detection of ZAP-70 was performed according to previously reported
methods (8). A cut-off point of 30%
positive cells was selected to discriminate CD38− from
CD38+ CLL. A cut-off point of 20% was used to
distinguish ZAP-70− from ZAP-70+ CLL.
Cytogenetic analysis
Interphase fluorescence in situ hybridization
(FISH) was carried out for the detection of trisomy 12 and
chromosome deletions at 13q14.3, 11q22.3 and 17p13.1 loci. A
chromosome 12-specific α-satellite probe was used to identify
trisomy 12. For the detection of a 13q14.3 deletion, a
locus-specific probe (LSI D13D25) was cohybridized with the 13q34
telomeric probe as an internal control for nullisomy. Dual-color
hybridizations using the appropriate centromere-specific probes and
unique sequence-specific probes for the ATM (LSI ATM) and TP53 (LSI
P53) loci were performed for the detection of 11q22.3 and 17p13.1
deletions, respectively. All probes were purchased from Vysis
(Abbott, Shanghai, China) and FISH procedures were performed
according to the manufacturer’s instructions. For each
hybridization, ≥200 interphase nuclei were assessed. Patients were
categorized into low- (13q14.3 deletion and normal), intermediate-
(trisomy 12) and high- (11q22.3 and 17p13.1 deletions) risk groups
for subsequent analysis.
Treatments
Treatment consisted of six 28-day courses of
intravenous F at 25 mg/m2 per day and C at 250
mg/m2 per day for the first three days of each treatment
course, with or without R at a dose of 375 mg/m2 on day
0 of each course. Ch was initially administered orally at 0.4 mg/kg
body weight (BW) on day one and was increased by 0.1 mg/kg for each
treatment course up to 0.8 mg/kg BW if treatment was well
tolerated. Maintenance therapy was performed with two cycles of the
original treatment course after achieving CR.
Statistical methods
Clinical data are presented using descriptive
statistics. The χ2 test was used to compare clinical
characteristics between groups. Overall survival (OS) was defined
as the time between the date of diagnosis and the date of the last
follow-up or death due to any cause. For univariate survival
analysis, the Kaplan-Meier method for incomplete observation was
used. The estimated survival curves were compared using the
log-rank test. A multivariate analysis of the potential factors
affecting the OS was performed using a step-wise Cox
proportional-hazard regression method. P<0.05 was considered to
indicate a statistically significant difference. All tests were
two-tailed with a multiple significance level of α=5%.
Results
Patients
Between January 2002 and December 2011, 210 patients
with CLL from a single institution in China were enrolled in this
study and followed up for survival. The main clinical
characteristics of the 210 patients in this study are shown in
Table I. The median follow-up for
the entire group was 68 months (range, 4–110 months). There were 95
males and 115 females in this study, whose age at the time of
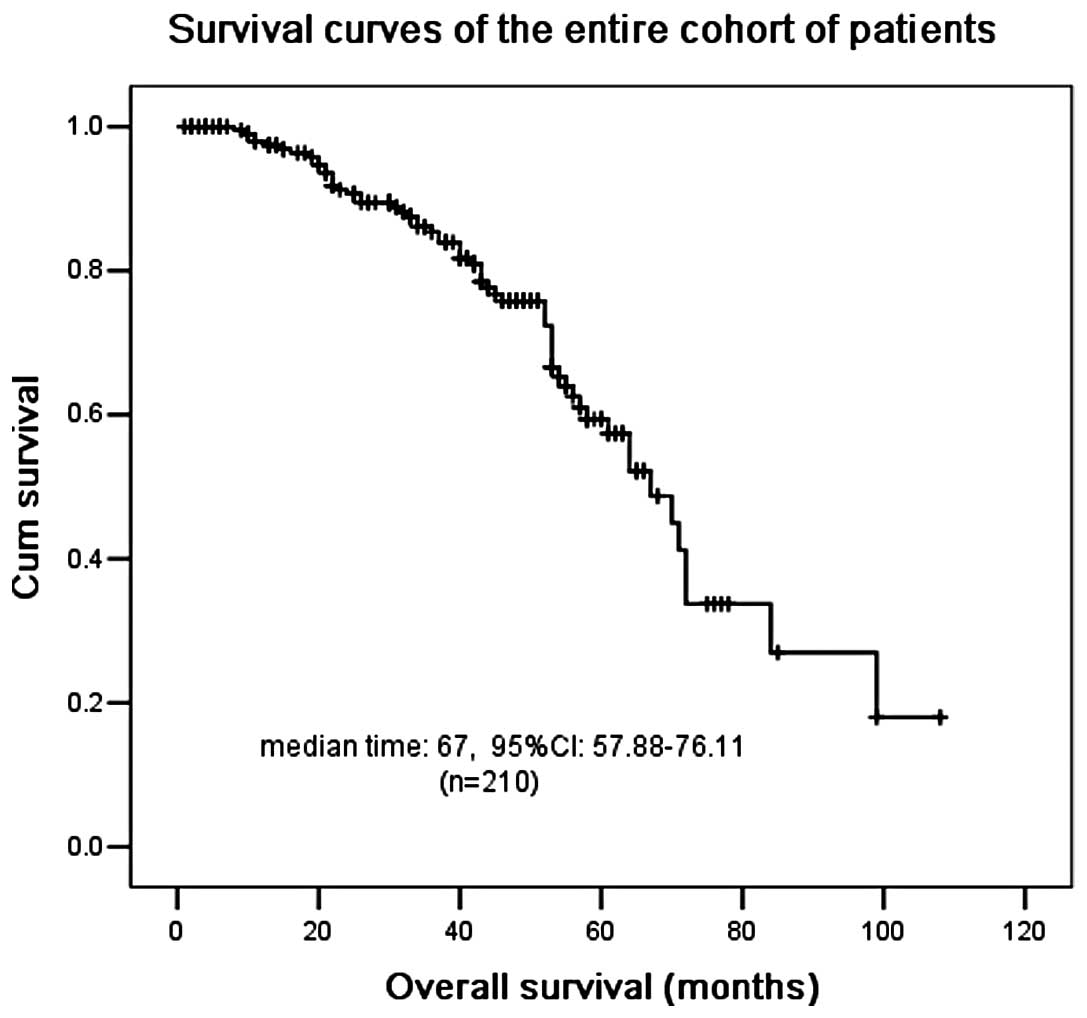
enrollment ranged from 35–92 years with a median age of 60.2
years (Fig. 1). Immunophenotypic
data, available for 202 of the 210 patients, showed that all cases
of leukemia were CD19+, 196/202 were CD5+,
188/202 were CD20+ [61/188 expressed (+) and 127/188
expressed (++)] and 200/202 were CD23+. All cases were
confirmed to be of the B-cell type. At the time of enrollment, 53
patients had stage A, 120 had stage B and 37 had stage C disease
according to the Binet system.
 | Table IClinical characteristics of the
patients with CLL. |
Table I
Clinical characteristics of the
patients with CLL.
| Variable | CR+PR (%) | P-valueb | Median OS, months
(95% CI) | P-valuec |
|---|
| Age, yearsa | | | | |
| ≤60 | 62.8 | 0.112 | 70 (61–78.9) | 0.031 |
| >60 | 74.2 | | 61 (54.9–73) | |
| Gender | | | | |
| Male | 71.6 | 0.652 | 69 (60.6–79) | 0.732 |
| Female | 67.8 | | 64 (57.1–72.3) | |
| Binet stage at
diagnosis | | | | |
| A | 73.6 | 0.398 | 77 (64.5–91.1) | 0.732 |
| B | 65.8 | | 65 (57.7–73.6) | |
| C | 75.7 | | 59 (53.3–65.9) | |
| ECOG | | | | |
| 0 | 68.9 | 0.858 | 67 (56–77.9) | 0.536 |
| 1 | 70.4 | | 61 (46.5–75.4) | |
| ≥2 | 80.0 | | 53 (27.3–78.6) | |
| LDH | | | | |
| ≤450 IU/l | 73.5 | 0.176 | 70 (52.3–87.6) | 0.055 |
| Elevated | 64.5 | | 61 (46.1–75.8) | |
| Serum β2-MG | | | | |
| Normal | 74.3 | 0.132 | 72 (57–86.9) | 0.028 |
| Elevated | 63.9 | | 59 (42.8–76.1) | |
| CD38 | | | | |
| Positive | 66.0 | 0.085 | 65 (58.6–73.2) | 0.175 |
| Negative | 79.6 | | 65 (58.3–70.8) | |
| ZAP-70 | | | | |
| Positive | 67.2 | 0.088 | 58 (52–63.9) | 0.723 |
| Negative | 83.3 | | 67 (59.5–74.4) | |
| IgVH mutational
status | | | | |
| Mutation | 72.7 | 0.714 | 70 (60.4–80) | 0.085 |
| No mutation | 68.7 | | 65 (58.2–72.3) | |
| Genomic
aberrations | | | | |
| Trisomy 12 | 66.7 | 0.761 | 61 (47.3–75) | 0.351 |
|
11q− | 54.5 | 0.749 | 37 (23.4–51) | 0.057 |
|
17p− | 40.0 | 0.008 | 33 (20.4–45.5) | 0.001 |
|
13q− | 33.3 | 0.150 | 49 (34.2–64.6) | 0.939 |
| F-based
treatment | | | | |
| Yes | 77.8 | 0.082 | 85 (72.3–98.4) | 0.062 |
| No | 65.2 | | 79 (63.5–93) | |
| R-based
treatment | | | | |
| Yes | 68.5 | 0.067 | 71 (68.4–73.5) | 0.218 |
| No | 58.5 | | 61 (54.6–67.3) | |
Treatment and associated adverse
events
The overall response rates [ORR, CR + partial
remission (PR)] were significantly different among the treatment
regimens (P<0.0001) and the lowest ORR of 44% was identified in
patients treated with Ch (95% CI, 0.30–0.48). The ORR was 68.5% in
patients treated with F and C (95% CI, 0.60–0.72) and this further
improved in patients treated with F, C and R (77.8%; 95% CI,
0.70–0.92). There was no significant difference in ORRs between
patients ≤60 years old and patients >60 years old (P=0.112).
The most frequently occurring adverse events for R-
or F-based treatments in this study were fatigue, hypersensitive
skin reactions, hematological toxicity and GI events (nausea,
vomiting and diarrhea). There were ten reports of tumor lysis
syndrome, all in patients who had received their first cycle of
chemotherapy. Neutropenia was observed in 25% of patients,
including grade 3 or 4 in 14% of patients, and thrombocytopenia was
observed in 9% of patients. During maintenance therapy, 54% of
patients had low Ig serum levels and 47 patients experienced grade
3 or 4 infectious episodes, including 26 patients with pneumonia,
10 with appendicitis, 6 cases of myositis and 5 of herpes zoster.
The hepatitis B virus was activated in 10% (21 cases) of patients
after R-based treatment. Severe infections were of particular
interest since they are a major cause of morbidity and mortality in
CLL patients. Notably, there were 39 patients treated with F, C and
R who experienced grade 3 and 4 infections, however, only eight
patients who received other treatments (including Ch) had grade 3
and 4 infections.
Survival analysis
The median OS for the entire cohort was 67 months
(Fig. 1). Univariate analysis
showed that an age of >60 years old, chromosome 17p deletion
(17p−) and elevated β2-MG levels were associated with a
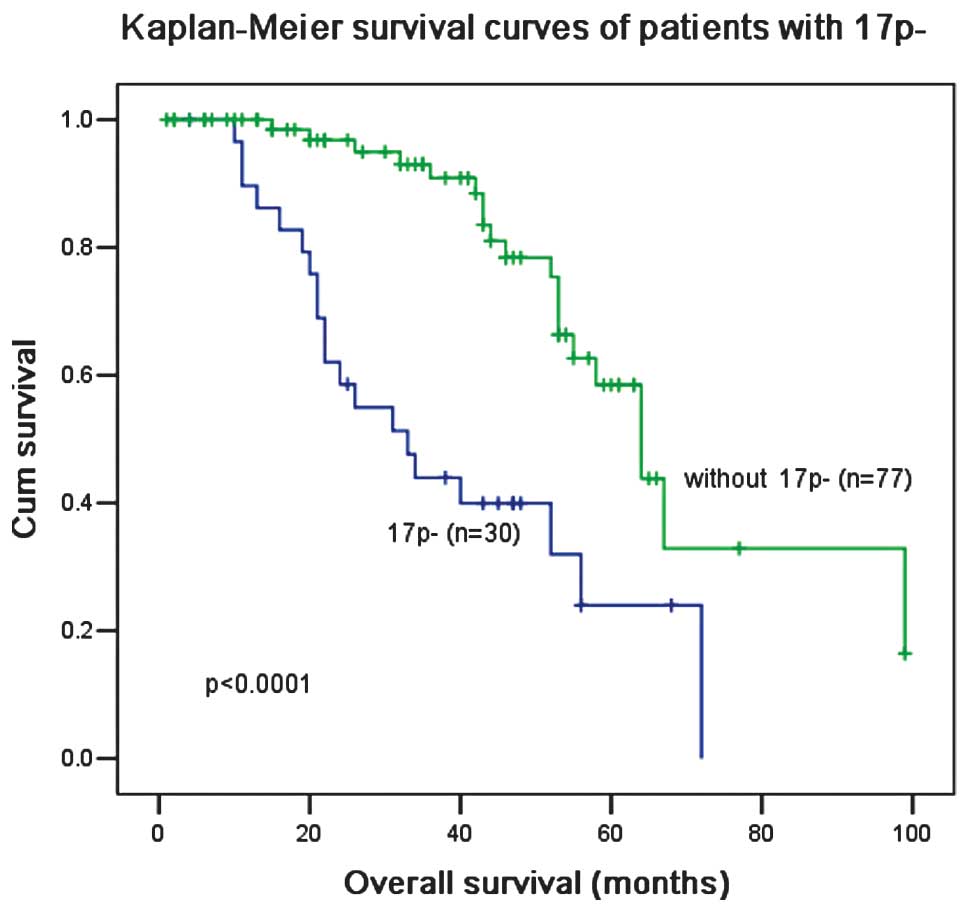
worse overall survival. The patients who harbored 17p−
had a significantly lower ORR (40 vs. 72%; P=0.008; Table I) and a shorter OS (33 vs. 64
months; P<0.0001; Fig. 2) than
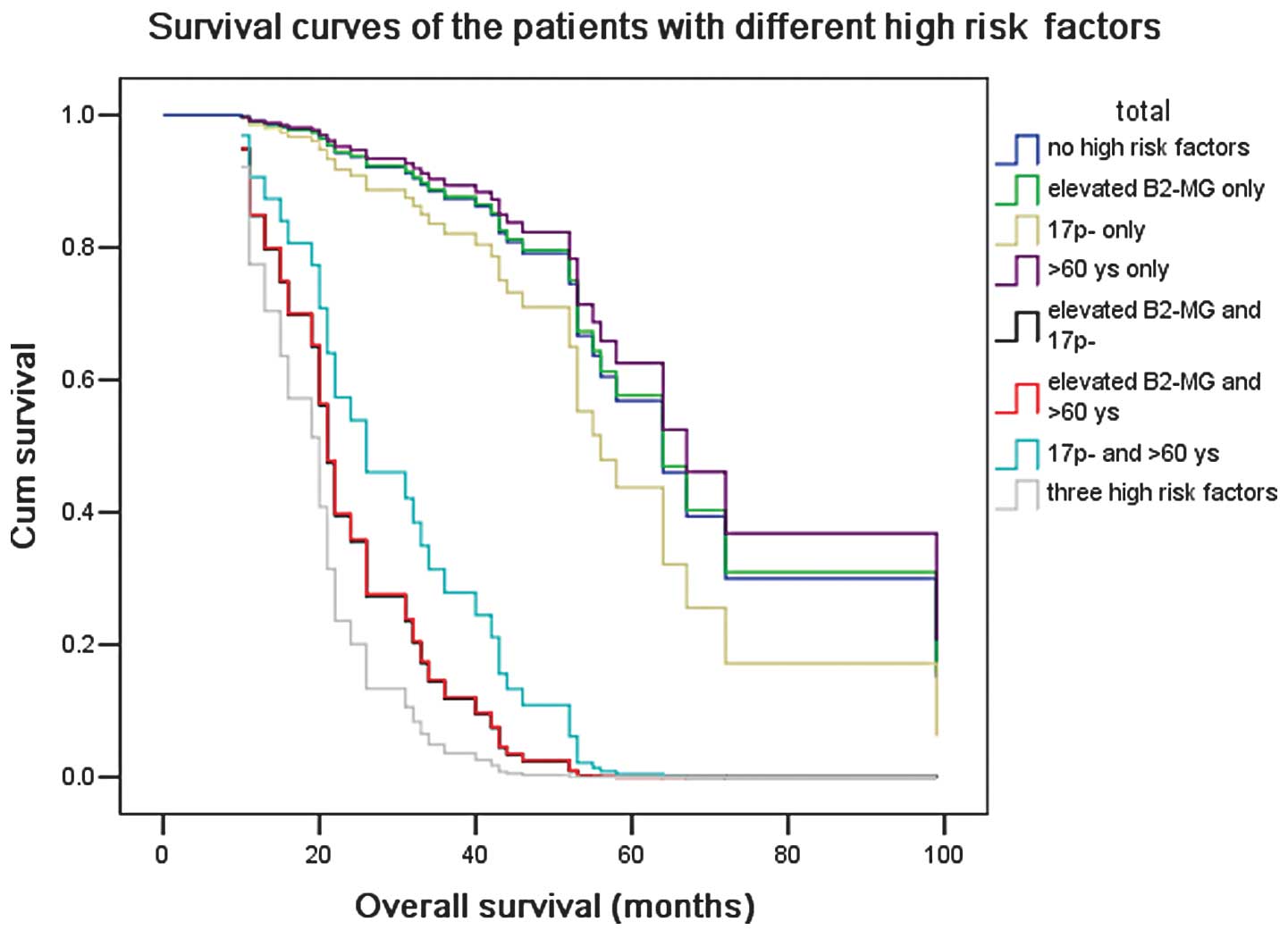
patients without 17p−. Patients with all three poor
prognostic factors had a worse median OS than patients with only
one or two factors (21–28 months vs. 54–62 months; P=0.02; Fig. 3).
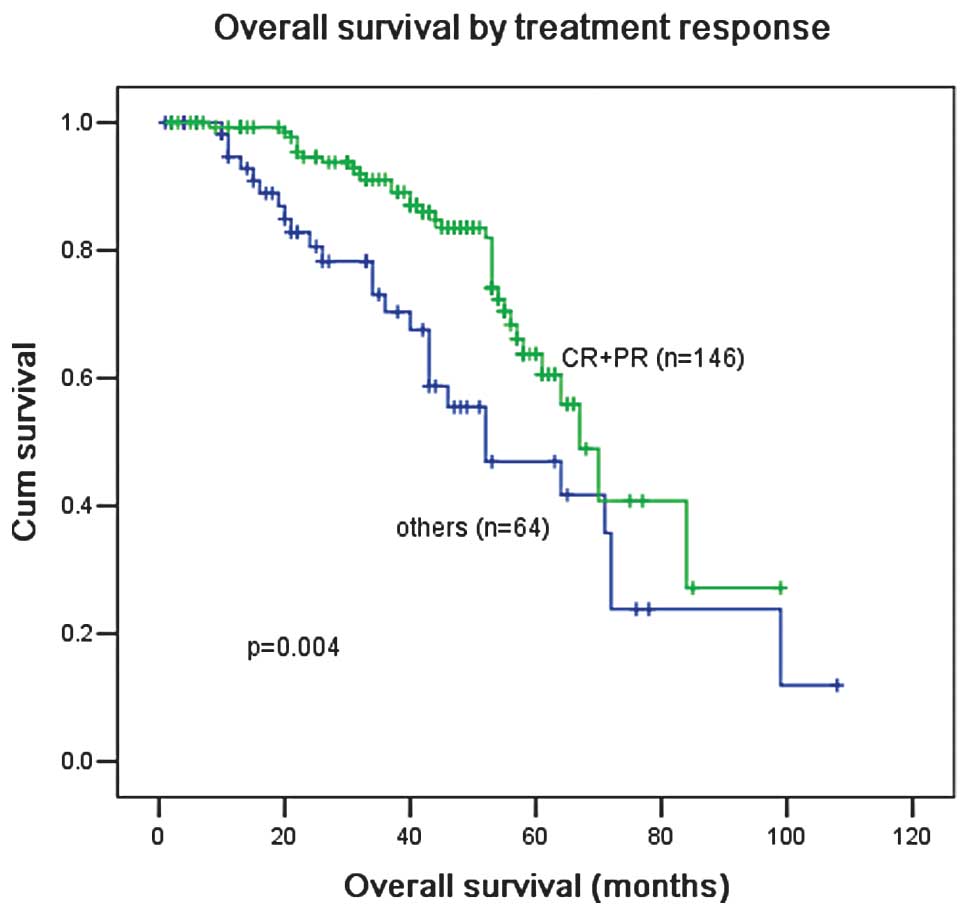
Patients who achieved CR or PR after four cycles of
therapy had a better OS than patients who failed to achieve CR or
PR (Fig. 4). The OS of patients was
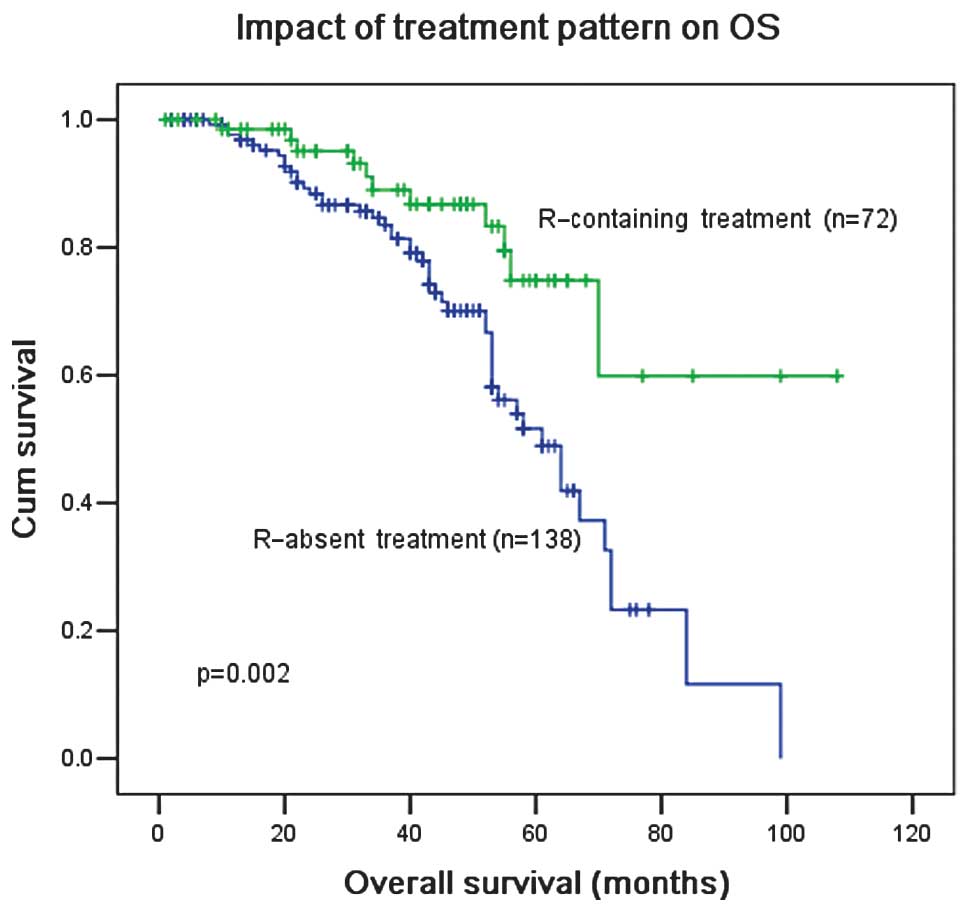
significantly improved with R-based treatment (P=0.002; Fig. 5).
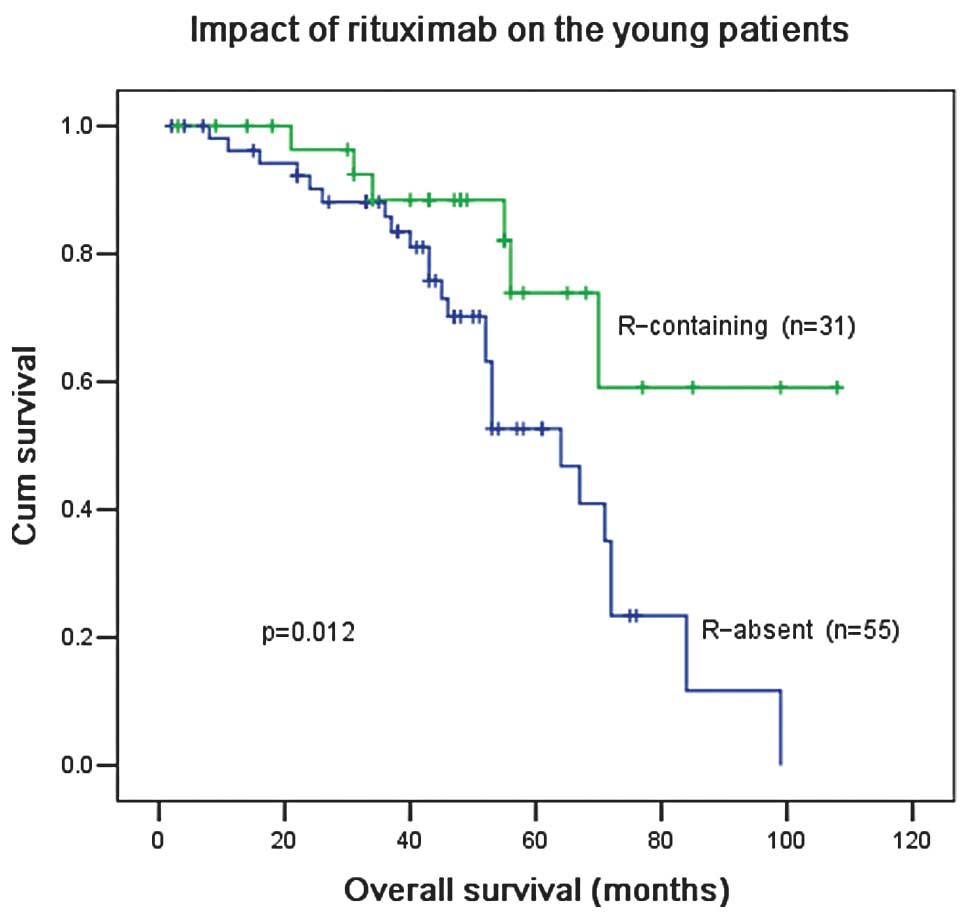
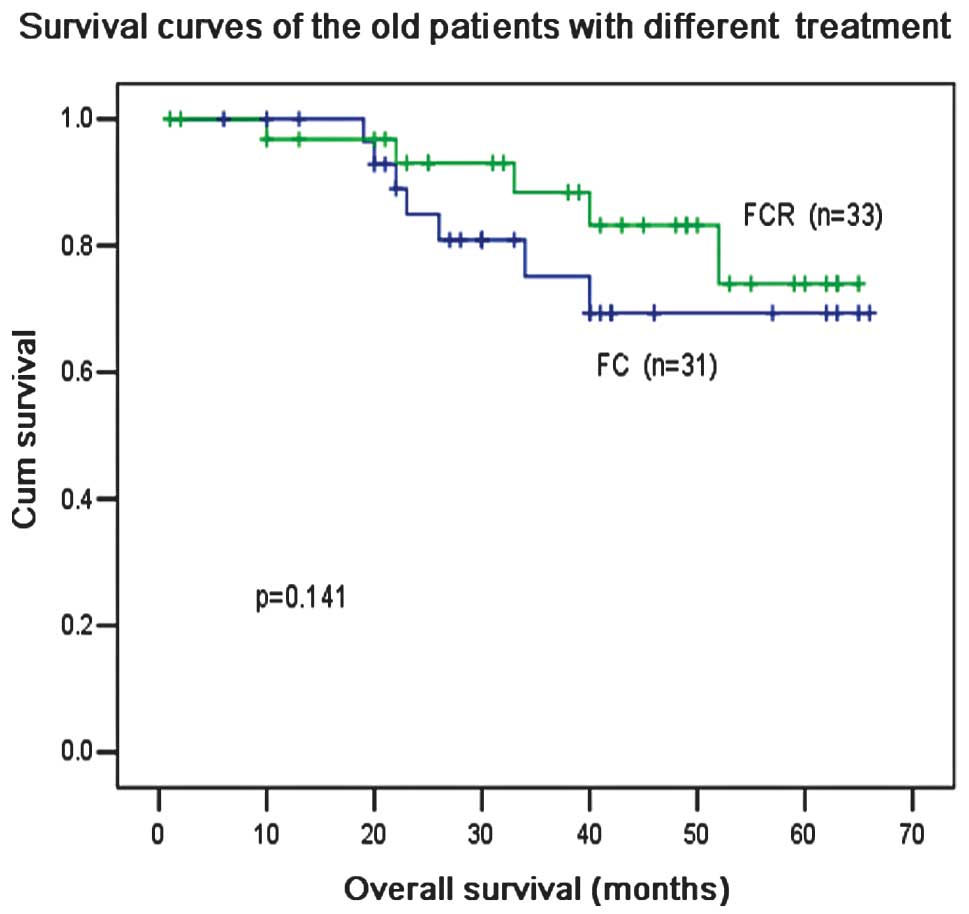
In patients ≤60 years old, R-based treatment
significantly improved the OS compared with treatment without R
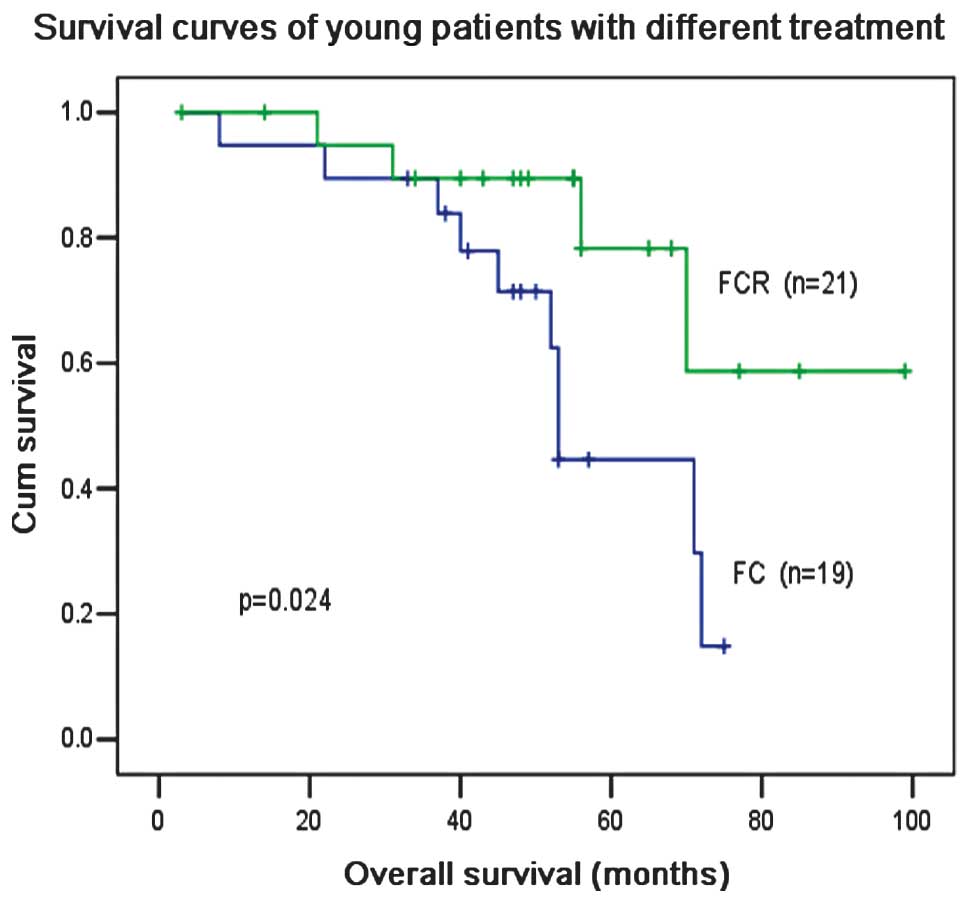
(P=0.012; Fig. 6). R treatment also
increased the OS compared with F and C treatment (P=0.024; Fig. 7), although F and C treatment
produced a higher (not significant) ORR than F, C and R treatment
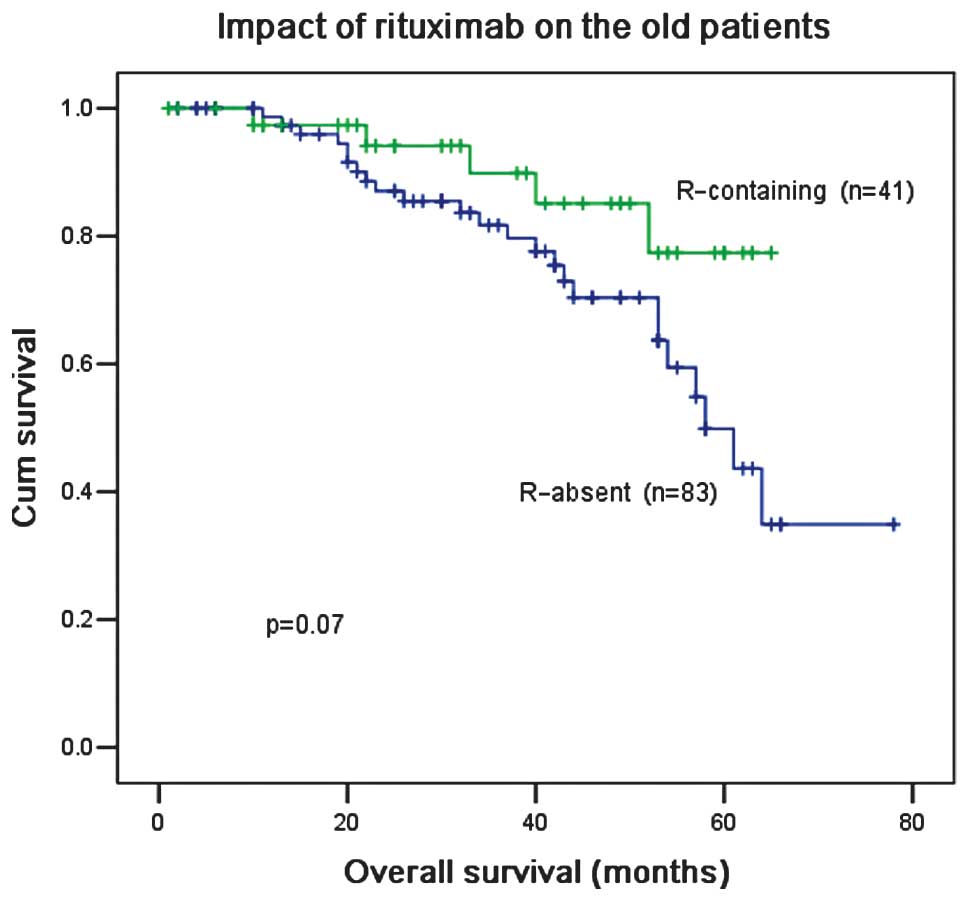
for patients ≤60 years old (87.5 vs. 83.9%; P=0.24; Table II). For patients >60 years old,
the ORR was slightly higher with F and C treatment compared with F,
C and R treatment, however, there was no difference in OS
irrespective of the administration of R (Figs. 8 and 9). In multivariate analysis,
17p− and treatments without R were independent
prognostic factors for a worse OS in younger patients, with hazard
ratios (HRs) of 3.23 (95% CI, 0.327–31.98) and 4.9 (95% CI,
0.79–31.3), respectively. Binet stage C and elevated β2-MG levels
were independent prognostic factors for a worse OS in older
patients, with a HR of 2.8 (95% CI, 1.4–10.2) and 1.14 (95% CI,
0.56–3.41), respectively (Table
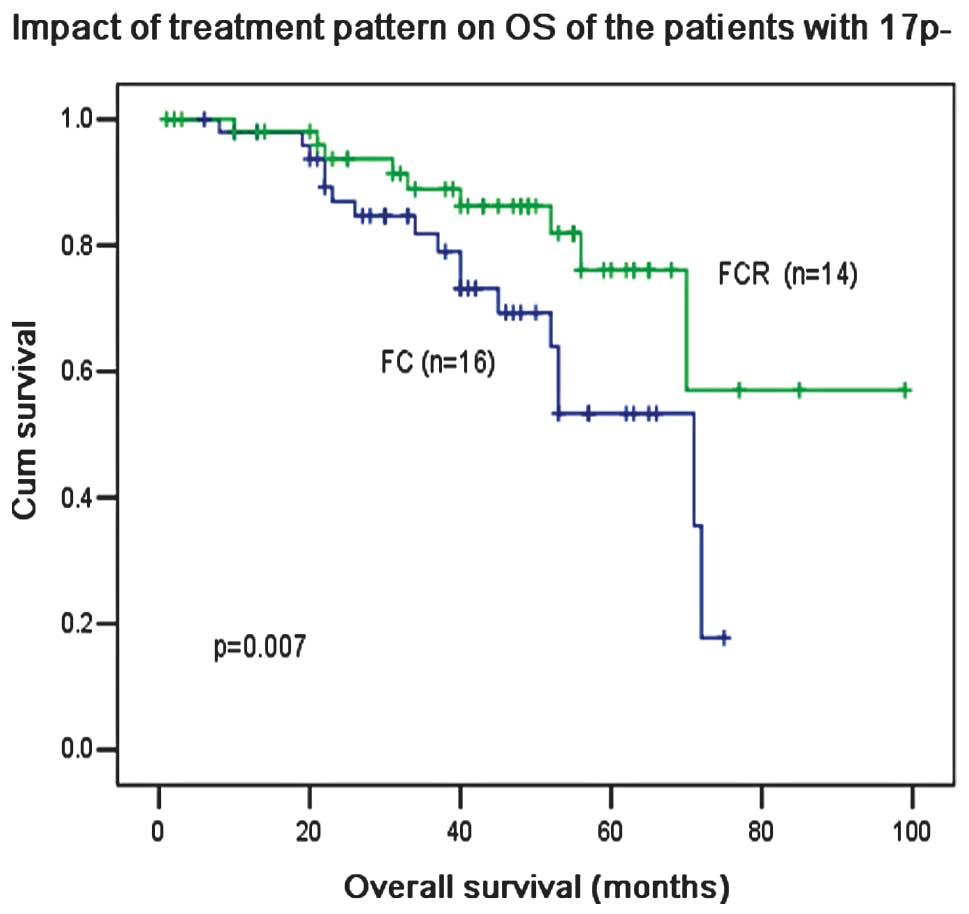
III). Notably, R-based treatment was able to overcome the poor
prognosis associated with 17p− and significantly
increased the 5-year OS from 52 to 74% (P=0.007, Fig. 10).
 | Table IIORR of the patients with different
treatments. |
Table II
ORR of the patients with different
treatments.
| Treatment | ≤60 years | >60 years |
|---|
|
|
|---|
| n | ORR (%) | P-valuea | n | ORR (%) | P-valuea |
|---|
| FC | 19 | 87.5 | 0.24 | 31 | 76.6 | 0.28 |
| FCR | 21 | 83.9 | | 33 | 73.2 | |
 | Table IIIComparison of the clinical
characteristics of CLL patients and their outcome association (OS)
according to age. |
Table III
Comparison of the clinical
characteristics of CLL patients and their outcome association (OS)
according to age.
| Clinical
characteristic | >60 years
group | ≤60 years
group |
|---|
|
|
|---|
| 5-year OS | HR (95% CI)a | P-valueb | 5-year OS | HR (95% CI)a | P-valueb |
|---|
| Overall | 0.506 | | | 0.59 | | |
| Binet Stage | | | | | | |
| A+B | 0.589 | 0.356
(0.098–0.667) | 0.003 | 0.659 | 0.616
(0.196–1.93) | 0.073 |
| C | 0.166 | 1 | | 0.543 | 1 | |
| LDH | | | | | | |
| Elevated | 0.5 | 10.97
(1.62–74.2) | 0.114 | 0.489 | 3.19
(0.6–17.4) | 0.064 |
| Normal | 0.591 | 1 | | 0.651 | 1 | |
| Serum β2-MG | | | | | | |
| Elevated | 0.476 | 1.14
(0.56–3.41) | 0.012 | 0.44 | 1.685
(0.123–3.81) | 0.051 |
| Normal | 0.57 | 1 | | 0.601 | 1 | |
| CD38 | | | | | | |
| Positive | 0.501 | 2.51
(0.89–7.47) | 0.438 | 0.52 | 1.477
(0.053–4.31) | 0.51 |
| Negative | 0.574 | 1 | | 0.622 | 1 | |
| ZAP-70 | | | | | | |
| Positive | 0.502 | 7.619
(1.018–57.04) | 0.148 | 0.57 | 2.95
(0.04–7.61) | 0.157 |
| Negative | 0.553 | 1 | | 0.631 | 1 | |
| IgVH mutation | | | | | | |
| Yes | 0.641 | 0.128
(0.029–0.57) | 0.067 | 0.676 | 0.244
(0.032–1.87) | 0.062 |
| No | 0.44 | 1 | | 0.512 | 1 | |
| FISH | | | | | | |
|
17p− | 0 (NA) | 2.34
(0.644–22.06) | 0.06 | 0 (NA) | 3.23
(0.327–31.98) | 0.001 |
|
11q− | 0.201 | 1.75
(0.53–7.88) | 0.251 | 0.392 | 1.84
(0.49–7.61) | 0.525 |
| Others | 0.59 | 1 | | 0.642 | 1 | |
| Treatment | | | | | | |
| F-containing | 0.591 | 0.02
(0.002–0.189) | 0.091 | 0.622 | 0.274
(0.053–1.41) | 0.123 |
| R-containing | 0.701 | 0.251
(0.038–1.66) | 0.052 | 0.739 | 0.203
(0.032–1.266) | 0.020 |
| Others | 0.501 | 1 | | 0.494 | 1 | |
Discussion
CLL is a mature B-cell neoplasm characterized by the
expansion of CD5+ small- to medium-sized lymphocytes in
the peripheral blood and is accompanied by other common disorders,
such as lymphadenopathy, splenomegaly and hepatomegaly (9). Although CLL mainly demonstrates
indolent behavior, a number of patients experience an aggressive
course and succumb to the disease within a few years of
diagnosis.
Combination therapies, such as F and C, have been
developed and chemoimmunotherapy (CIT), which combines purine
nucleoside analogs with or without alkylating agents and anti-CD20
monoclonal antibodies, have been used in previous years. Several
studies have confirmed that the anti-CD20 monoclonal antibody R
sensitizes CLL cells to the apoptotic effects of F (3–5). In
the present study, CIT increased the ORR in patients and also
caused a longer OS than cytotoxic chemotherapy alone. Similar or
higher CR rates have been reported with combinations of F with C
(2,10) or R (11) or F with C and R (3,4). The
OS rates of patients 10 years after R and F-based CIT was
equivalent to those demonstrated in CLL patients from Western
countries (12,13).
Rituximab is a chimeric monoclonal antibody directed
against the CD20 antigen and has become the standard drug used with
chemotherapy for treating various B-cell lymphomas. In CLL, a low
expression of the CD20 antigen on leukemic cells and poor response
rates to a standard dose of R led to the initial expectation that R
may not generate sufficient clinical benefits in this disease
(14). However, another study
demonstrated that higher doses of R used alone improved response
rates (15). In this study, we
observed a high percentage of CD20+ tumors (188 in 202
cases) and high expression levels of CD20 on CLL cells, including
61/188 that expressed CD20(+) and 127/188 that expressed CD20(++).
The expression of CD20 was similar to other B-cell neoplasms. The
majority of patients in this cohort did not receive a
dose-escalation of R as this expensive antibody is not covered by
health insurance in China. However, the results in Fig. 3 show that a routine dose of R in
combination with chemotherapy achieved high response rates and a
long OS in patients with CLL and may also have additive or
synergistic effects in patients with 17p−. There is a
difference between Western and Chinese patients with CLL. These
results suggest that the patients with 17p− may benefit
from R-based CIT, which is consistent with results from a previous
study (16).
The addition of R to F-containing regimens
significantly improves the OS in younger patients, confirming the
importance of this agent in current first-line CLL regimens for
treating younger patients. This study showed that after R-based
CIT, younger patients gained a greater benefit for 5-year OS (59%)
than older patients (50.6%). According to SEER data, the 5-year
survival rate of patients <55 years old in the United States is
88% (17). However, when compared
with their age-matched control population, the life expectancy of
younger patients with CLL is significantly reduced. While a younger
age alone should not be considered as a reason to initiate therapy,
the therapeutic goal for a young patient requiring therapy should
focus on improving survival by achieving CR. The importance of
achieving CR is emphasized by the finding that a better quality of
remission is associated with longer survival times and notably,
there were 39 patients who received R-based CIT that experienced
grade 3 and 4 infections, compared with only eight patients who had
received other treatments (including Ch) experiencing such
infections. This difference may be explained by different
etiologies. Infections which occur during Ch treatment may be
correlated with transient neutropenia, whereas R and F treatments
may be associated with prolonged B- or T-cell depletion (18).
Several clinical and pathological factors, including
age, Binet stage and β2-MG and LDH levels, have been associated
with prognosis. Biologically, an unfavorable course may be
predicted by a variety of parameters, including the IgVH mutation
status (19), ZAP-70 expression
(20), genomic aberrations
identified by FISH and serological proliferation markers. In
univariate analysis, age >60 years, 17p− and elevated
β2-MG levels were associated with a worse OS in this study. Serum
β2-MG levels have shown a strong prognostic effect in previous
studies (13) and we confirmed this
in the older patients of this study. The patients with normal β2-MG
levels have a higher OS at five years compared with patients who
had higher β2-MG levels. The presence of 17p− was shown
to be a strong negative prognostic indicator of progression-free
and overall survival (3). Patients
with this deletion had a significantly shorter OS in our study,
irrespective of the treatment administered (Table I). Therefore, assessment of β2-MG
levels and cytogenetic changes before treatment may be assayed to
provide data for predicting the outcome of a patient. The
combination of age, 17p− and β2-MG levels may be useful
to classify these patients.
Our study had limitations, as it was a retrospective
single center study and had a small sample size. Hence, there was a
chance of selection bias. However, we used standard statistical
techniques to perform comparisons and carried out univariate and
multivariate analysis in a non-selected population that received a
uniform diagnostic and therapeutic approach.
In conclusion, younger patients with CLL benefited
from R treatment, implying that the age of a patient is important
in their response to therapy and survival.
Acknowledgements
This study was supported in part by a
Grant of New Century Talent in Fujian (No. JA10128) to Z.S.X.
References
|
1
|
Keating MJ: Chronic lymphocytic leukemia.
Semin Oncol. 26:107–114. 1999.
|
|
2
|
Catovsky D, Richards S, Matutes E, et al
UK National Cancer Research Institute (NCRI); Haematological
Oncology Clinical Studies Group; NCRI Chronic Lymphocytic Leukaemia
Working Group: Assessment of fludarabine plus cyclophosphamide for
patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a
randomised controlled trial. Lancet. 370:230–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hallek M, Fischer K, Fingerle-Rowson G, et
al International Group of Investigators; German Chronic Lymphocytic
Leukaemia Study Group: Addition of rituximab to fludarabine and
cyclophosphamide in patients with chronic lymphocytic leukaemia: a
randomised, open-label, phase 3 trial. Lancet. 376:1164–1174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tam CS, O’Brien S, Wierda W, et al:
Long-term results of the fludarabine, cyclophosphamide, and
rituximab regimen as initial therapy of chronic lymphocytic
leukemia. Blood. 112:975–980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bosch F, Abrisqueta P, Villamor N, et al:
Rituximab, fludarabine, cyclophosphamide, and mitoxantrone: a new,
highly active chemoimmunotherapy regimen for chronic lymphocytic
leukemia. J Clin Oncol. 27:4578–4584. 2009. View Article : Google Scholar
|
|
6
|
Duke VM, Gandini D, Sherrington PD, et al:
V(H) gene usage differs in germline and mutated B-cell chronic
lymphocytic leukemia. Haematologica. 88:1259–1271. 2003.PubMed/NCBI
|
|
7
|
Del Poeta G, Maurillo L, Venditti A, et
al: Clinical significance of CD38 expression in chronic lymphocytic
leukemia. Blood. 98:2633–2639. 2001.PubMed/NCBI
|
|
8
|
Del Principe MI, Del Poeta G, Buccisano F,
et al: Clinical significance of ZAP-70 protein expression in B-cell
chronic lymphocytic leukemia. Blood. 108:853–861. 2006.
|
|
9
|
Hallek M, Cheson BD, Catovsky D, et al
International Workshop on Chronic Lymphocytic Leukemia: Guidelines
for the diagnosis and treatment of chronic lymphocytic leukemia: a
report from the International Workshop on Chronic Lymphocytic
Leukemia updating the National Cancer Institute-Working Group 1996
guidelines. Blood. 111:5446–5456. 2008. View Article : Google Scholar
|
|
10
|
Leporrier M, Chevret S, Cazin B, et al
French Cooperative Group on Chronic Lymphocytic Leukemia:
Randomized comparison of fludarabine, CAP, and ChOP in 938
previously untreated stage B and C chronic lymphocytic leukemia
patients. Blood. 98:2319–2325. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Byrd JC, Peterson BL, Morrison VA, et al:
Randomized phase 2 study of fludarabine with concurrent versus
sequential treatment with rituximab in symptomatic, untreated
patients with B-cell chronic lymphocytic leukemia: results from
Cancer and Leukemia Group B9712 (CALGB 9712). Blood. 101:6–14.
2003. View Article : Google Scholar
|
|
12
|
Guarini A, Gaidano G, Mauro FR, et al:
Chronic lymphocytic leukemia patients with highly stable and
indolent disease show distinctive phenotypic and genotypic
features. Blood. 102:1035–1041. 2003. View Article : Google Scholar
|
|
13
|
Wierda WG, O’Brien S, Wang X, et al:
Prognostic nomogram and index for overall survival in previously
untreated patients with chronic lymphocytic leukemia. Blood.
109:4679–4685. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huhn D, von Schilling C, Wilhelm M, et al:
Rituximab therapy of patients with B-cell chronic lymphocytic
leukemia. Blood. 98:1326–1331. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O’Brien SM, Kantarjian H, Thomas DA, et
al: Rituximab dose-escalation trial in chronic lymphocytic
leukemia. J Clin Oncol. 19:2165–2170. 2001.PubMed/NCBI
|
|
16
|
Schlette EJ, Admirand J, Wierda W, et al:
p53 expression by immunohistochemistry is an important determinant
of survival in patients with chronic lymphocytic leukemia receiving
frontline chemo-immunotherapy. Leuk Lymphoma. 50:1597–1605. 2009.
View Article : Google Scholar
|
|
17
|
Altekruse SF, Kosary CL, Krapcho M, et al
National Cancer Institute: SEER Cancer Statistics Review,
1975–2007. http://seer.cancer.gov/csr/1975_2007.
Accessed July 1, 2011.
|
|
18
|
Cheson BD: Infectious and
immunosuppressive complications of purine analog therapy. J Clin
Oncol. 13:2431–2448. 1995.PubMed/NCBI
|
|
19
|
Hamblin TJ, Orchard JA, Gardiner A, et al:
Immunoglobulin V genes and CD38 expression in CLL. Blood.
95:2455–2457. 2000.PubMed/NCBI
|
|
20
|
Rassenti LZ, Jain S, Keating MJ, et al:
Relative value of ZAP-70, CD38, and immunoglobulin mutation status
in predicting aggressive disease in chronic lymphocytic leukemia.
Blood. 112:1923–1930. 2008. View Article : Google Scholar : PubMed/NCBI
|