Introduction
Follicular dendritic cells (FDCs) are non-lymphoid,
non-phagocytic antigen-presenting cells of the immune system and
are able to capture, process and present antigens and immune
complexes to B and T cells. FDCs belong to the dendritic cell
family which may be divided into 4 categories based on
immunophenotypes: interdigitating dendritic, indeterminate,
Langerhans and follicular dendritic cells (1). FDCs are normally located in the
germinal centers of primary and secondary follicles where they form
a tight meshwork with other cells. In addition, FDCs may be located
extranodally in acquired lymphoid tissue which is the foundation
condition for the occurrence of extranodal FDCS (2). The possibility of a follicular
dendritic cell tumor was first suggested by Lennert in 1978
(3). A primary neoplasm of FDC
origin in a lymph node was first described by Monda et al in
1986 (4), while the name, FDCS, was
first proposed by Chan et al in 1997 (5).
FDCS is a rare neoplasm. At present, ∼200 cases have
been reported in the English-language literature. The majority of
cases were observed in lymph nodes and less than one-third were
extranodal (6). The pharyngeal
region, along with the abdominal/pelvic cavity, was one of the
preferred sites of extranodal FDCS (7). The present study reports 3 cases of
pharyngeal FDCS treated at the First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China) over the last 10 years.
With regard to the diagnosis and therapeutic modalities, 49 cases
reported in the English-language literature were reviewed.
Materials and methods
Samples
Tissue specimens from 3 patients, who were all
treated at the First Affiliated Hospital of Zhengzhou University
over the last decade, were collected and fixed in 10% buffered
formalin, then dehydrated, embedded in paraffin and cut into
4-μm-thick sections for hematoxylin and eosin (HE) staining
and light microscopy.
Immunohistochemistry
Immunohistochemical reactions were performed on the
paraffinized sections using the EnVision method. The diagnostic
antibodies used included antibodies against CD21, CD23, CD35,
podoplanin (D2-40), CXCL-13, S100 protein, vimentin, epithelial
membrane antigen (EMA), cytokeratin (CK, AE1/AE3), CD163, leukocyte
common antigen (LCA, CD45), CD1a, CD68, CD34, CD3 and CD20. Ki-67
(MIB-1) antigen was detected to show the proliferative activity of
each lesion.
In situ hybridization
In situ hybridization for EBV-encoded RNA
(EBER) was also performed on the formalin-fixed, paraffin-embedded
tissue sections and the probes used were purchased from Beijing
Zhongshan Golden Bridge (Beijing, China).
Literature review
A literature search was performed using MEDLINE on
PubMed (www.ncbi.nlm.nih.gov/pubmed) with the
term ‘follicular dendritic cell sarcoma’ combined with ‘extranodal’
and ‘pharyngeal’. Efforts were made to identify cases that were
reported more than once in various settings and only the entry with
the most up-to-date information was included in such cases. Only
FDCS of the pharyngeal region were included in Table III.
 | Table IIIClinical characteristics of 49
patients with pharyngeal FDC sarcoma from literature (case
4–52). |
Table III
Clinical characteristics of 49
patients with pharyngeal FDC sarcoma from literature (case
4–52).
| Case no. | Reference | Age (years) | Gender | Size (cm) | Sites | Initial
diagnosis | Initial
treatment | Recurrence, DFT
(months) | Status | Follow-up
(months) |
|---|
| 4 | Chan et
al(38), 1994 | 44 | M | 1.5 | Tonsil | FDC tumor | Surg | NA | NED | 36 |
| 5 | Chan et
al(38), 1994 | 63 | F | 3×3×2 | Soft palate | Acinic cell
carcinoma | Surg + RT | NA | NED | 54 |
| 6 | Perez-Ordonez et
al(39), 1996 | 62 | F | NA | Tonsil | NA | Surg | NA | NED | 12 |
| 7 | Nayler et
al(40), 1996 | 18 | F | 4×2×2 | Tonsil | FDC tumor | Surg + ChT | NA | NA | NA |
| 8 | Chan et
al(5), 1997 | 32 | M | NA | Tonsil | NA | Surg + RT | LR & DM (LN),
54 | AWD | 54 |
| 9 | Chan et
al(5), 1997 | 40 | F | 7×3×2 | Parapharyngeal
space | NA | Surg | LR, 12 | AWD | 12 |
| 10 | Beham-Schmid et
al(41), 1998 | 44 | M | 2 | Nasopharynx | FDC tumor | Surg + RT +
ChT | NA | NED | 20 |
| 11 | Desai et
al(20), 1999 | 45 | F | 6×3×3 | Parapharyngeal
space | Ectopic
meningioma | Surg | LR, 31 | NED | 57 |
| 12 | Araujo et
al(25), 1999 | 14 | M | 1.5×1.0×0.5 | Palate | Fibrous
histiocytoma | Surg | NA | NED | 5 |
| 13 | Chan et
al(14), 2001 | 23 | M | 1 | Nasopharynx | No specific
inflammation | Surg | NA | NED | 36 |
| 14 | Vargas et
al(42), 2002 | 54 | F | 3 | Tonsil, CLN | FDC tumor | Surg | NA | NED | 8 |
| 15 | Vargas et
al(42), 2002 | 54 | F | 6 | Parapharyngeal
space, parotid | FDC tumor | Surg + RT | LR, 6 | AWD | 8 |
| 16 | Biddle et
al(2), 2002 | 48 | M | 3.5×2×2 | Tonsil | FDC sarcoma | Surg | NA | NED | 8 |
| 17 | Biddle et
al(2), 2002 | 48 | F | 1.5×1.2×1.0 | Tonsil, CLN | Low-grade
malignancy | Surg | NA | NED | 6 |
| 18 | Biddle et
al(2), 2002 | 33 | M | NA | Pharynx | Malignant
schwannoma | Surg + RT | DM (lung), 10 | AWD | 10 |
| 19 | Tish et
al(36), 2003 | 51 | M | NA | Tonsil | FDC sarcoma | Surg + RT | NA | NED | 60 |
| 20 | Satoh et
al(26), 2003 | 16 | M | 3.0×2.5 | Parapharyngeal
space, palate | Low-grade
malignancy | Surg + RT +
ChT | NA | NED | 24 |
| 21 | Wang et
al(27), 2003 | 59 | M | 3 | Pharynx |
NESCTWEF* | Surg | NA | NED | 48 |
| 22 | Dominguez-Malagon
et al(9), 2004 | 29 | F | 4.8 | Parapharyngeal
space | Malignant
meningioma | Surg + RT | LR, 12 | STD | 132 |
| 23 | Dominguez-Malagon
et al(9), 2004 | 48 | M | 1.5 | Tonsil, CLN | FDC sarcoma | Surg + RT | NA | NED | 36 |
| 24 | Dominguez Malagon
et al(9), 2004 | 26 | M | NA | Parapharyngeal
space | FDC sarcoma | Surg + RT +
ChT | DM (lung), 36 | AWD | 72 |
| 25 | Idrees et
al(28), 2004 | 77 | F | NA | Tonsil | SCC | Surg + RT | DM (LN, lung),
96 | AWD | 96 |
| 26 | Georglass et
al(43), 2004 | 61 | M | 5×4 | Hypopharynx,
CLN | FDC sarcoma | Surg | NA | NA | NA |
| 27 | Grogg et
al(44), 2004 | 57 | F | NA | Tonsil, CLN,
ALN | NA | Surveillance | NA | AWD | 8 |
| 28 | Bothra et
al(29), 2005 | 45 | M | NA | Tonsil | UD-carcinoma | Surg | NA | NED | 12 |
| 29 | Bothra et
al(29), 2005 | 45 | M | NA | Tonsil | UD-carcinoma | Surg | NA | NED | 12 |
| 30 | Bothra et
al(29), 2005 | 34 | M | NA | Tonsil | FDC sarcoma | Surg | LR, 120 | AWD | 120 |
| 31 | Chou et
al(45), 2005 | 61 | M | NA | Soft palate | NA | Surg | NA | NED | 60 |
| 32 | Aydin et
al(33), 2006 | 76 | F | 3.5×3.5×1.5 | Tonsil | FDC sarcoma | Surg + RT | NA | NED | 48 |
| 33 | Clement et
al(30), 2006 | 27 | F | 4×3×2 | Tonsil | Nerve sheath
tumor | Surg + RT | NA | NED | 6 |
| 34 | Shia et
al(31), 2006 | 69 | F | NA | Tonsil | SCC | Surg + RT | DM (LN, lung),
96 | AWD | 108 |
| 35 | McDuffieet
al(46), 2007 | 59 | F | 4 | Tonsil | FDC sarcoma | Surg + RT | NA | NED | 18 |
| 36 | Fan et
al(32), 2007 | 48 | F | NA | Tonsil | Malignant
lymphoma | Surg + RT +
ChT | LR&DM (LN),
180 | AWD | 180 |
| 37 | Fan et
al(32), 2007 | 42 | M | NA | Nasopharynx | FDC sarcoma | Surg + RT +
ChT | DM (LN), 48 | AWD | 144 |
| 38 | Alexander et
al(47), 2007 | 69 | M | 3 | Parapharyngeal
space | Paraganglioma | Surg | LR, 12 | AWD | 12 |
| 39 | Soriano et
al(17), 2007 | 33 | M | NA | Nasopharynx | NA | Surg + RT | LR, 10 | STD | 14 |
| 40 | Encabo et
al(48), 2008 | 36 | F | 2.3×2.3 | Nasopharynx |
Teratocarcinosarcoma | Surg + RT | NA | NED | 27 |
| 41 | Vaideeswar et
al(49), 2009 | 50 | M | 2×2 | Tonsil | UD carcinoma | Surg | NA | NED | 48 |
| 42 | Duan et
al(8), 2010 | 54 | F | 3×3×2 | Nasopharynx | UD-carcinoma | Surg + RT | NA | NED | 18 |
| 43 | Duan et
al(8), 2010 | 41 | M | 3×3×2 | Nasopharynx | UD carcinoma | Surg | NA | NED | 7 |
| 44 | Duan et
al(8), 2010 | 41 | M | 3×3×2 | Tonsil | FDC sarcoma | Surg | NA | NED | 9 |
| 45 | Duan et
al(8), 2010 | 39 | F | 2×2×1 | Soft palate | Ectopic
meningioma | Surg | NA | NED | 40 |
| 46 | Li et
al(7), 2010 | 60 | M | 5 | Tonsil | Granuloma | Surg + RT | NA | NED | 86 |
| 47 | Li et
al(7), 2010 | 35 | F | 5 | Parapharyngeal
space | Nasopharyngeal
sarcoma | Surg | LR, 2 | STD | 12 |
| 48 | Li et
al(7), 2010 | 28 | F | 6 | Parapharyngeal
space | FDC sarcoma | Surg + RT +
ChT | DM (lung), 14 | AWD | 22 |
| 49 | Li et
al(7), 2010 | 55 | M | 2 | Tonsil | FDC sarcoma | Surg + RT | LR, 18 | AWD | 21 |
| 50 | Young et
al(50), 2010 | 65 | M | 3×2×1.7 | Tonsil | FDC sarcoma | Surg + RT | NA | NED | 24 |
| 51 | Suchitha et
al(51), 2010 | 63 | M | 4.2×4×2 | Tonsil | FDC sarcoma | Surg + RT | NA | NED | 8 |
| 52 | Karabulut et
al(37), 2012 | 70 | F | 3×3×2 | Nasopharynx | FDC sarcoma | Surg | LR, 12 | AWD | 46 |
Statistical analysis
Statistical analyses were performed using the
Statistical Analysis System (SAS) 9.2. Disease-free survival (DFS)
and overall survival (OS) rates were analyzed using the
Kaplan-Meier method. Log-rank tests were used to analyze the
equality of survival function over strata. P<0.05 was considered
to indicate statistically significant differences.
Results
Clinical data
As shown in Table I,
the 3 patients were all female, with ages ranging between 36 and 64
years (median, 59 years). Of the 3 cases, 2 were tonsil lesions
with case 1 being a left-tonsil lesion with left cervical lymph
node involvement, while case 3 was a left-tonsil lesion. Case 2 was
a mass in the parapharyngeal space. A mass was detected in the
oropharynx and slight dysphagia symptoms were experienced in all 3
cases. Tumor resection was performed in all 3 cases and the
symptoms were relieved. The sizes of the tumors ranged between 3.0
and 6.0 cm as measured in the longitudinal dimension, with an
average of 4.5 cm. All the tumors were initially misdiagnosed as
non-specific inflammation (case 1), a benign tumor (case 2) or
squamous cell carcinoma (case 3).
 | Table IClinical characteristics. |
Table I
Clinical characteristics.
| Case | Age (years) | Gender | Site | Symptom | Size (cm) | Initial
diagnosis | Initial
treatment | Recurrence, DFT
(months) | Status | Follow-up
(months) |
|---|
| 1 | 36 | F | Tonsil, CLN | Oropharyngeal mass,
slight dysphagia, 1 month | 3.0×2.5×1.5 | Non-specific
inflammation | Surgery | LR, 6 | AWD | 15 |
| 2 | 64 | F | Parapharyngeal
space | Oropharyngeal mass,
dysphagia, 12 months | 6.0×4.0×3.0 | Squamous cell
carcinoma | Surgery, ChT | NA | STD | 7 |
| 3 | 59 | F | Tonsil | Oropharyngeal mass,
dysphagia, dyspnea, 2 months | 4.5×4.0×2.0 | Benign tumor | Surgery | LR, 17 | STD | 24 |
There was only 1 patient (case 1) who remained alive
in the follow-up 7 to 24 months. Case 2 succumbed to the disease 7
months after initial treatment with combined surgery and
chemotherapy without recurrence. Case 3 succumbed 7 months after
local recurrence despite a second resection followed by
radiotherapy (200 cGy x 25 times; total 5,000 cGy). Case 1 relapsed
locally in the left neck lymph nodes 6 months after surgery, then
salvage therapy was performed, including 4 courses of the ‘CHOP’
adjuvant chemotherapy regimen according to the following schedule:
cyclophosphamide 750 mg/m2 i.v (day 1), vincristine 1.4
mg/m2 i.v (day 1), doxorubicin 40 mg/m2 i.v
(day 1) and prednisone 100 mg p.o. (days 1–5) and 1 course of
radiotherapy with a total of 5,600 cGy (200 cGy x 28 times).
Subsequently, complete remission (CR) was achieved. The longest
disease-free time (DFT) among the 3 present cases was 17
months.
Histopathology
In case 1, the tumor was confined by fibrous tissue
and showed clear boundaries. The tumor cells were ovoid- to
spindle-shaped and arranged in sheet-like or fascicular patterns.
In case 2, the tumor cells showed diffusing sheet-like
distributions, partially in the storiform pattern, and were
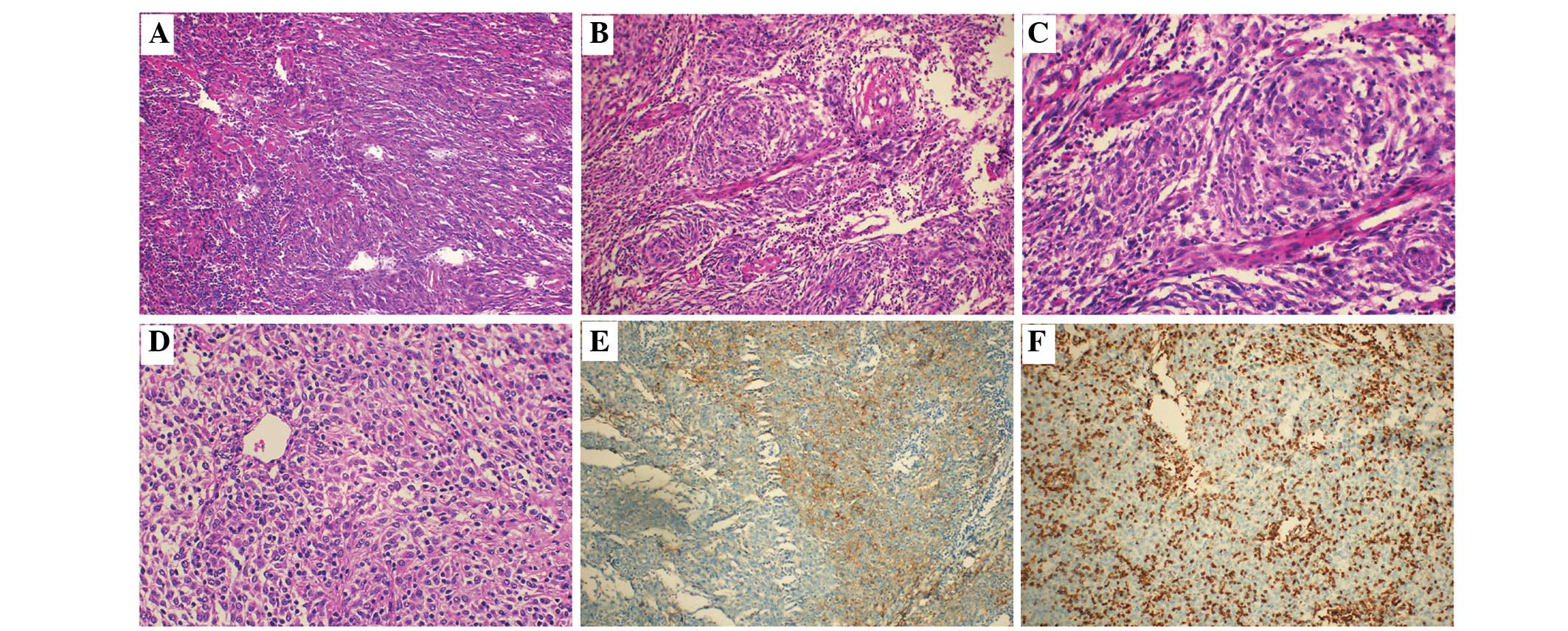
separated by collagenous fibers (Fig.
1A). In case 3, whorl structures formed by tumor cells were
observed (Fig. 1B). In all 3 cases,
irregular round or spindle nuclei, containing delicate chromatin
and small nucleoli, were observed (Fig.
1D). Indistinct cell borders and slightly eosinophil-stained
cytoplasm were the most common features. Infiltrating small
lymphocytes and tissue cells were distributed in the background
(Fig. 1C).
Immunohistochemistry and in situ
hybridization
As shown in Table
II, the immunohistochemical stains in all 3 cases were positive
for CD21, CD35, CD23 and vimentin, but negative for CK (positive
minority in case 3) and S100 protein. The expression of D2-40 was
observed in partial or focal tumor cells in all cases while CXCL-13
was absent (Fig. 1E). Case 1 was
slightly positive for EMA. CD163 and LCA were positive in the
background cells(Fig. 1F). The
Ki-67-labeling index (Ki-67 LI) of all the lesions ranged between
30 and 40%. Furthermore, the in situ hybridizations for EBER
were negative in all cases. Certain antibodies were stained in 1
case but not stained in the others, as follows: in case 3, the
immunohistochemical reaction for CD1a was negative, while CD3 and
CD20 were positive in the background cells and negative in tumor
cells; CD34 was negative in the tumor of case 2.
 | Table IIImmunohistochemical
characteristics. |
Table II
Immunohistochemical
characteristics.
| Case | CD21 | CD35 | CD23 | D2 40 | CXCL 13 | S-100 | Vimentin | EMA | CK | CD163 | LCA | CD1a | CD68 | CD34 | CD3 | CD20 | Ki-67 (%) | EBER |
|---|
| 1 | + | +++ | +++ | + | − | − | + | − | − | +b | +b | 0 | 0 | 0 | 0 | 0 | 35 | − |
| 2 | ++ | + | +++ | + | − | − | + | − | − | +b | +b | 0 | 0 | − | 0 | 0 | 40 | − |
| 3 | + | + | ++ | + | − | − | + | + | + | +b | +b | − | − | 0 | − | − | 30 | − |
Literature review of pharyngeal FDC
sarcomas
The following summaries were obtained from a total
of 52 cases of FDCS in the pharyngeal region, including the 3
present cases and 49 cases identified through a MEDLINE search
(Table III).
The patients ranged between 14 and 77 years old
(median, 48 years; average, 47 years). The female/male ratio was
1:1.08 (25:27). The tumors tended to occur in young to middle aged
adults without any gender difference. The FDCSs occurred in various
anatomical parts of the pharyngeal region. The most common site was
the tonsils, with 27 cases (52%). Other sites included the
parapharyngeal space (10/52, 19%), nasopharynx (8/52, 15%), palate
(4/52, 8%), pharynx (2/52, 4%) and hypopharynx (1/52, 2%). The
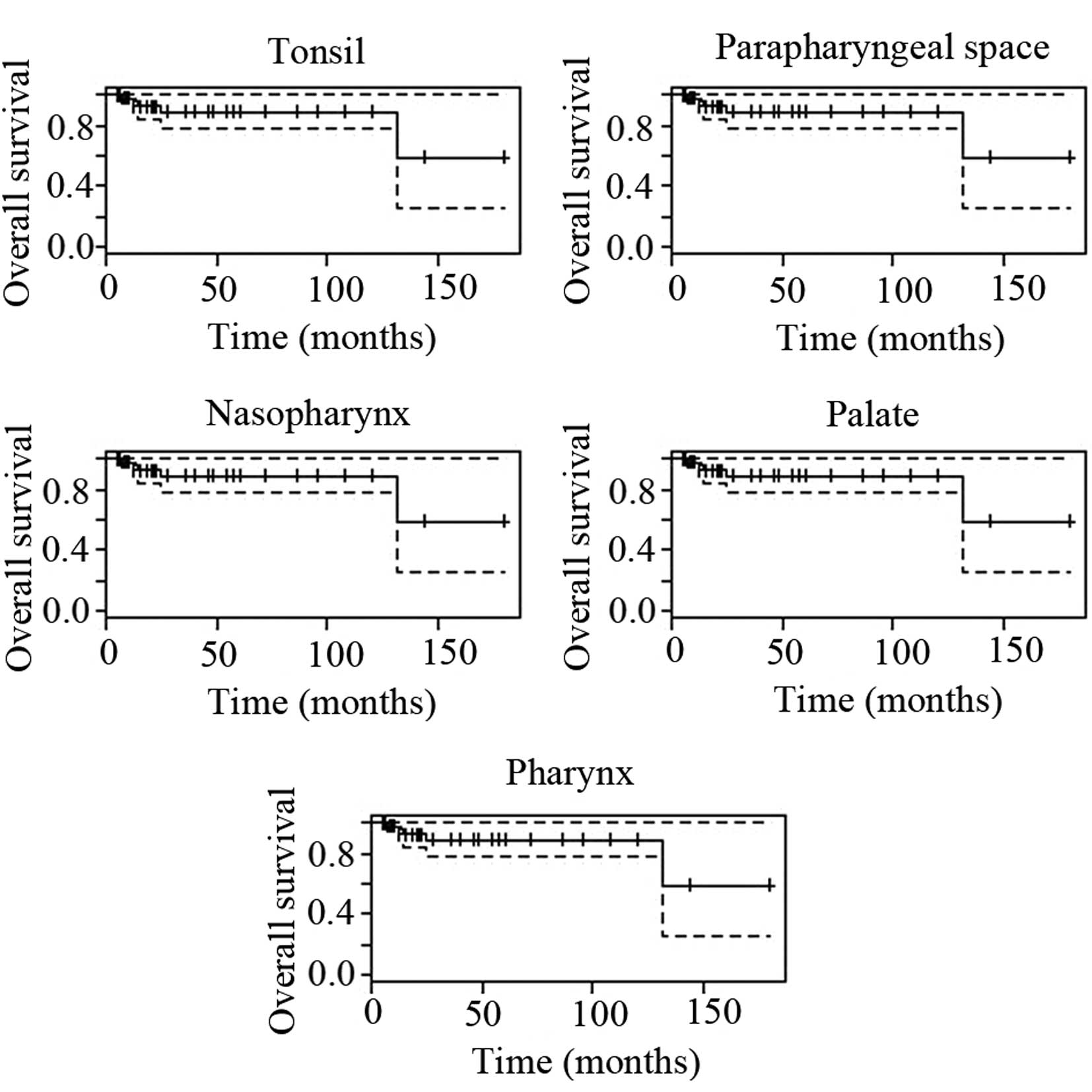
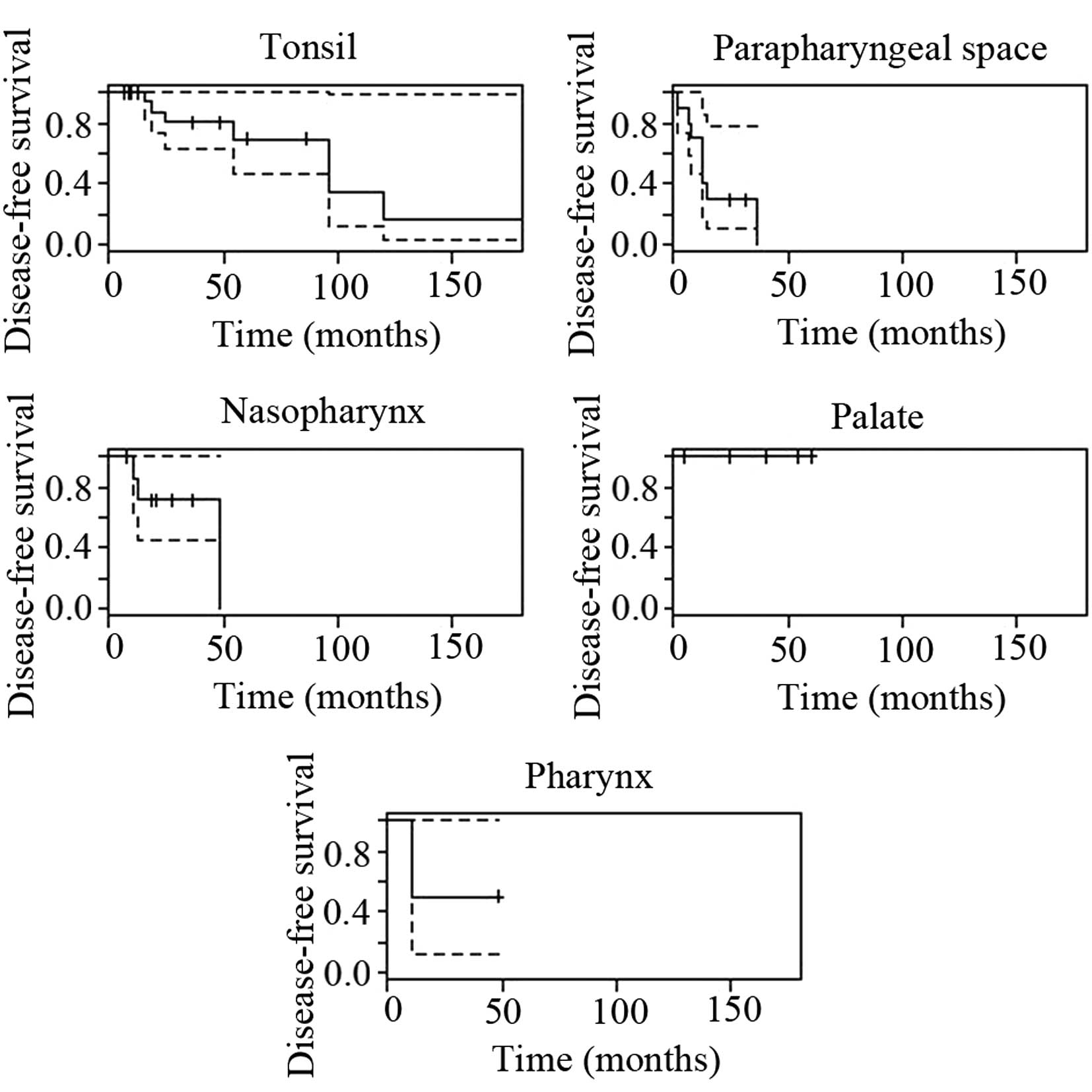
tumors in the various anatomical regions showed distinct prognoses
(Table IV) and their survival
curves are shown in Figs. 2 and
3. The recurrence, metastasis and
mortality rates in the tonsils were 30.8, 15.4 and 3.9%,
respectively. Of the cases with metastasis, all (4/4, 100%)
involved the cervical lymph nodes, while a number (2/4, 50%) also
involved the lungs. The FDCSs in the parapharyngeal space showed
the worst prognosis with recurrence, metastasis and mortality rates
of 80, 20 and 30%, respectively. Furthermore, all the cases (2/2,
100%) of metastasis from the parapharyngeal space involved the
lungs. Due to the limited number of cases, the results in Table IV remain to be confirmed by a
larger-scale survey.
 | Table IVSurvival rates. |
Table IV
Survival rates.
| | DFS rate (%)
| OS rate (%)
| | | | P-value
|
|---|
| Cases | Frequency (n/total,
%) | 2-year | 5-year | 2-year | 5-year | Recurrence rate
(%) | Metastasis rate
(%) | Mortality rate
(%) | DFS | OS |
|---|
| Available
total | 50, 100 | 66.2 | 51.3 | 88.6 | 88.6 | 40 | 16 | 10 | - | - |
| Surgery alone | 23/50, 46 | 67.4 | 56.2 | 84.7 | 84.7 | 34.8 | 0 | 8.7 | 0.5513 | 0.7193 |
| Surgery +
RT/ChT | 26/50, 52 | 73.8 | 66.4 | 91.4 | 91.4 | 46.2 | 30.8 | 7.7 | | |
| Size <4 cm | 23/35, 66 | 81.5 | - | 100 | - | 17.4 | 0 | 0 | 0.0446 | 0.0086 |
| Size ≤4 cm | 12/35, 34 | 36.8 | 36.8 | 59.7 | 59.7 | 58.3 | 8.3 | 33.3 | | |
| Tonsil | 27/52, 52 | 95.8 | 66.4 | 95.3 | 88.6 | 30.8 | 15.4 | 3.9 | - | - |
| Parapharyngeal
space | 10/52, 19 | 40.0 | 40.0 | 95.3 | 88.6 | 80 | 20 | 30 | - | - |
| Nasopharynx | 8/52, 15 | 71.4 | 71.4 | 95.3 | 88.6 | 37.5 | 12.5 | 12.5 | - | - |
| Palate | 4/52, 8 | 100 | 100 | 95.3 | 88.6 | 0 | 0 | 0 | - | - |
| Pharynx | 2/52, 4 | 50.0 | 50.0 | 95.3 | 88.6 | 50 | 50 | 0 | - | - |
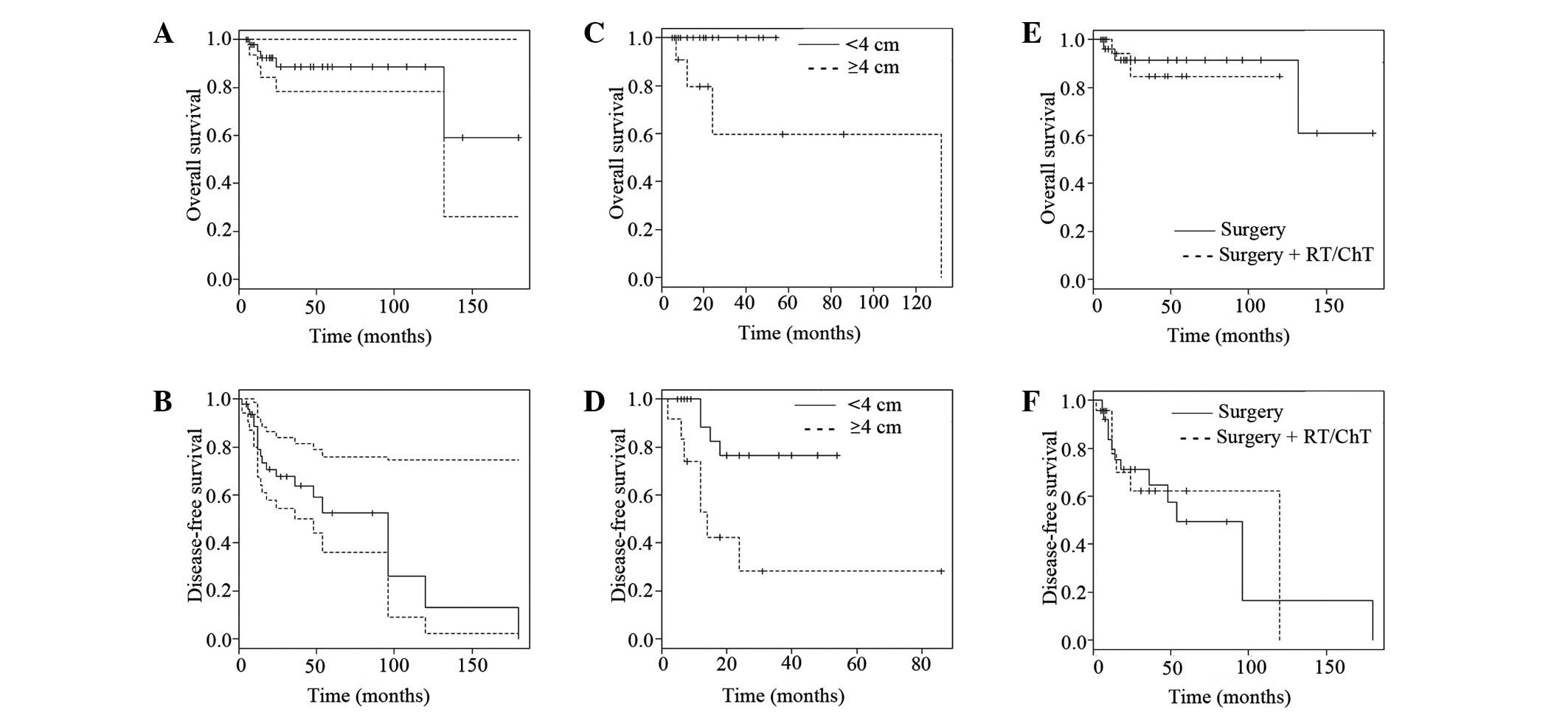
To assess the prognosis, the size of the initial
tumor was analyzed. The sizes varied between 1 and 7 cm, with an
average of 3.4 cm, in the longitudinal dimension. The large tumors
(≥4 cm) accounted for 34% (12/35) of cases and had recurrence,
metastasis and mortality rates of 58.3, 8.3 and 33.3%,
respectively. However, the small tumors (<4 cm, 66%, 23/35)
exhibited recurrence, metastasis and mortality rates of 17.4, 0 and
0%, respectively. The 2-year DFS and OS rates of the large tumors
were 36.8 and 59.7%, respectively, in the follow-up of 6 to 132
months, while those of the small tumors were 81.5 and 100% in the
follow-up of 5 to 54 months.
Since the longest follow-up of the small tumors was
54 months (<60 months), the 5-year DFS and OS rates were not
suitable, while those of the large tumors were 36.8 and 59.7%,
respectively. Further statistical analysis of the size was
performed among the 35 available tumors and indicated that large
tumors (≥4 cm) had a poorer prognosis compared with small tumors
(<4 cm) with regard to DFS (P=0.0446, P<0.05) and OS
(P=0.0086, P<0.05) rates (Table
IV, Fig. 4C and D; Kaplan-Meier
estimation, log-rank test). Notably, 58.3% of the large FDCSs
(7/12) occurred in the parapharyngeal space, among which 85.7%
(6/7) exhibited recurrence. To a certain degree, this accounted for
the poor prognosis of tumors in the parapharyngeal space. While
41.2% of the large sarcomas (5/12) occurred in the tonsils, only 1
case (1/5, 25%) locally relapsed.
In the 50 cases with follow-up data, the follow-up
time ranged between 5 and 180 months, with a median of 23 months
and an average of 39 months. A total of 20 cases (20/50, 40%)
relapsed, including 12 (12/50, 24%) of local recurrence and 8
(8/50, 16%) of distant metastasis. Additionally, 5 patients (5/50,
10%) succumbed to the disease. The disease-free time varied from 2
to 180 months (median, 18 months; mean, 32 months). The recurrence,
metastasis and mortality rates were 40, 16 and 10%, respectively.
The 2- and 5-year DFS rates for the entire group were 66.2 and
51.3%, respectively, while the 2- and 5-year OS rates were 88.6 and
88.6%, respectively (Kaplan-Meier estimation; Table IV, Fig.
4A and B).
Initially, nearly all the cases (51/52, 98.1%)
underwent surgery to remove the tumor, with the exception of 1 case
(1/52, 1.9%) where the patient opted to be surveilled. A total of 2
patients were lost to follow-up. Adjuvant treatment (radiotherapy
and/or chemotherapy) was administered post-operatively to over half
of the cases (26/50, 52%), among which 12 (12/26, 46%) suffered
recurrence (local recurrence and/or metastasis), 8 (8/26, 31%)
suffered metastasis (lung, lymph nodes) and 2 (2/26, 8%) succumbed
to the disease in the follow-up of 6 to 180 months. For the group
who received surgery and adjuvant therapy, the recurrence,
metastasis and mortality rates were 46.2, 30.8 and 7.7%,
respectively. The 2- and 5-year DFS rates were 73.8 and 66.4%,
respectively, while the 2- and 5-year OS rates were 91.4 and 91.4%,
respectively. Among the surgery alone group, recurrence was
observed in 8 cases (8/23, 35%), no cases of metastasis were
detected and 2 cases (2/23, 9%) succumbed to the disease in the
follow-up of 5 to 120 months. The recurrence, metastasis and
mortality rates were 34.8, 0 and 8.7%, respectively. The 2- and
5-year DFS rates were 67.4 and 56.2%, respectively, while the 2-
and 5-year OS rates were 84.7 and 84.7%, respectively (Table IV). The disease-free time of
patients who received adjuvant treatment (range, 6 to 180 months;
mean, 39 months) was longer compared with the patients who received
surgery alone (range, 2 to 120 months; mean, 24 months). However,
the survival curves (Fig. 4E and F)
suggested that the survival rates were not significantly different
(P>0.05, log-rank test).
Discussion
FDCS of the pharyngeal region is an extremely rare
neoplasm which has been detected in the tonsils, palate,
parapharyngeal space, nasopharynx, pharynx and hypopharynx, with
the majority of distant metastasis occurring in the cervical or
axillary lymph nodes and lungs (8).
Similar to other body regions, pharyngeal FDCS occurs at a wide
range of ages and has an adult predominance with equal gender
distribution (9). The tumor usually
presents as an enlarging solid mass and local pharyngeal disorders,
including foreign body sensation, dysphagia, naso-obstruction and
even intermittent bleeding (8).
The etiology and pathogenesis of FDCS are not clear.
It is possible that certain FDCSs, particularly in hepatic and
splenic lesions (10–12), are associated with EBV infection,
although the association is not evident in pharyngeal tumors as the
in situ hybridizations for EBV-encoded RNA were negative in
the study by Duan et al(8),
as well as the present study. Pauwels et al(13) and Chan et al(14) agreed that FDCS developed according
to a hyperplasia-dysplasia-neoplasia sequence in follicular
dendritic cells.
The diagnosis of pharyngeal FDCS depends on
pathology. Histologically, the typical lesion characteristics are
whorl, storiform and fascicular arrangements of oval to spindle
tumor cells with indistinct cell borders, possible syncytial growth
patterns and pale eosinophilic cytoplasm (5,15,16).
Infiltrating small lymphocytes, which may gather around blood
vessels, creating a cuffing pattern (5,16), may
be observed in the background. The nuclei of the tumor cells may be
elongated or oval with delicate dispersed chromatin and small
nucleoli (5,16). The cells may also be multinucleated.
Nuclear atypia and mitotic activity vary among different lesions
(5,16).
Due to the previously mentioned morphological
variability, immunohistochemical confirmation is essential. As
demonstrated in the literature, the most sensitive and specific
markers for FDCS are CD21, CD23 and CD35 (17). They are consistently positive and
should be the first-line markers. D2-40 and CXCL13 were also
considered to be effective by Xie et al(18) and Vermi et al(19) and should be observed together with
other immunohistochemical makers including vimentin, S-100 protein,
EMA and CD68, which were variably positive (17,20).
CD1a, HMB45 and CD34 are specifically negative while CK is
occasionally positive in FDCS (2,15,21).
Among the three present cases, CD21, CD23, CD35 and D2-40 were all
distinctly positive. CD3 and CD20 (17) have been noted to be negative in FDC
tumor cells but positive in the background lymphocytes, but were
replaced by LCA (leukocyte common antigen) (20) and CD163 in the present study.
Moreover, EGFR was observed to be overexpressed and may be a
therapeutic target (17). Clusterin
was reported to be an important marker in the differential
diagnosis of FDCS (22). Several
other variable antibodies, including desmoplakin, fascin, bcl-2 and
p53 and certain nonspecific markers, including smooth muscle actin
(SMA), CD30, lysozyme and myeloperoxidase (MPO), are used
clinically (5,7,17,20,21,23,24).
The Ki-67 antigen expression levels were low to moderate (15). EBER staining by in situ
hybridization was negative in all pharyngeal FDCS cases, according
to the statistics of Duan et al(8). In the three present cases, the Ki-67
LIs were 35, 40 and 30%, respectively, and none of the cases were
positive for EBER.
Misdiagnosis occurs frequently due to the rarity of
pharyngeal FDCS tumors and similar histopathological
characteristics shared with other common pharyngeal tumors,
including ectopic meningioma, undifferentiated carcinoma, nerve
sheath tumor, inflammatory sarcoma, squamous cell carcinoma,
lymphoma and fibrous histiocytoma (2,5,9,14,20,25–32).
All three present cases were misdiagnosed. The misdiagnosis rate in
the present survey was 57% (26/46), higher than the 30% reported by
Shia et al(31) in the whole
body. Among the mistaken diagnoses, ectopic meningioma accounted
for 12% (3/26) and undifferentiated carcinoma for 19% (5/26).
Ectopic meningioma and FDCS feature syncytial cells arranged in
whorl patterns and the tumor cells are negative for CK. However,
lymphocytes and positive reactions for CD21, CD68 and CD35 are not
observed in meningiomas (33).
Similar to FDCS, undifferentiated carcinoma shows syncytial cells
with vesicular nuclei and infiltrating lymphocytes. However,
immunohistochemical markers positive for CD21, CD35 and CD23 and
negative for CK, may be used to distinguish undifferentiated
carcinoma from FDCS (8). Whenever
pharyngeal tumors are identified, we recommend differential
diagnosis and specific monoclonal antibody staining for FDCS.
FDCS is considered to be a sarcoma of
intermediate-grade malignancy, which was demonstrated by Chan et
al(5) in the observation of 17
cases. Prior to Chan et al’s study, it was regarded as an
indolent tumor similar to a low-grade soft tissue sarcoma with a
tendency for local recurrence and a low risk of metastasis
(2,25). However, after analyzing 20 cases,
Domínguez-Malagón et al(9)
suggested that, in the pharyngeal region, FDCS is a low-grade
carcinoma with recurrence, metastasis and mortality rates of 25, 25
and 5%, respectively. Duan et al(8) also suggested this and the rates of
their 41-case analysis were 23, 21 and 3%, respectively. In the
present study of 52 cases, the overall recurrence, metastasis and
mortality rates were 40, 16 and 10%, respectively. The 2- and
5-year DFS rates were 66.2 and 51.3%, respectively. This
demonstrated that extranodal FDCS of the pharyngeal region remains
low-grade with recurrence tendencies.
FDCSs of various anatomical sites in the pharyngeal
region were also analyzed. The most common site was the tonsils,
where 52% of all cases occurred. The site with the poorest
prognosis was the parapharyngeal space, with recurrence, metastasis
and mortality rates of 80, 20 and 30%, respectively. The
recurrence, metastasis and mortality rates of FDCS at five sites
were calculated and survival curves were shown for the first time.
However, larger-scale research is required due to the limited
number of cases in certain sites, particularly the nasopharynx,
palate and pharynx.
As a result of the rarity of FDCS, the assessment of
pharyngeal FDCS prognoses remains difficult. Li et
al(7) proposed a model for
recurrence risk assessment according to the tumor size and
histological grade. By this model, extranodal FDC sarcomas were
divided into low-, intermediate- and high-risk groups and the
recurrence rates were 16, 46 and 73%, respectively, while the
mortality rates were 0, 4 and 45%. In the present study,
statistical analyses suggested that large tumors (≥4 cm in the
longitudinal dimension) had a worse outcome compared with small
tumors (<4 cm).
There is not yet a consensus with regard to the
optimal therapeutic modality due to the limited number of case
reports and absence of prospective studies of treatments and
outcomes. Surgery or combination therapy (surgery followed by
chemotherapy and/or radiotherapy) are the choices for the initial
treatment. Radical surgery (dissection of nodal lesions or wide
local excision of extranodal lesions) is essential for localized
FDC tumors, as demonstrated by De Pas et al(34). Chera et al(35) analyzed 67 cases of FDCS in the head
and neck region and recommended postoperative radiotherapy. The
total doses ranged between 6,000 and 7,000 cGy. With regard to
chemotherapy, the agents most commonly used are combination
regimens designed for the treatment of non-Hodgkin’s lymphoma, such
as the CHOP (cyclophosphamide, doxorubicin, vincristine and
prednisone) or CHOP-like regimens (36). Other regimens, such as sarcoma
regimens, usually include a combination of doxorubicin and
ifosfamide or gemcitabine, while a taxane may also be selected
(17). Generally, 3 to 4 courses of
chemotherapy were performed and partial or complete responses were
achieved. However, it is not clear whether adjuvant treatment
(chemotherapy and/or radiotherapy following surgery) is essential.
Adjuvant treatment made no difference according to Duan et
al(8) who analyzed 42 cases in
the pharyngeal region. However, Karabulut et al(37) suggested that it was better to
administer radiotherapy following resection to prevent local
recurrence. According to the results of the present 52-case study,
the average disease-free time of patients who underwent adjuvant
treatment (range, 6 to 180 months; mean, 39 months) was longer
compared with patients who underwent surgery alone (range, 2 to
120; mean, 24 months). The recurrence and mortality rates of the
group who underwent surgery and adjuvant therapy were 46.2 and
7.7%, respectively, while those of the surgery alone group were
34.8 and 8.7%. However, the survival curves exhibited no
statistically significant differences in DFS and OS rates between
the two groups. From our clinical experience, postoperative
adjuvant therapy appears to contribute to a longer disease-free
time and is recommended. However, this remains to be clarified by
larger-scale prospective studies of the treatments and outcomes of
FDCS.
For accurate identification and effective therapy
for FDCS, more attention should be paid to diagnosis and
treatment.
Acknowledgements
The authors would like to thank Jian
Chai (Social Medicine Research Center, Henan Provincial Population
and Family Planning Reseach Institute, China) for performing the
statistical analysis.
References
|
1
|
Favara BE, Feller AC, Pauli M, et al:
Contemporary classification of histiocytic disorders. The WHO
Committee On Histiocytic/Reticulum Cell Proliferations
Reclassification Working Group of the Histiocyte Society. Med
Pediatr Oncol. 29:157–166. 1997.
|
|
2
|
Biddle DA, Ro JY, Yoon GS, Yong YW, Ayala
AG, Ordonez NG and Ro J: Extranodal follicular dendritic cell
sarcoma of the head and neck region: three new cases, with a review
of the literature. Mod Pathol. 15:50–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lennert K: Malignant Lymphomas Other Than
Hodgkin’s Disease: Histology, Cytology, Ultrastructure, Immunology.
Springer-Verlag; New York, NY: 1978
|
|
4
|
Monda L, Warnke R and Rosai J: A primary
lymph node malignancy with features suggestive of dendritic
reticulum cell differentiation: a report of 4 cases. Am J Pathol.
122:562–572. 1986.PubMed/NCBI
|
|
5
|
Chan JK, Fletcher CD, Nayler SJ and Cooper
K: Follicular dendritic cell sarcoma: clinicopathologic analysis of
17 cases suggesting a malignant potential higher than currently
recognized. Cancer. 79:294–313. 1997. View Article : Google Scholar
|
|
6
|
Youens KE and Waugh MS: Extranodal
follicular dendritic cell sarcoma. Arch Pathol Lab Med.
132:1683–1687. 2008.PubMed/NCBI
|
|
7
|
Li L, Shi YH, Guo ZJ, et al:
Clinicopathological features and prognosis assessment of extranodal
follicular dendritic cell sarcoma. World J Gastroenterol.
16:2504–2519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duan GJ, Wu F, Zhu J, Guo DY, et al:
Extranodal follicular dendritic cell sarcoma of the pharyngeal
region: a potential diagnostic pitfall, with literature review. Am
J Clin Pathol. 133:49–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Domínguez-Malagón H, Cano-Valdez AM,
Mosqueda-Taylor A and Hes O: Follicular dendritic cell sarcoma of
the pharyngeal region: histologic, cytologic, immunohistochemical,
and ultra-structural study of three cases. Ann Diagn Pathol.
8:325–332. 2004.PubMed/NCBI
|
|
10
|
Cheuk W, Chan JK, Shek TW, et al:
Inflammatory pseudotumor-like follicular dendritic cell tumor: a
distinctive low-grade malignant intra-abdominal neoplasm with
consistent Epstein-Barr virus association. Am J Surg Pathol.
25:721–731. 2001. View Article : Google Scholar
|
|
11
|
Chen TC, Kuo TT and Ng KF: Follicular
dendritic cell tumor of the liver: a clinicopathologic and
Epstein-Barr virus study of two cases. Mod Pathol. 14:354–360.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bai LY, Kwang WK, Chiang IP and Chen PM:
Follicular dendritic cell tumor of the liver associated with
Epstein-Barr virus. Jpn J Clin Oncol. 36:249–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pauwels P, Dal Cin P, Vlasved LT, Aleva
RM, van Erp WF and Jones D: A chromosomal abnormality in hyaline
vascular Castleman’s disease: evidence for clonal proliferation of
dysplastic stromal cells. Am J Surg Pathol. 24:882–888. 2000.
|
|
14
|
Chan AC, Chan KW, Chan JK, Au WY, Ho WK
and Ng WM: Development of follicular dendritic cell sarcoma in
hyaline-vascular Castleman’s disease of the nasopharynx: tracing
its evolution by sequential biopsies. Histopathology. 38:510–518.
2001.
|
|
15
|
Pileri SA, Grogan TM, Harris NL, et al:
Tumors of histiocytes and accessory dendritic cells: An
immunohistochemical approach to classification from the
International Lymphoma Study Group based on 61 cases.
Histopathology. 41:1–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perez-Ordoñez B and Rosai J: Follicular
dendritic cell tumor: review of the entity. Semin Diagn Pathol.
15:144–154. 1998.PubMed/NCBI
|
|
17
|
Soriano AO, Thompson MA, Admirand JH, et
al: Follicular dendritic cell sarcoma: a report of 14 cases and a
review of the literature. Am J Hematol. 82:725–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie Q, Chen L, Fu K, et al: Podoplanin
(d2-40): a new immunohistochemical marker for reactive follicular
dendritic cells and follicular dendritic cell sarcomas. Int J Clin
Exp Pathol. 1:276–284. 2008.PubMed/NCBI
|
|
19
|
Vermi W, Lonardi S, Bosisio D, et al:
Identification of CXCL13 as a new marker for follicular dendritic
cell sarcoma. J Pathol. 216:356–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Desai S, Deshpande RB and Jambhekar N:
Follicular dendritic cell tumor of the parapharyngeal region. Head
Neck. 21:164–167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaideeswar P, George SM, Kane SV, et al:
Extranodal follicular dendritic cell sarcoma of the tonsil - case
report of an epithelioid cell variant with osteoclastic giant
cells. Pathol Res Pract. 205:149–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grogg KL, Macon WR, Kurtin PJ and
Nascimento AG: A survey of clusterin and fascin expression in
sarcomas and spindle cell neoplasms: strong clusterin
immunostaining is highly specific for follicular dendritic cell
tumor. Mod Pathol. 18:260–266. 2005. View Article : Google Scholar
|
|
23
|
Orii T, Takeda H, Kawata S, Maeda K and
Yamakawa M: Differential immunophenotypic analysis of dendritic
cell tumours. J Clin Pathol. 63:497–503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cyriac S, Praveenkumar D, Majhi U and
Sagar TG: Follicular dendritic cell sarcoma of the neck with an
aggressive and fatal course. J Cancer Res Ther. 6:114–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Araújo VC, Martins MT, Salmen FS and
Araújo NS: Extranodal follicular dendritic cell sarcoma of the
palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
87:209–214. 1999.
|
|
26
|
Satoh K, Hibi G, Yamamoto Y, Urano M,
Kuroda M and Nakamura S: Follicular dendritic cell tumor in the
oropharyngeal region: report of a case and a review of the
literature. Oral Oncol. 39:415–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Kong Y, Lu H and Xu Y: Two cases
of extranodal follicular dendritic cell sarcoma. Chin Med J (Engl).
116:794–797. 2003.PubMed/NCBI
|
|
28
|
Idrees MT, Brandwein-Gensler M, Strauchen
JA, Gil J and Wang BY: Extranodal follicular dendritic cell tumor
of the tonsil: report of a diagnostic pitfall and literature
review. Arch Otolaryngol Head Neck Surg. 130:1109–1113. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bothra R, Pai PS, Chaturvedi P, Majeed TA,
Singh C, Gujral S and Kane SV: Follicular dendritic cell tumor of
tonsil: is it an underdiagnosed entity? Indian J Cancer.
42:211–214. 2005.PubMed/NCBI
|
|
30
|
Clement P, Saint-Blancard P, Minvielle F,
Le Page P and Kossowski M: Follicular dendritic cell sarcoma of the
tonsil: a case report. Am J Otolaryngol. 27:207–210. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shia J, Chen W, Tang LH, et al: Extranodal
follicular dendritic cell sarcoma: clinical, pathologic and
histogenetic characteristics of an underrecognized disease entity.
Virchows Arch. 449:148–158. 2006. View Article : Google Scholar
|
|
32
|
Fan YS, Ng WK, Chan A, Chan GS, Tsang J,
Chim CS and Ip P: Fine needle aspiration cytology in follicular
dendritic cell sarcoma: a report of two cases. Acta Cytol.
51:642–647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aydin E, Ozluoglu LN, Demirhan B and
Arikan U: Follicular dendritic cell sarcoma of the tonsil: case
report. Eur Arch Otorhinolaryngol. 263:1155–1157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Pas T, Spitaleri G, Pruneri G, et al:
Dendritic cell sarcoma: an analytic overview of the literature and
presentation of original five cases. Crit Rev Oncol Hematol.
65:1–7. 2008.PubMed/NCBI
|
|
35
|
Chera BS, Orlando C, Villaret DB and
Mendenhall WM: Follicular dendritic cell sarcoma of the head and
neck: case report and literature review. Laryngoscope.
118:1607–1612. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tisch M, Hengstermann F, Kraft K, von
Hinüber G and Maier H: Follicular dendritic cell sarcoma of the
tonsil: report of a rare case. Ear Nose Throat J. 82:507–509.
2003.PubMed/NCBI
|
|
37
|
Karabulut B, Orhan KS, Guldiken Y and
Dogan O: Follicular dendritic cell sarcoma of the nasopharynx. Int
J Oral Maxillofac Surg. 41:218–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chan JK, Tsang WY, Ng CS, Tang SK, Yu HC
and Lee AW: Follicular dendritic cell tumors of the oral cavity. Am
J Surg Pathol. 18:148–157. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perez-Ordonez B, Erlandson RA and Rosai J:
Follicular dendritic cell tumor: report of 13 additional cases of a
distinctive entity. Am J Surg Pathol. 20:944–955. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nayler SJ, Verhaart MJ and Cooper K:
Follicular dendritic cell tumor of the tonsil. Histopathology.
28:89–92. 1996. View Article : Google Scholar
|
|
41
|
Beham-Schmid C, Beham A, Jakse R, Auböck L
and Höfler G: Extranodal follicular dendritic cell tumour of the
nasopharynx. Virchows Arch. 432:293–298. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vargas H, Mouzakes J, Purdy SS, Cohn AS
and Parnes SM: Follicular dendritic cell tumor: an aggressive head
and neck tumor. Am J Otolaryngol. 23:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Georgalas C, Kanagalingam J, Gallimore A
and O’Flynn P: Follicular dendritic cell sarcoma arising from the
hypopharynx. J Laryngol Otol. 118:317–318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Grogg KL, Lae ME, Kurtin PJ and Macon WR:
Clusterin expression distinguishes follicular dendritic cell tumors
from other dendritic cell neoplasms: report of a novel follicular
dendritic cell marker and clinicopathologic data on 12 additional
follicular dendritic cell tumors and 6 additional interdigitating
dendritic cell tumors. Am J Surg Pathol. 28:988–998. 2004.
|
|
45
|
Chou YY, How SW and Huang CH: Follicular
dendritic cell sarcoma of the soft palate. J Formos Med Assoc.
104:843–847. 2005.PubMed/NCBI
|
|
46
|
McDuffie C, Lian TS and Thibodeaux J:
Follicular dendritic cell sarcoma of the tonsil: a case report and
literature review. Ear Nose Throat J. 86:234–235. 2007.PubMed/NCBI
|
|
47
|
Alexander AA, Zapanta PE and Khan A:
Diagnosis and recurrence of follicular dendritic cell sarcoma.
Otolaryngol Head Neck Surg. 137:832–834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Encabo RS, McHugh J, Carrau RL, Kassan A
and Heron D: Follicular dendritic cell sarcoma of the nasopharynx.
Am J Otolaryngol. 29:262–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vaideeswar P, George SM, Kane SV,
Chaturvedi RA and Pandit SP: Extranodal follicular dendritic cell
sarcoma of the tonsil: case report of an epithelioid cell variant
with osteoclastic giant cells. Pathol Res Pract. 205:149–153. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Eun YG, Kim SW and Kwon KH: Follicular
dendritic cell sarcoma of the tonsil. Yonsei Med J. 51:602–604.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Suchitha S, Sheeladevi CS, Sunila R and
Manjunath GV: Extra nodal follicular dendritic cell tumor. Indian J
Pathol Microbiol. 53:175–177. 2010. View Article : Google Scholar : PubMed/NCBI
|