Introduction
Human papillomavirus (HPV) infection has been
globally reported as being involved in tumors in several types of
cancer, including genital mutilation cancer, penis cancer, lung
cancer, head and neck tumors, gastric cancer, breast cancer, colon
cancer, skin cancer and esophageal cancer. Numerous studies have
shown that HPV infection is closely related to squamous cell
carcinoma (SCC) and it has been determined as an important factor
in the induction of cervical SCC. Preliminary studies have been
carried out to determine whether HPV exists in SCCs in non-cervical
sites (1). Studies have been
carried out in various geographical locations, therefore, sample
volumes, means of detection, virus types and the distribution of
HPV subtypes are often detected in SCCs from different sites. Few
studies have characterized the distribution of the specific
subtypes of HPV in the varying grades of SCCs from different
sites.
This study aimed to explore the correlation between
the different subtypes of HPV and the different sites of non-
cervical SCCs in 511 patients from Qingdao, China, using a
polymerase chain reaction (PCR) detection method. The high
prevalence of HPV6/16 and the lack of HPV18 in esophageal SCC and
lung SCC may point to specific virus-tissue interactions.
Materials and methods
Specimen selection
Surgical resection specimens (n=511) were retrieved
from Qingdao Hiser Medical Group and Qingdao Center Hospital
between 2006 and 2011. All patients belonged to low socioeconomic
strata and the majority of these were agricultural workers. None of
the cases had been treated with radio- or chemotherapy prior to
surgery. The specimens consisted of 27 tongue SCC, 79
nasopharyngeal SCC, 196 lung SCC, 185 esophageal SCC and 24 rectal
SCC cases. The specimens for the control group, which were cut from
the margins of the tissues in the same cases, were confirmed as
non-tumor tissue by pathology. There were 397 specimens from males,
while 114 were from females. According to Broders’ classification,
the histopathological staging of all SCC cases were as follows: 238
stage I, 234 stage II, 30 stage III and 9 stage IV cases. Their
ages ranged from 26–81 years (mean, 63 years). None of the cases
had distant metastasis. All specimens underwent a regular dewaxing
process and were cut continuously into 4-μm-thick sections for HPV
testing. The histology from all cases was reviewed by two
pathologists who confirmed the diagnosis. Discrepant cases were
resolved by histological evaluation by a third pathologist.
HPV testing
HPV genotyping test kit
DNA was extracted from each of the 511
paraffin-embedded specimens using the HPV genotyping test kit
according to the manufacturer’s instructions (Asian Research Centre
of Molecular Diagnostic, Co., Ltd., Shenzhen, China). The kit
applied DNA-chip technology based on in vitro amplification
combined with PCR reverse dot blotting.
The kit used special primers to obtain 23 types of
HPV amplification products by PCR. It was hybridized with probes,
which had 5 low-risk genotypes and 17 high-risk genotypes fixed in
the membrane. It determined the HPV genotype by its hybridization
signal.
HPV DNA extraction
Exfoliated SCC cells were collected and added to 50
μl lysate and centrifuged at 13,000 rpm for 10 min after a boiling
water bath for 10 min. The supernatant was reserved for template
DNA.
PCR amplification
Template DNA (5 μl) was added to the PCR mix. PCR
conditions were as follows: 50°C for 15 min, denaturation at 95°C
for 10 min followed by 40 cycles of 94°C for 30 sec, 42°C for 1 min
and 30 sec, 72°C for 30 sec and 72°C for 6 min.
Hybridization
The PCR product and membrane were incubated for ≥1 h
and 30 min in a hybridization incubator and then kept in a boiling
water bath for 10 min in 5 ml solution A. The DNA became purified
after this step.
Membrane wash
The membranes were washed in a hybridization
incubator for 5 min in solution B at 51°C to remove proteins and
other contaminants.
Color agent
After washing the membrane, it was incubated for 30
min in solution A (2X SSC, 0.1% SDS) mixed with POD (Solution A:POD
= 2000:1). It was then colored for at least 30 min in color liquid,
which was added to 19 ml solution C (1M 100 ml sodium citrate), 1
ml TMB and 10 μl 3% H2O2. The color liquid
was removed and deionized water was added. The HPV genotype was
determined by its hybridization signal.
The film articles were placed on the reading
instrument scanner and the results were saved. The wet film article
was placed into an airtight seal in a hermetic sealing bag and
stored at 2–8°C in a refrigerator for preservation.
Statistical analysis
Statistical analysis was performed using Fisher’s
exact test and Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
HPV infection in various sites of
SCC
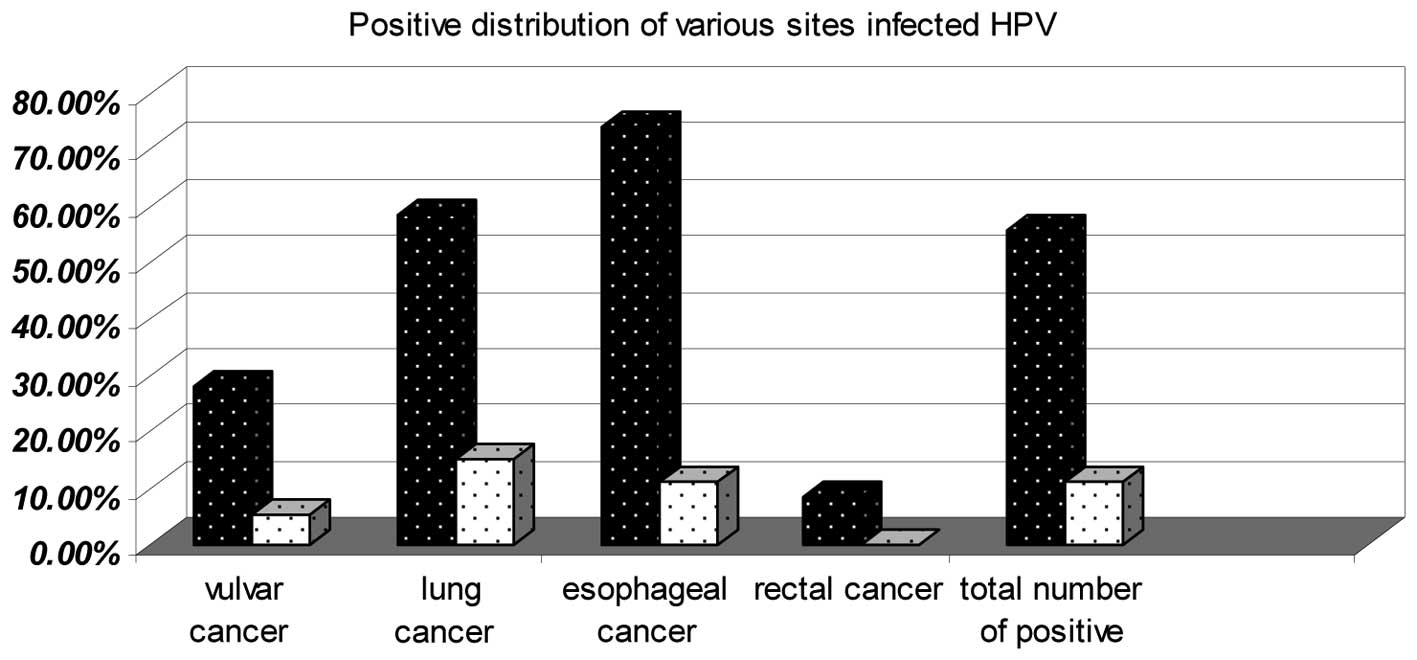
Overall, HPV was detected in 285 of 511 (55.77%) SCC
tissues compared with 55 of the 511 (10.76%) normal tissues. The
HPV-positive distribution for the tissue from each SSC site was
higher than for the normal tissue at its corresponding site. The
HPV-positive distribution in 137 of 185 (74.05%) esophageal SCCs
and 114 of 196 (58.16%) lung SCCs was higher than for the other SCC
sites (Fig. 1).
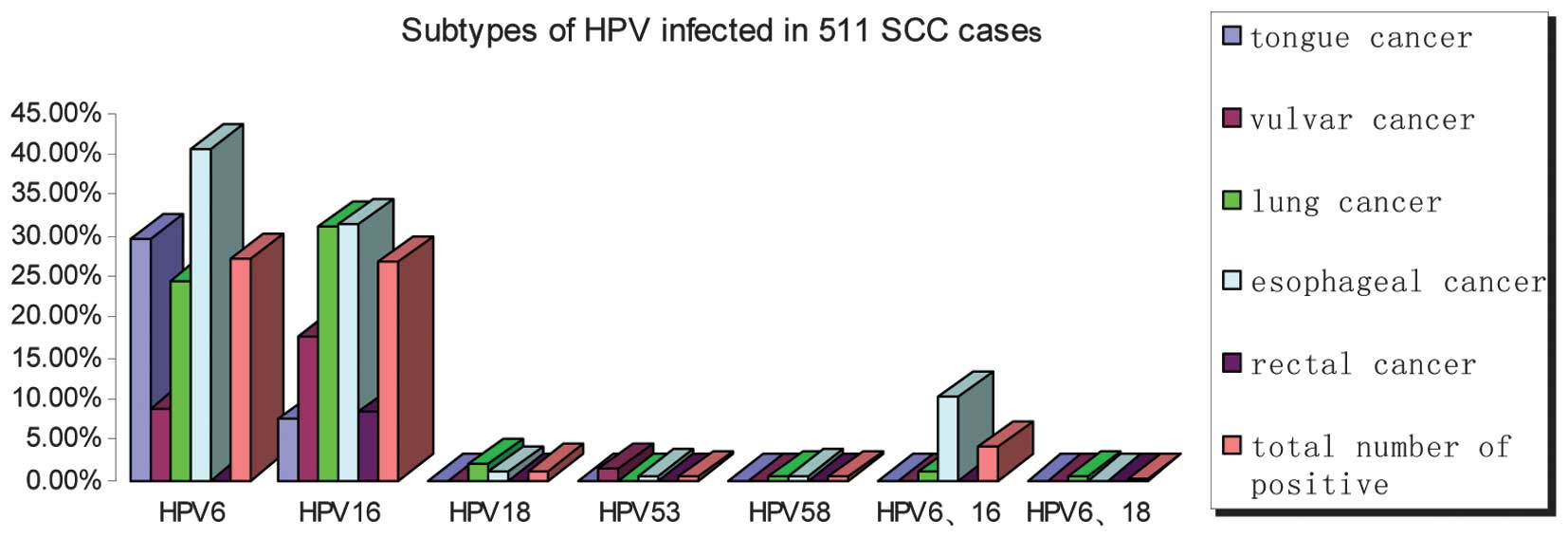
Distribution of HPV subtypes in various
sites of SCC
Five HPV genotypes were identified, including HPV6,
16, 18, 53 and 58. High-risk HPV was composed of HPV16, 18, 53 and
58, whereas low-risk HPV was HPV6 only. Overall, 147 of 285
(51.58%) cases were positive for high-risk HPV, of which 137
(48.07%) were associated with HPV16, 6 (2.11%) with HPV18, 2
(0.70%) with HPV53 and 2 (0.70%) with HPV58. The number of cases
with low-risk HPV positivity (138/285, 48.42%) was higher compared
with other HPV types and the majority of cases had esophageal
cancer. HPV6 and HPV16 comprised a large proportion of the
HPV-infected cases (Fig. 2).
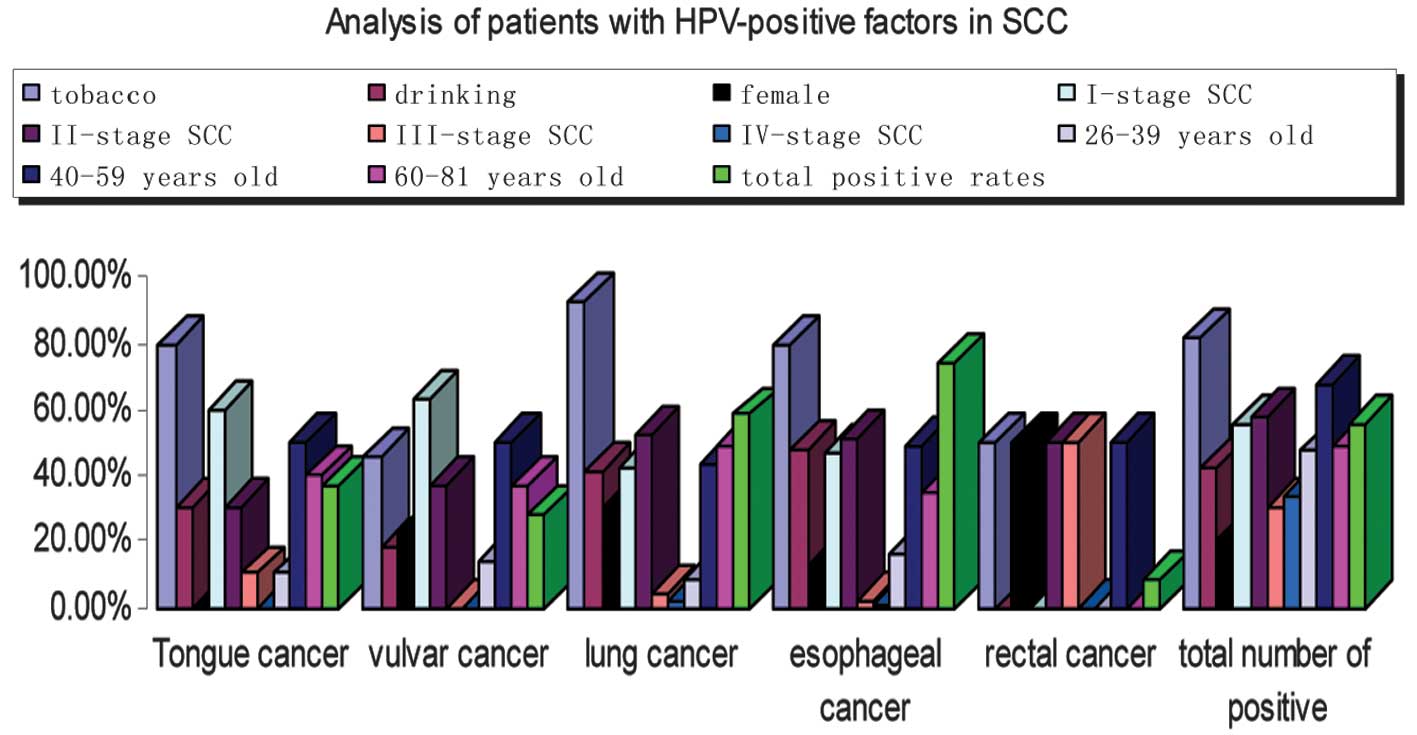
Patients with HPV-positive factors in
SCC
Follow-up information was available for 285
HPV-positive patients who were diagnosed with SCC stages I-IV
(Fig. 3), including 129 (45.26%)
with stage I, 92 (32.28%) with stage II, 53 (18.60%) with stage III
and 11 (3.86%) with stage IV. Tobacco (82.11%), drinking (42.11%)
and being middle-aged (67.17%) were important factors, however
tobacco was the most important (Fig.
3).
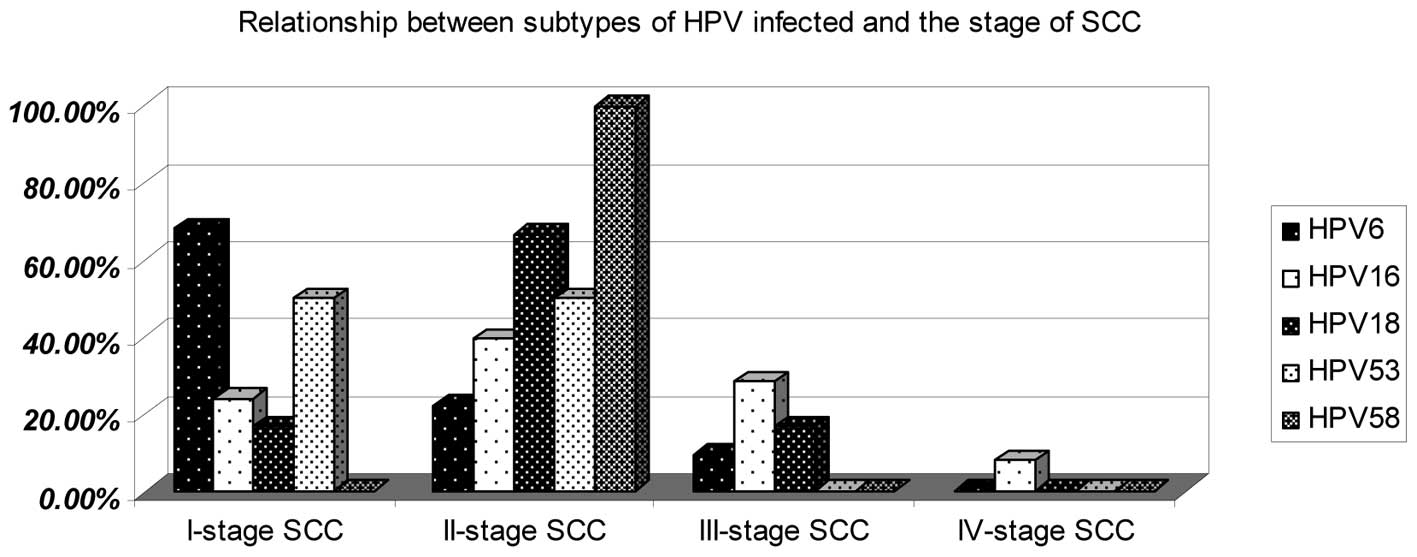
Association between subtypes of HPV
infection and the stage of SCC
There were 137 HPV16-infected cases, of which 33
(24.09%) were in stage I, 54 (39.42%) in stage II, 39 (28.47%) in
stage III and 11 (8.03%) in stage IV. For HPV6-infected cases,
there were 94 (68.12%) in stage I, 31 (22.46%) in stage II, 13
(9.42%) in stage III and 0 (0%) in stage IV. There was 1 (16.67%)
case in stage I, 4 (66.67%) in stage II and 1 (16.67%) in stage III
for HPV18-infected cases. There were only two cases infected by
HPV53, 1 of which was in stage I and the second was in stage II.
Only two cases were infected by HPV58 and these were in stage II
(Fig. 4).
Discussion
Cancer incidence in the Qingdao area has increased
rapidly, particularly tongue, nasopharyngeal, lung, esophageal and
colorectal cancer. These five types cancers are in the top ten most
commonly diagnosed tumors. Of the top ten, squamous cell carcinomas
are the most commonly observed types of tumor. Studies
investigating the correlation between non-cervical SCC and
different subtypes of HPV infection remain limited, despite their
increasing incidence in the last 20 years. Studies have reported
that HPV-positive tumors have a better response to therapy
(2). Numerous studies indicate that
a possible use of HPV diagnosis is as an additional aid for
survival prognosis, at least for localizations such as tonsils or
for females rather than males, where an improved clinical outcome
has been demonstrated (3–5). However, there have been no further
studies with regard to the specific type of positive HPV which is
useful for determining the prognosis of SCC. Whether the high- or
low-risk HPV type is better for accurate prognosis remains unclear.
HPV vaccines have been used in numerous areas as one of the
effective measures to prevent cervical SCC. Whether it is possible
to administer them as a conventional measure to prevent SCC in
other sites is unknown. A previous study showed that the subtypes
of HPV infection presented with a regional distribution (6). The main factors which affect the
correlation between HPV infection and non-cervical SCC in Qingdao
and the correlation between these factors and the cases of SCC with
HPV infections have yet to be explored. Therefore, we conducted the
present study in Qingdao, China.
In the present study, we collected and analyzed 511
non-cervical SCC specimens for the presence of HPV infection using
PCR and non-isotopic in situ hybridization. In the
noncervical SCC samples, 19.30% (285/511) were positive for HPV
detection, while 10.76% (55/285) of the control samples were
positive. The presence of HPV infection was significant in
noncervical SCC cases (P<0.05). HPVs have been categorized by
their genotype into low- and high-risk types, according to the risk
of the virus causing SCC of the uterine cervix (7). In this study, we hypothesized that HPV
infection is closely correlated with the high- and low-risk types
of SCC. In 2007, the American Cancer Society announced the use of
the HPV vaccine to prevent cervical SCC in females (8). The quadrivalent vaccine for HPV types
6/11/16/18 was successful (9). This
study revealed a significant positive rate of HPV6 and HPV16 in
cervical SCC patients, which indicates that it may be possible to
administer the HPV vaccine for the prevention and treatment of
SCC.
HPV invades basal cells through small amounts of
epithelial damage. The virus particle binds with receptors on the
cell surface, enters into the cell and transfers to the nucleus,
where the released virus gene is replicated, resulting in
chromosomal changes of the host cell. The cell with DNA damage may
be the foundation for the induction of cancer (10). Studies have shown that high- and
low-risk types of HPV infection are present in a variety of the SCC
subtypes, including HPV16, 18 and 6 (11,12).
However, whether HPV infection was correlated with epithelial tumor
differentiation was not determined. The total positive rate of the
high-risk type was 51.58%, of which 48.07% of cases were
HPV16-infected. The positive rate of HPV16 infection was 100% in
stage IV SCC, 66.67% in stage III, 58.70% in stage II and 25.58% in
stage I. The total positive rate of the low-risk type was 48.42%
and this consisted of the HPV6 subtype only. In stage IV SCC, the
positive rate of HPV6 infection was 0.00%, while there were 24.53%
HPV6-positive cases in stage III, 33.70% in stage II and 72.87% in
stage I. The distribution in all stages of non-cervical SCC between
high- and low-risk types was clear; low-risk types were mainly
distributed in the higher grades of differentiation and high-risk
types were mainly distributed in the lower-level differentiated
SCCs. This is in agreement with numerous studies which show high
expression rate of high risk HPV infection in SCC patients
(6,13). Statistical analysis demonstrated
that the high-risk HPV subtype was the most important factor that
was proportional to the malignant degree of SCC.
Infection of the uterine cervix with any HPV
genotype is associated with high-risk sexual behavior, particularly
if started at a younger age. Persistent infection of the uterine
cervix with high-risk HPV genotypes, particularly HPV16 and HPV18,
is essential for the development of SCC (14). This study showed that tobacco
(234/285) was more important than three other factors which were
also associated with HPV infection in SCC; drinking, age and gender
(P<0.05). The positive rate of HPV infection among middle-aged
patients (133/198) was higher than for younger and older ages
(P<0.05).
With regard to incidence and prevalence, esophageal
cancer exhibits marked geographical variations due to unknown
factors between countries, in addition to between different regions
of the same country. According to the World Health Organization,
incidence rate spectra are located between Western Africa at the
low-risk end and China at the high-risk end, including the apparent
‘Asian esophageal cancer belt’ (15). The prevalence of HPV6
(75/137) and HPV16 (58/137) is high in esophageal SCC, while for
HPV18 (2/137) it is low. The prevalence of HPV6 (48/114) and HPV16
(61/114) is also high in lung SCC, while HPV18 (4/114) is also low.
The two subtypes of HPV infection in esophageal and lung SCC had
significant differences when compared with other sites of SCC
(P<0.05). The high prevalence of HPV6/16 and the lack of HPV18
in esophageal and lung SCC may point to specific virus-tissue
interactions.
This study demonstrated that the high-risk HPV
subtype was the most important factor associated with the malignant
degree of SCC. The study provided a theoretical basis for the
preventative treatment of non-cervical SCC using HPV vaccines.
Further study is required to determine the effect of HPV on
survival in patients when observed in combination with other
prognostic factors.
Acknowledgements
The authors would like to thank
Professor Guangdong Zhou and Doctor Zhiyong Xu for their assistance
in editing this manuscript. This study was supported in part by a
generous grant from the Health Research Fund of Qingdao City and
the Research Fund from Clinical Medicine Doctoral of Shandong
University, China.
References
|
1
|
Gao ZD and Pan Q: Research progress in
relevance of HPV infection and non-cervical squamous cell
carcinoma. Zhong Liu Fang Zhi Za Zhi. 18:1816–1820. 2011.(In
Chinese).
|
|
2
|
Dayyani F, Etzel CJ, Liu M, et al:
Meta-analysis of the impact of human papillomavirus (HPV) on cancer
risk and overall survival in head and neck squamous cell carcinomas
(HNSCC). Head Neck Oncol. 2:152010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Syrjänen KJ and Syrjänen SM:
Papillomavirus Infections in Human Pathology. Wiley & Sons; New
York, NY: pp. 1–10. 2000
|
|
4
|
Cox JT: Human papillomavirus testing in
primary cervical screening and abnormal Papanicolaou management.
Obstet Gynecol Surv. 61(Suppl 1): S15–S25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muñoz N, Bosch FX, Castellsagué X, et al:
Against which human papillomavirus types shall we vaccinate and
screen? The international perspective. Int J Cancer. 111:278–285.
2004.
|
|
6
|
Kreimer AR, Clifford GM, Boyle P and
Franceschi S: Human papillomavirus types in head and neck squamous
cell carcinomas worldwide: a systematic review. Cancer Epidemiol
Biomarkers Prev. 14:467–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muñoz N, Bosch FX, de Sanjosé S, et al:
Epidemiologic classification of human papillomavirus types
associated with cervical cancer. N Engl J Med. 348:518–527.
2003.
|
|
8
|
Saslow D, Castle PE, Cox JT, et al:
American Cancer Society Guideline for human papillomavirus (HPV)
vaccine use to prevent cervical cancer and its precursors. CA
Cancer J Clin. 57:7–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joura EA, Leodolter S, Hernandez-Avila M,
et al: Efficacy of a quadrivalent prophylactic human papillomavirus
(types 6, 11, 16, and 18) L1 virus-like-particle vaccine against
high-grade vulval and vaginal lesions: a combined analysis of three
randomised clinical trials. Lancet. 369:1693–1702. 2007. View Article : Google Scholar
|
|
10
|
zur Hausen H: Papillomaviruses and cancer:
from basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002.PubMed/NCBI
|
|
11
|
Boyd AS, Stasko TS and Tang YW: Basaloid
squamous cell carcinoma of the skin. J Am Acad Dermatol.
64:144–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cubilla AL, Lloveras B, Alejo M, et al:
The basaloid cell is the best tissue marker for human
papillomavirus in invasive penile squamous cell carcinoma: a study
of 202 cases from Paraguay. Am J Surg Pathol. 34:104–114. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong AK, Chan RC, Aggarwal N, et al: Human
papillomavirus genotypes in anal intraepithelial neoplasia and anal
carcinoma as detected in tissue biopsies. Mod Pathol. 23:144–150.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyashita M, Agdamag DM, Sasagawa T, et
al: High-risk HPV types in lesions of the uterine cervix of female
commercial sex workers in the Philippines. J Med Virol. 81:545–551.
2009. View Article : Google Scholar : PubMed/NCBI
|