Introduction
Epithelial-to-mesenchymal transition (EMT) is an
important event during tumorigenesis (1). The functional loss of E-cadherin
protein, a key protein maintaining epithelial cell-cell adhesion,
is a key marker in the EMT process (2). Several transcriptional repressors,
such as the zinc finger factors Snail, Slug, ZEB1 and ZEB2, the
basic helix-loop-helix (bHLH) factor E47 and Twist have been
identified as strong repressors of E-cadherin expression and have
been implicated in tumor progression (2).
Human high-mobility group A2 (HMGA2) is a
chromatin-binding protein which contains three AT-hook domains that
enable its binding to the minor groove of DNA. HMGA2 organizes
protein complexes on enhancers of various genes to regulate gene
expression and cell differentiation (3,4). HMGA2
protein is overexpressed in many types of cancer, such as lung
cancer (5), ovarian cancer
(6), breast cancer (7), oral squamous cell carcinoma (8), pancreatic cancer (9) and colorectal cancer (10). Previous studies have demonstrated
how HMGA2 promotes cancerogenesis at the molecular level. For
example, it is well-known that human telomerase reverse
transcriptase (hTERT) is essential for tumor cell proliferation and
self-renewal. HMGA2 increases hTERT transcription to promote
tumorigenesis (11). Certain micro
RNAs (miRNAs) are able to target HMGA2 gene, which inhibits
tumorigenesis through the downregulation of HMGA2 protein (12). The role of HMGA2 that controls EMT
has been well-described. Transforming growth factor-b (TGF-β)
induces the expression of HMGA2 by activating transcription factor
Smad. HMGA2 associates with Smad complexes to induce Snail and
Twist expression, two established regulators of EMT, which finally
leads to mesenchymal transition (13,14).
It is not known whether HMGA2 regulates EMT in human
hepatocellular carcinoma (HCC) cell lines; furthermore, the
mechanism(s) have not been fully elucidated.
Material and methods
Cell culture
PLC/PRF/5, Huh-7, HepG2, and Hep3B cells
(nonmetastatic or low metastatic potential human HCC cell lines)
used in this study were obtained from the American Type Culture
Collection (ATCC; Rockville, MD, USA) and MHCC97-H cells human HCC
cell lines with high metastatic potential were established at the
Liver Cancer Institute, Zhongshan Hospital, Fudan University,
Shanghai, China (15). All cells
were cultured in the corresponding medium supplemented with 10%
fetal bovine serum (FBS) and 1% penicillin-streptomycin and were
maintained in a 37°C incubator with 5% CO2.
The study was approved by the Ethics Committee of
Jun Xie Hospital, Nanjing, China.
Transfection
HepG2 cells were transiently transfected with
HA-tagged human HMGA2 using FuGENE HD (Roche Applied Science,
Mannheim, Germany). Transient transfections of MHCC97-H cells with
siRNA against human Hmga2 (ON-TARGETplus SMARTpool L-013495;
Dharmacon, Pittsburgh, PA, USA) or non-targeting siRNA control were
done using DharmaFECT1 siRNA transfection reagent (Dharmacon). The
effectiveness of gene overexpression or silencing was determined
using real-time PCR and western blot analysis.
Real-time PCR
Total-RNA was extracted using the RNeasy mini kit
(Qiagen, Valencia, CA, USA) and the concentration detected using a
biological spectrophotometer. Real-time PCR analysis was performed
according to the manufacturer’s instructions (Quant SYBR-Green PCR
kit, Tiangen Biotech, Beijing, China). Primers for mouse
glyceraldehyde-3′-phosphate dehydrogenase (Gapdh) were used
as a reference. The primers for Gapdh were
5′-TGTGTCCGTCGTGGATC TGA-3′ (sense) and 5′-CCTGCTTCACCACCTTCTTGA-3′
(antisense). The primers for Hmga2 were 5′-TCCCTCTAA
AGCAGCTCAAAA-3′ (sense) and 5′-ACTTGTTGTGGC CATTTCCT-3′
(antisense). Gene expression levels were determined with the
comparative Ct method using Gapdh as a reference. The
control condition was set to 1 or 100% and expression levels are
presented as bar graphs of means ± standard error of the mean
(SEM).
Western blot analysis
A total of 1×107 cells were collected.
The cells were lysed and the protein concentrations were measured
using a BCA Protein Assay Reagent kit (Pierce, Rockford, USA). A 20
μg aliquot of the protein was subjected to 10%
SDS-polyacrylamide gel electrophoresis (PAGE), and transferred to a
polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA,
USA). After being blocked by incubation overnight in PBST
containing 5% dry nonfat milk, the PVDF membrane was incubated with
the indicated primary antibodies (1:1,000 dilution) for 2 h and
incubated with a horseradish-peroxidase-conjugated secondary
antibody (1:100 dilution; Proteintech, Chicago, IL, USA) for 1 h.
Immunoreactive bands were visualized using an enhanced
chemiluminescence (ECL) detection system (Amersham, Arlington
Heights, IL, USA). β-actin was detected simultaneously as a loading
control (anti-β-actin, 1:1,000 dilution; Kangchen, Beijing,
China).
Invasion and migration assay
For the invasion assay, cell culture inserts (8
μm, 24-well format, Becton-Dickinson Labware, Franklin
Lakes, NJ, USA) were evenly coated with diluted Matrigel (1:5
dilution with blank medium). Cells (1×105) were added to
the upper chamber and the lower chamber was filled with 300
μl medium containing 10% FBS. The culture was maintained for
24 h. The cell migration assay was similar to the invasion assay,
except that inserts were not coated with Matrigel and the culture
was maintained for 24 h. Cells were fixed with 4% formaldehyde for
10 min and stained with 0.5% crystal violet for 10 min. The cells
on the upper side of the filters were removed with cotton-tipped
swabs. The cells on the underside of the filters were counted under
a ×20 objective lens in five randomly selected fields. The results
are presented as the fold change when compared with vector control
cells.
Statistical analysis
All experiments were performed in trip-licate.
Results are expressed the mean ± SEM. A two-tailed Student’s t-test
was performed to analyze the statistical significance of
differences between experimental groups using the SPSS 11.5
software statistical package (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
result.
Results
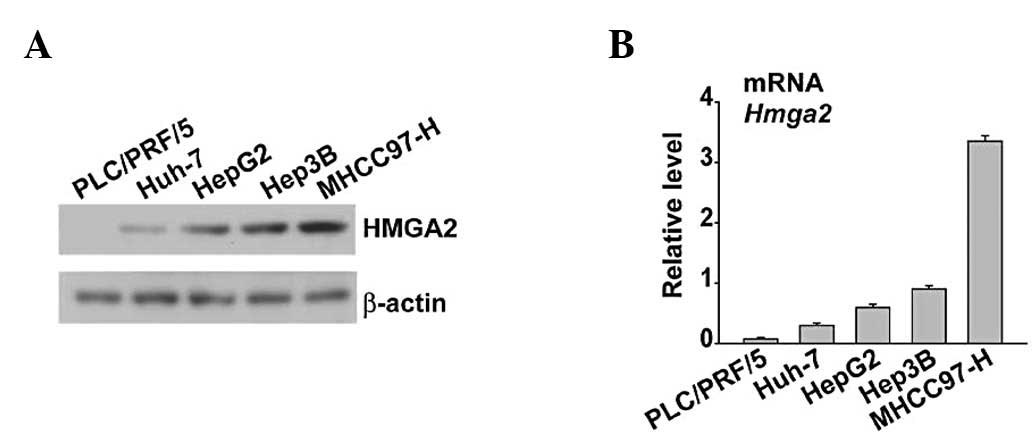
Expression of HMGA2 in five HCC cell
lines at the mRNA and protein level
To establish the correlation between HMGA2
expression and HCC cell lines, five HCC cell lines were evaluated
for HMGA2 mRNA expression using quantitative PCR (qPCR) and
expression at protein level using western blot analysis. HMGA2 was
highly expressed in MHCC97-H cells that were characterized as high
metastatic potential (Fig. 1A and
B). In contrast, the nonmetastatic or low metastatic potential
cells almost completely lacked HMGA2 or showed decreased expression
(Fig. 1A and B).
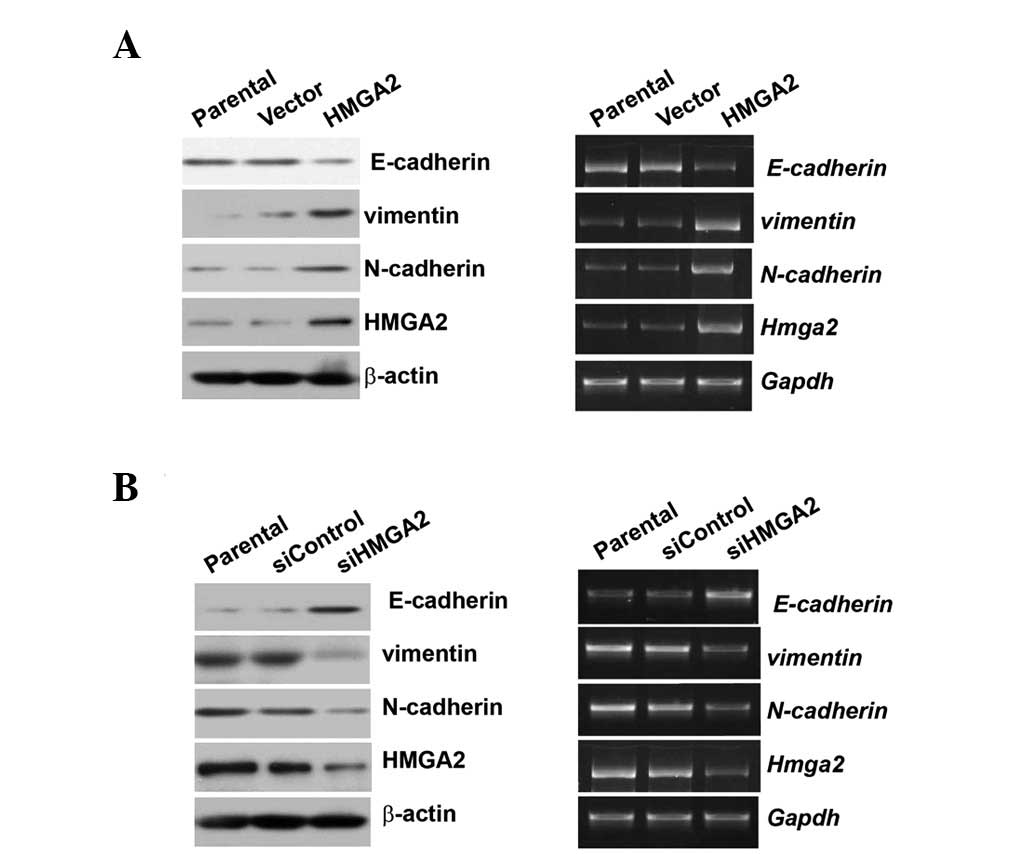
Overexpression of HMGA2 promotes, but
silencing inhibits EMT
To verify whether HMGA2 regulates EMT in HCC cell
lines, HA-HMGA2 was transiently transfected into low metastatic
HepG2 cells or the expression of HMGA2 was knocked down using siRNA
in MHCC97-H cells. HMGA2 overexpression induced downregulation of
the epithelial marker E-cadherin expression at the protein and mRNA
levels; furthermore, mesenchymal genes such as vimentin or
N-cadherin expression at the protein and mRNA levels were induced
by HMGA2 overexpression (Fig. 2A).
Whether HMGA2 knockdown also affects EMT marker variation was
examined. HMGA2 knockdown could increase E-cadherin expression and
decrease vimentin or N-cadherin expression at both the protein and
mRNA levels (Fig. 2B).
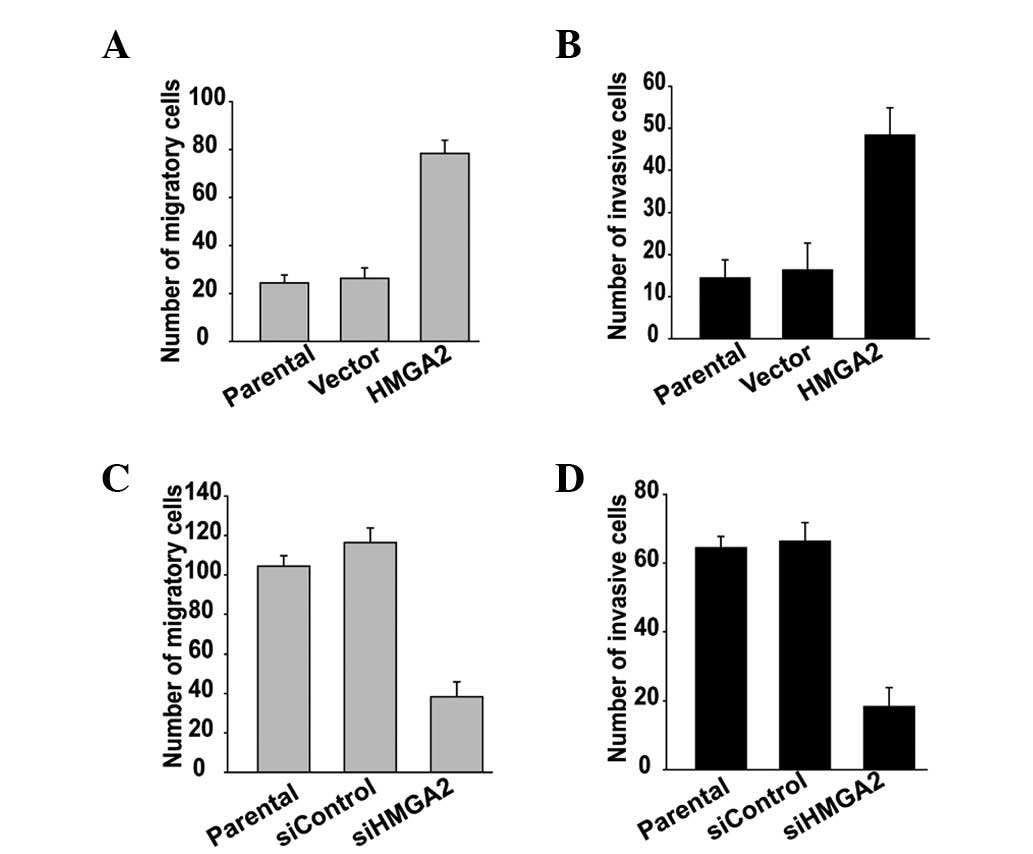
Ectopic expression of HMGA2 promotes, but
depletion blocks tumor cell migration and invasion
The regulatory effect of HMGA2 on migratory and
invasive ability was examined using transwell migration and
invasion assays. HMGA2 overexpression promotes the migration and
invasion of HepG2 cells (Fig. 3A and
B), whereas knockdown of HMGA2 blocked the migration and
invasion of MHCC97-H cells (Fig. 3C and
D). These data suggest that HMGA2 participates in the
regulation of HCC cell migration and invasion.
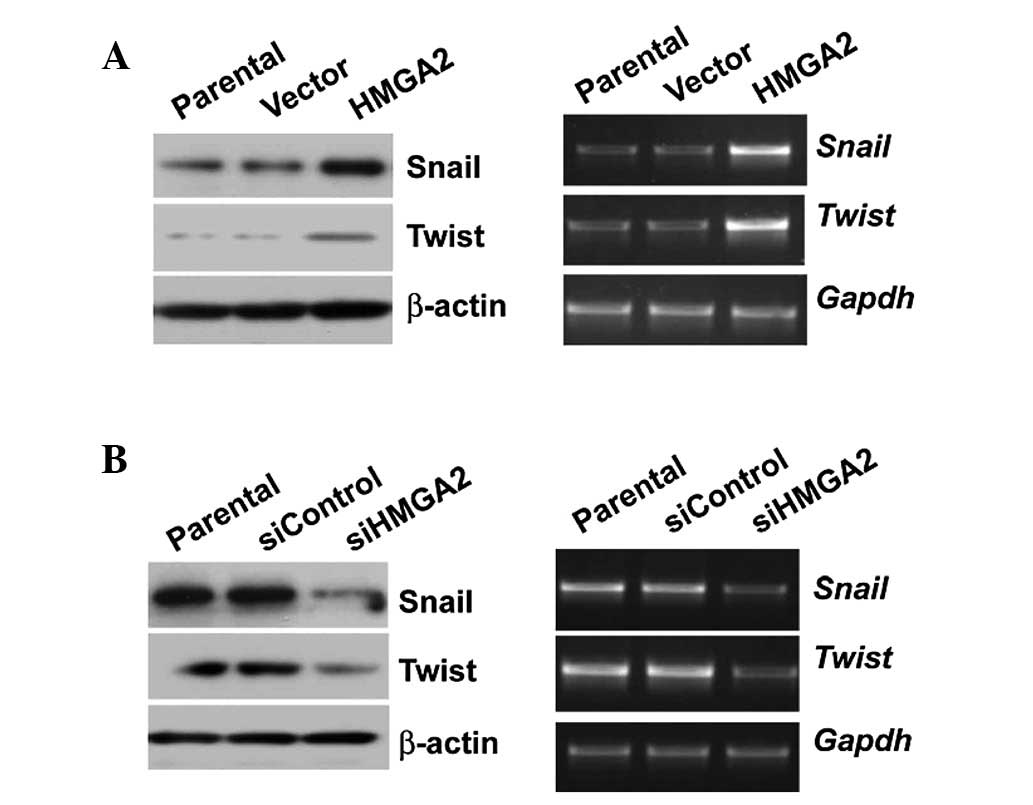
HMGA2 regulates the expression of Twist
and Snail at both mRNA and protein level
To explore how HMGA2 promotes migration and invasion
by inducing EMT at the molecular level, Twist and Snail, two strong
inducers of EMT, were used. Overexpression of HMGA2 significantly
upregulated Snail and Twist expression at both the mRNA and protein
level (Fig. 4A). In contrast, HMGA2
knockdown decreased the expression of the two molecules (Fig. 4B).
Discussion
Hmga2 is a member of the HMGA family that
encodes a chromatin-associated protein (3). In humans, the Hmga2 gene is
located at chromosome 12q14 and encodes a 109 amino acid protein.
In embryonic tissues, HMGA2 is essential for normal cardiac
development (16) and increases the
frequency and self-renewal of fetal and young-adult stem cells
(17), thereby regulating cell
growth and differentiation. Moreover, the role of HMGA2 that
participates in the process of tumorigenesis has been studied
extensively. The functional significance of HMGA2 in HCC cell
lines, however, is still not clear. Elucidating its functional
roles and molecular mechanisms involved in the tumorigenesis may,
therefore, be helpful for the early diagnosis and development of
malignancies.
In this study, the expression of HMGA2 in five HCC
cell lines was examined at the mRNA level using real-time PCR and
at the protein level by western blot analysis. The level of HMGA2
expression among the five HCC cell lines coincided with their
invasiveness, which was consistent with a previous observation that
increased expression of HMGA2 correlated with increased
invasiveness in tumor tissues (18). Many successive steps are required
for a growing benign hyperplasia to evolve into a fully malignant
and metastatic cancer (1). EMT is a
critical event that enables cancer cells to invade the local
tissue, acquire competence for intravasation and generate progeny
with tumor-initiating capacities (2). During EMT, differentiated epithelial
cells lose their cell-cell adhesions, become more motile and
exhibit mesenchymal features. For example, loss of E-cadherin
expression, a key molecule of the adherens junction and a tumor
suppressor gene (CDH1) and induction of vimentin-based intermediate
filaments are two of the many established hallmarks of the EMT
process (3). To demonstrate the
role of HMGA2 in EMT, the expression of HMGA2 was silenced using
siRNA in MHCC97-H cells with highly invasive potential and high
HMGA2 expression. Concurrently, we overexpressed HMGA2 in HepG2
cells with low invasion and HMAG2 low expression. The variation of
HMGA2 expression was found to correlate significantly with the
expression of several putative EMT markers. In addition, assessment
of the invasive potential following transfection with HMGA2-siRNA
demonstrated that the rate of cell migration was significantly
reduced compared with that in siControl and mock control samples,
suggesting that HMGA2 may be an important contributor to the
invasion of tumor cells, and that the expression level of HMGA2
influences the metastatic behavior of HCC cells.
The large number of cellular events that
characterize the mesenchymal transition are thought to be
collectively regulated by a group of transcription factors that
coordinate the transcriptional program of EMT. These
transcriptional regulators are the zinc finger factors Snail,
Snail2 (also known as Slug), ZEB1, ZEB2 and the basic
helix-loop-helix factors E47 and Twist1 (Twist) (4). Previously, Moustakas and his group
demonstrated that HMGA2 binds directly to the Twist or Snail
promoter to induce EMT using HepG2 cells. To further confirm the
conclusion and explore the molecular mechanism by which HMGA2
induces EMT, it was assumed that HMGA2 upregulates the expression
of Twist and Snail in HCC cell lines. Indeed, HMGA2 was found to
increase the expression of the two proteins at both the mRNA and
protein level. Since it was also demonstrated that HMGA2 regulates
the TGF-β signaling pathway, future research should be carried out
to elucidate whether HMGA2 has correlations with TGF-β in EMT in
HCC cell lines.
In conclusion, the present study is the first to
show that HMGA2 effectively regulates EMT to prompt invasion and
metastasis in HCC cells. The function of HMGA2 as an oncoprotein
may be associated with several important molecules involved in
invasion and metastasis of cancer cells. These results further
indicate that HMGA2 may serve as a potential target for the
development of therapies for HCC, although additional detailed
studies in vivo are required.
Acknowledgements
This study was supported in part by
grants from the Nanjing military medical scientific research
projects (10MA053) and Zhejiang Provincial Natural Science
Foundation of China (LY12H31003).
References
|
1
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qin Q, Xu Y, He T, Qin C and Xu J: Normal
and disease-related biological functions of Twist1 and underlying
molecular mechanisms. Cell Res. 22:90–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fusco A and Fedele M: Roles of HMGA
proteins in cancer. Nat Rev Cancer. 7:899–910. 2007. View Article : Google Scholar
|
|
4
|
Sgarra R, Zammitti S, Lo Sardo A, et al:
HMGA molecular network: from transcriptional regulation to
chromatin remodeling. Biochim Biophys Acta. 1799:37–47. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sarhadi VK, Wikman H, Salmenkivi K, et al:
Increased expression of high mobility group A proteins in lung
cancer. J Pathol. 209:206–212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shell S, Park SM, Radjabi AR, et al: Let-7
expression defines two differentiation stages of cancer. Proc Natl
Acad Sci USA. 104:11400–11405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Langelotz C, Schmid P, Jakob C, et al:
Expression of high-mobility-group-protein HMGI-C mRNA in the
peripheral blood is an independent poor prognostic indicator for
survival in metastatic breast cancer. Br J Cancer. 88:1406–1410.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyazawa J, Mitoro A, Kawashiri S, Chada
KK and Imai K: Expression of mesenchyme-specific gene HMGA2 in
squamous cell carcinomas of the oral cavity. Cancer Res.
64:2024–2029. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dangi-Garimella S, Krantz SB, Barron MR,
et al: Three-dimensional collagen I promotes gemcitabine resistance
in pancreatic cancer through MT1-MMP-mediated expression of HMGA2.
Cancer Res. 71:1019–1028. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Liu X, Li AY, et al:
Overexpression of HMGA2 promotes metastasis and impacts survival of
colorectal cancers. Clin Cancer Res. 17:2570–2580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li AY, Lin HH, Kuo CY, et al:
High-mobility group A2 protein modulates hTERT transcription to
promote tumorigenesis. Mol Cell Biol. 31:2605–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palmieri D, D’Angelo D, Valentino T, et
al: Downregulation of HMGA-targeting microRNAs has a critical role
in human pituitary tumorigenesis. Oncogene. 31:3857–3865. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan EJ, Thuault S, Caja L, Carletti T,
Heldin CH and Moustakas A: Regulation of transcription factor Twist
expression by the DNA architectural protein high mobility group A2
during epithelialto-mesenchymal transition. J Biol Chem.
287:7134–7145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thuault S, Tan EJ, Peinado H, Cano A,
Heldin CH and Moustakas A: HMGA2 and Smads co-regulate SNAIL1
expression during induction of epithelial-to-mesenchymal
transition. J Biol Chem. 283:33437–33446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Tang ZY, Ye SL, et al: Establishment
of cell clones with different metastatic potential from the
metastatic hepatocellular carcin oma cell line MHCC97. World J
Gastroenterol. 7:630–636. 2001.PubMed/NCBI
|
|
16
|
Monzen K, Ito Y, Naito AT, et al: A
crucial role of a high mobility group protein HMGA2 in
cardiogenesis. Nat Cell Biol. 10:567–574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishino J, Kim I, Chada K and Morrison SJ:
Hmga2 promotes neural stem cell self-renewal in young but not old
mice by reducing p16Ink4a and p19Arf expression. Cell. 135:227–239.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fabjani G, Tong D, Wolf A, et al: HMGA2 is
associated with invasiveness but not a suitable marker for the
detection of circulating tumor cells in breast cancer. Oncol Rep.
14:737–741. 2005.PubMed/NCBI
|