Introduction
Gastric cancer (GC) is the fourth most common type
of cancer in the world. The incidence rates for GC are highest in
males from Northeast Asia (Japan, Korea and China) (1), with up to 69 cases per 100,000
individuals per year. The carcinoembryonic antigen, CEA, and the
carbohydrate antigens (CA) 19-9 and 72-4, have previously been used
as tumor markers (2–3). Specifically, they have been used as
reliable markers for monitoring tumor progression and the response
to treatments for GC, including chemotherapy and radiation therapy
(4). In the majority of patients
with CA 19-9- and CA 72-4-positive GC, a decrease in these marker
levels is correlated with a positive clinical outcome following
successful resection and treatment (5). Consequently, these antigens are not so
accurate as tumor progression predictors for GC diagnosis and
follow-up. Therefore, it is important to develop additional markers
for the screening and follow-up of patients with GC. Considerable
efforts have been dedicated to the identification of sensitive and
specific markers for GC (6–9). If the clinical predictors that
identify patients with a low-risk of cancer recurrence following
surgical resection were determined, then low-risk patients would be
able to avoid unnecessary post-operative chemotherapy, thus
improving their quality of life.
Ezrin-radixin-moesin-binding phosphoprotein 50
(EBP50) is a 358-amino acid protein containing two
post-post-synaptic density-95/disc-large/zonula occludens-1 (PDZ)
domains. EBP50 functions as a linker between membrane proteins and
the cytoskeleton network and is involved in various types of cancer
(10–11). EBP50 is important in cancer
progression as it regulates cell proliferation and migration
(12). Several studies have
observed that EBP50 is a novel marker for various types of cancer,
including breast cancer, and that EBP50 is able to predict the
clinical behavior of these tumors (11). In a previous study, EBP50 was
positively associated with tumor grade, prognosis and the estrogen
receptor in the circulatory lymphocytes and breast cancer tissues
(13).
EBP50 has been shown to be expressed in gastric
parietal cells, as opposed to the mucous epithelium of the stomach
which only expresses ezrin (14).
However, to the best of our knowledge, there is no available data
concerning EBP50 expression in GC. On the basis of data from
previous cancer studies, we hypothesized that the EBP50 protein
expression level was correlated with the progression of GC.
Therefore, quantum dot (QD) and immunohistochemistry (IHC) assays
were performed to investigate the prognostic value of EBP50 in
GC.
Materials and methods
Patients
The present study included 101 GC patients (29
females and 72 males, aged 24–81 years and of Chinese nationality)
diagnosed and treated at the General Surgery Department of Renmin
Hospital of Wuhan University (Wuhan, China) between 2000 and 2005.
Resected tissues were fixed in formalin, then embedded in paraffin.
Stage IV GC tissue was unavailable due to a requirement for surgery
in the affected patients (Table I).
The tumor staging was based on a histopathological analysis and
clinical assessment, according to the TNM (tumor-node-metastases)
classification. Patients were staged according to the American
Joint Committee on Cancer-International Union Against Cancer
classification (15). For
statistical analysis, the GC patients were divided into two groups,
with 47 cancer patients in the stage I–II group and 54 patients in
the stage III group. The patients were also subdivided into four
groups depending on the degree of gastric wall invasion
(T1, T2, T3 and T4) and
four other groups depending on the nodal involvement
(N0, N1, N2 and N3).
The number of patients in these analyzed subgroups is shown in
Table I. The present study was
approved by the Ethics Committee of Wuhan University. Consent was
received from all patients and all clinical investigations were
performed according to the principles of the Declaration of
Helsinki.
 | Table ICharacteristics of the patients with
GC. |
Table I
Characteristics of the patients with
GC.
| Tested group | Number (%) |
|---|
| Age, years | |
| ≤50 | 24 (23.8) |
| >50 | 77 (76.2) |
| Gender | |
| Male | 72 (71.3) |
| Female | 29 (28.7) |
| Differentiation | |
| Well | 16 (15.8) |
| Moderate | 37 (36.6) |
|
Undifferentiated | 48 (47.6) |
| Tumor size | |
| T1 | 8 (7.9) |
| T2 | 21 (20.8) |
| T3 | 55 (54.5) |
| T4 | 17 (16.8) |
| Nodal metastasis | |
| N0 | 33 (32.7) |
| N1 | 48 (47.5) |
| N2 | 13 (12.9) |
| N3 | 7 (6.9) |
| Stage | |
| I–III | 47 (46.1) |
| III | 54 (53.9) |
IHC analysis
The GC tissues were fixed in 10% buffered formalin,
embedded in paraffin and cut into 4-μm sections. The
sections were deparaffinized in xylene, rehydrated in a series of
descending ethanol concentrations and incubated in 0.03% hydrogen
peroxide for 10 min. Antigen retrieval was performed in 10 mM
sodium citrate buffer (pH 6.0) for 15 min. The tissue sections were
then incubated with an anti-EBP50 antibody (1:800 dilution,
PA5-17045; Thermo Scientific, Rockford, IL, USA) at room
temperature for 40 min. Following incubation, the specimens were
washed with 0.5% Tween, 0.1 M Tris-base, 0.9% NaCl, (TBS-T; pH 7.6)
and incubated with peroxidase- labeled polymer at room temperature
for 30 min. The samples were then washed with TBS-T buffer and
incubated with freshly prepared 3,3′-diaminobenzidine
tetrahydrochloride (DAB) and substrate-chromogen buffer at room
temperature for 8 min. Immunohistochemical reactions were developed
in freshly prepared DAB (DAB kit; Fujian Maixin Biological
Technology Ltd., Fujian, China) at room temperature for 8 min, then
lightly counterstained with hematoxylin prior to mounting.
QD fluorescence IHC
The specimen treatment by QD-IHC was similar to
conventional IHC. The QD-IHC assay was performed according to the
manufacturer’s instructions (Wuhan Jiayuan Quantum Dot Co., Ltd.,
Wuhan, China). Antigen retrieval was performed in citric acid (10
mM, pH 6.0) at 95°C for 10 min and the samples were cooled for 30
min. For the antibody binding, the specimens were first incubated
in a 2% bovine serum albumin buffer (Sigma, St. Louis, MO, USA) at
37°C for 30 min and then at 4°C overnight with poly-rabbit
anti-EBP50 antibodies (1:800 dilution, PA5-17045; Thermo
Scientific, Rockford, IL, USA). The specimens were then washed
three times with TBS-T for 5 min each wash and incubated in
biotinylated goat anti-rabbit or anti-mouse IgG (1:100 dilution,
Jackson ImmunoResearch, West Grove, PA, USA) at 37°C for 30
min.
For the QD conjugation, the antibody-bound specimens
were incubated in 2% BSA buffer again at 37°C for 10 min, then
incubated with QDs conjugated to streptavidin (QD-SA; 1:200
dilution in 2% BSA; Wuhan Jiayuan Quantum Dot Co., Ltd.) at 37°C
for 30 min, washed three times with TBS-T for 5 min and finally
sealed with 90% glycerin (Sigma).
The QD signal was detected with an Olympus BX51
fluorescence microscope equipped with an Olympus Micro DP 72
camera. The signal was red, target-specific, bright and
photo-stable. The images for each specimen were analyzed using the
WuDa Image Analysis System 2003, a multifunctional pathology
analysis software package developed by two of the present study
authors (16–18). For further quantification and
statistical analysis, the numerical calculations for the two key
variables in EBP50 detection, the fluorescence intensity and the
distribution area, were based on spectral unmixing and the QDs were
obtained as the final results.
Scoring of QD-IHC and IHC
Using a 400X objective lens, ≥100 cells were
randomly selected and counted from five representative fields of
each core by two independent observers blinded to the samples
identities. The scoring of the QD-IHC and IHC was based on the
percentage of positive levels as follows: No staining or weak
staining in <10% of the tumor cells (0); weak staining in
>10% of the tumor cells (1+); complete staining of the membrane
with weak or moderate intensity in >10% of the tumor cells (2+);
and marked staining in >10% of the tumor cells (3+) (19).
Statistical analysis
All statistical analysis was performed using SPSS
software, Version 13.0 (SPSS Inc., Chicago, IL, USA). The
differences between the two groups, based on the
clinicopathological factors, were statistically analyzed using the
Student’s t-test and the Chi-squared test. The survival rates were
calculated according to the Kaplan-Meier method, using a log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Detection of EBP50 expression by IHC
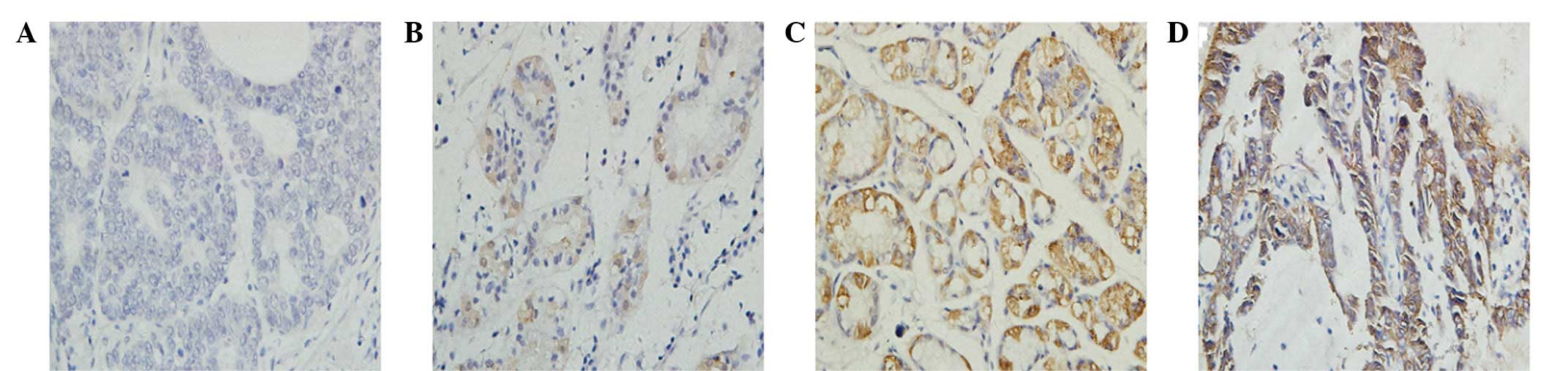
The EBP50 protein was overexpressed in the majority
of gastric carcinoma tissues (63/101, 62.4%), of these, 37 tissue
samples (36.6%) were scored as 1+, 16 (15.8%) were scored as 2+ and
10 (9.9%) were scored as 3+ (Fig.
1). The expression of EBP50 detected by IHC was associated with
tumor size and the male gender (P<0.05). The expression of EBP50
in the patients with GC increased with the tumor stage and was
highest in the male patients (Table
II).
 | Table IICorrelations between EBP50 protein
expression and the clinical significance of 101 cases of GC. |
Table II
Correlations between EBP50 protein
expression and the clinical significance of 101 cases of GC.
| IHC assessment of
EBP50 | QD score of
EBP50 |
|---|
|
|
|---|
| Tested group | Negative | Positive | Total | P-value | Number | QD (X±SD) | P-value |
|---|
| Age, years | | | | | | | |
| ≤50 | 10 | 14 | 24 | 0.640 | 24 | 11.810±3.937 | 0.456 |
| >50 | 28 | 49 | 77 | | 77 | 13.250±3.407 | |
| Gender | | | | | | | |
| Male | 21 | 51 | 72 | 0.006 | 72 | 13.477±6.355 | 0.933 |
| Female | 17 | 12 | 29 | | 29 | 9.433±5.687 | |
| Differentiation | | | | | | | |
| Well | 6 | 10 | 16 | 0.916 | 16 | 26.446±6.675 | 0.148 |
| Moderate | 13 | 24 | 37 | | 37 | 11.633±4.846 | |
|
Undifferentiated | 19 | 29 | 48 | | 48 | 36.135±11.039 | |
| Tumor size | | | | | | | |
| T1 | 6 | 2 | 8 | 0.020 | 8 | 33.708±17.851 | 0.029 |
|
T2 | 9 | 12 | 21 | | 21 | 6.548±4.192 | |
|
T3 | 21 | 34 | 55 | | 55 | 28.210±7.202 | |
|
T4 | 2 | 15 | 17 | | 17 | 41.935±8.167 | |
| Nodal
metastasis | | | | | | | |
|
N0 | 18 | 15 | 33 | 0.099 | 33 | 28.747±4.021 | 0.717 |
|
N1 | 15 | 33 | 48 | | 48 | 20.057±8.102 | |
|
N2 | 3 | 10 | 13 | | 13 | 29.271±10.734 | |
|
N3 | 2 | 5 | 7 | | 7 | 25.645±15.087 | |
| Stage | | | | | | | |
| I–III | 35 | 12 | 47 | 0.537 | 47 | 28.467±9.127 | 0.918 |
| III | 43 | 11 | 54 | | 54 | 25.941±15.540 | |
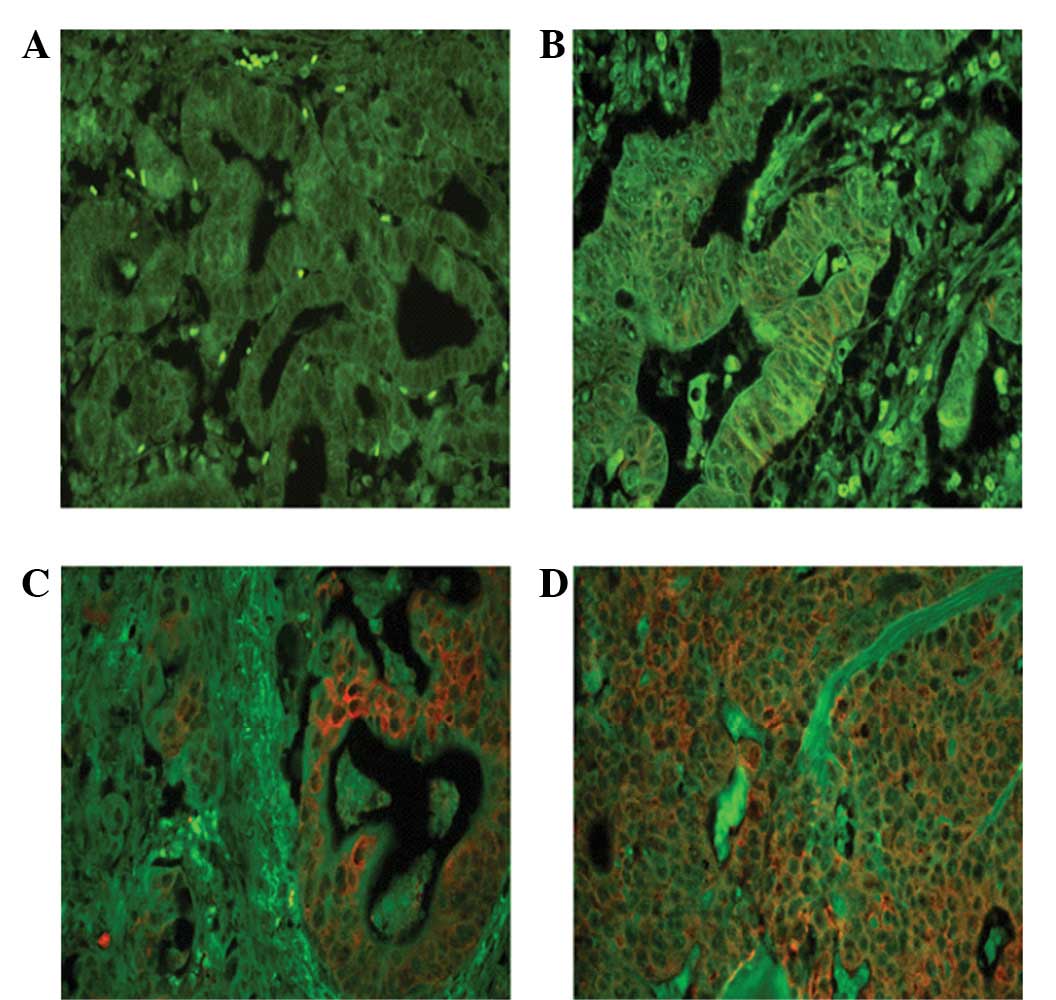
Detection of EBP50 expression by QD
analysis
As shown in Fig. 2,
the bright-red QD fluorescence specifically labeled tumor cells
without nonspecific binding. The green background was from tissue
autofluorescence. The score of the QDs was associated with the
tumor size of the gastric carcinoma (P<0.05; Table II).
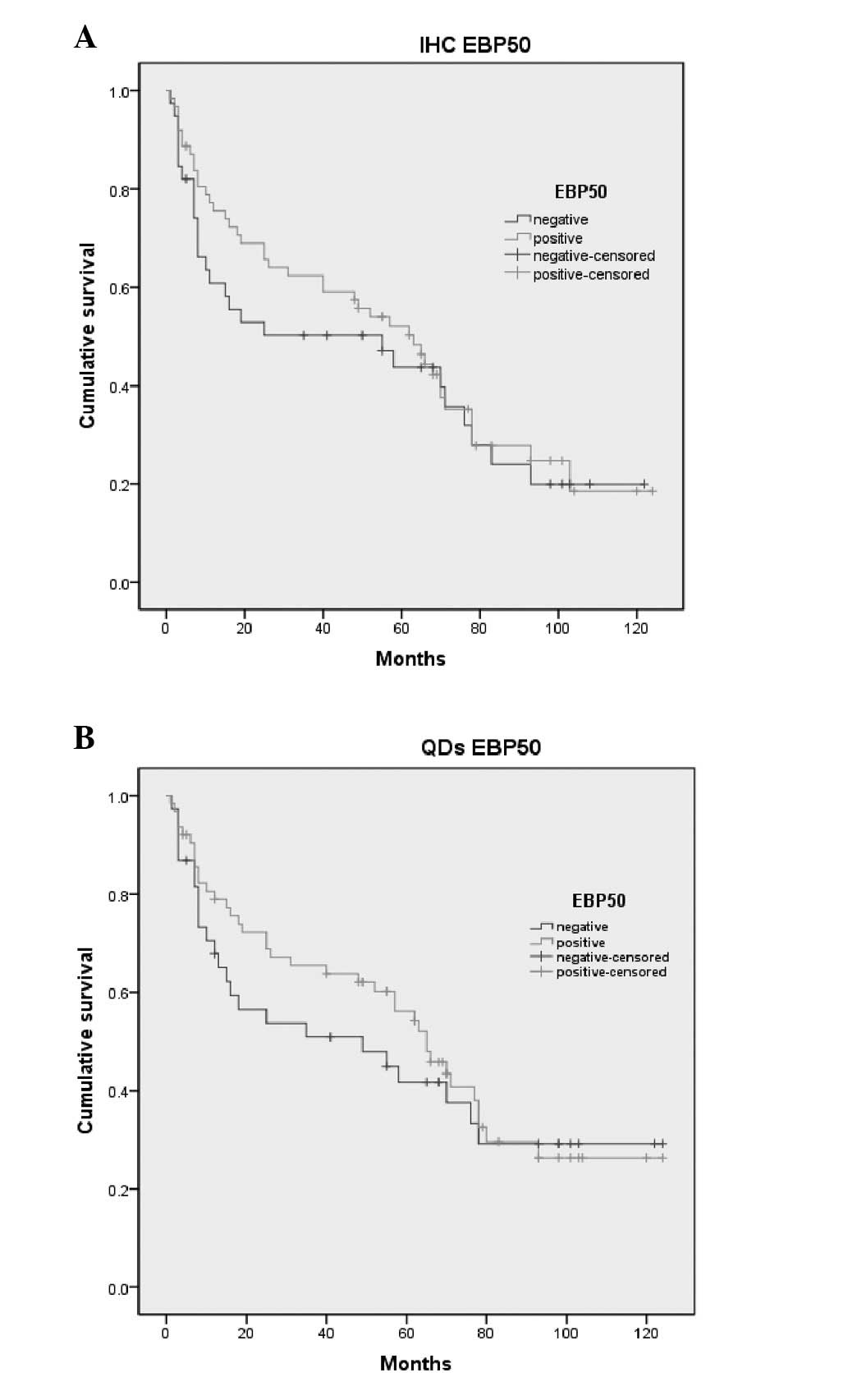
Survival analysis
A survival analysis was performed on 101 patients
who had survived for more than one month post-surgery. A total of
38 patients succumbed to GC within 124 months, while 63 had
survived survived to January 1, 2012. The survival curves created
according to the IHC and QD results of the EBP50 expression are
shown in Fig. 3. The association
between the expression of EBP50 and the mean survival rates was
assessed by the Kaplan-Meier method. The survival rates with
overexpression of EBP50 was higher than the negative one, however
as P>0.05, there was no association between the survival rates
and expression of EBP50. (IHC, 50.5 vs. 58.1 months, P>0.05; QD,
55.4 vs. 63.2 months, P>0.05; Fig.
3).
Discussion
EBP50 may play an essential role in carcinogenesis,
including that of breast cancer, colorectal cancer and hepatocellar
carcinoma. The present study demonstrated that EBP50 is
overexpressed in GC and that it is a novel marker of GC, as
observed in previous studies (20–22).
The expression of EBP50 was also observed to be correlated with the
male gender and the tumor stage. In the present study, the bright
red QD fluorescence specifically labeled the GC cell membranes
without non-specific binding. Previous studies have demonstrated
that EBP50 is important in cancer cell proliferation, invasion and
metastasis. Therefore, we hypothesized that the EBP50 protein
expression level may be correlated with the prognosis of GC.
However, no significant correlation was observed between the
expression of EBP50 and the overall survival rate of the GC
patients. The observable differences in these studies may be a
result of the usage of varying antibodies and scoring systems or,
alternatively, may reflect the heterogeneity of GC between the
various ethnicities. Gastric tumorigenesis is a multistep process
that is initiated by benign and atypical hyperproliferation, is
established as in situ carcinoma, progresses into invasive
carcinoma and culminates in metastatic disease (1,20–21).
However, the progression from in situ to invasive carcinoma
is poorly understood. It is important to identify additional
promising tumor markers to improve screening strategies for GC. In
the present study, EBP50 immunoreactivity was significantly
associated with the male gender and tumor invasion (T stage). These
results suggest that EBP50 expression is associated with several
malignant clinicopathological features of GC, although it is not a
valuable predictor for the prognosis of GC patients.
Immunofluorescence labeling is a standard technique
that is widely used in the biomedical field for the detection of
biological macromolecules in tissue sections. However, at present,
the available fluorescent labels are not stable and become
irreversibly photobleached under high-intensity illumination. We
used a validated QD-IHC protocol for quantifying EBP50 expression
in formalin-fixed, paraffin- embedded specimens, which provided an
accurate, sensitive and convenient approach. In the present study,
605-nm QD-SA conjugated probes were used for the detection of EBP50
expression. These probes have several advantages that are
prerequisites for clinical application (22,23).
First, the probes are more stable than QDs conjugated to
antibodies. Second, the biotin-avidin staining system is commonly
used in molecular pathology and is highly sensitive, therefore,
QD-SA probes may be incorporated into the current detection system
easily and conveniently.
In conclusion, the overexpression of EBP50 is
correlated with the male gender and with tumor size in GC, but not
with the patient survival time. Additionally, QD-IHC is as good as
IHC for the detection of tumor markers when considering the
accuracy of detecting the protein location, the photostability and
the duration of the fluorescence lifetime.
References
|
1
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar
|
|
2
|
Kodera Y, Yamamura Y, Torii A, Uesaka K,
Hirai T, Yasui K, et al: The prognostic value of preoperative serum
levels of CEA and CA19-9 in patients with gastric cancer. Am J
Gastroenterol. 91:49–53. 1996.PubMed/NCBI
|
|
3
|
Tocchi A, Costa G, Lepre L, Liotta G,
Mazzoni G, Cianetti A and Vannini P: The role of serum and gastric
juice levels of carcinoembryonic antigen, CA19.9 and CA72.4 in
patients with gastric cancer. J Cancer Res Clin Oncol. 124:450–455.
1998. View Article : Google Scholar
|
|
4
|
Hummel R, Hussey DJ and Haier J:
MicroRNAs: predictors and modifiers of chemo- and radiotherapy in
different tumour types. Eur J Cancer. 46:298–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamao T, Kai S, Kazami A, Koizumi K, Handa
T, Takemoto N and Maruyama M: Tumor markers CEA, CA19-9 and CA125
in monitoring of response to systemic chemotherapy in patients with
advanced gastric cancer. Jpn J Clin Oncol. 29:550–555. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Macdonald JS, Smalley SR, Benedetti J,
Hundahl SA, Estes NC, Stemmermann GN, et al: Chemoradiotherapy
after surgery compared with surgery alone for adenocarcinoma of the
stomach or gastroesophageal junction. N Engl J Med. 345:725–730.
2001. View Article : Google Scholar
|
|
7
|
Ichikawa D, Koike H, Ikoma H, Ikoma D,
Tani N, Otsuji E, et al: Detection of aberrant methylation as a
tumor marker in serum of patients with gastric cancer. Anticancer
Res. 24:2477–2481. 2004.PubMed/NCBI
|
|
8
|
Tani N, Ichikawa D, Ikoma D, Tomita H, Sai
S, Ikoma H, et al: Circulating cell-free mRNA in plasma as a tumor
marker for patients with primary and recurrent gastric cancer.
Anticancer Res. 27:1207–1212. 2007.PubMed/NCBI
|
|
9
|
Li N, Zhang J, Liang Y, Shao J, Peng F,
Sun M, et al: A controversial tumor marker: is SM22 a proper
biomarker for gastric cancer cells? J Proteome Res. 6:3304–3312.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shibata T, Chuma M, Kokubu A, Sakamoto M
and Hirohashi S: EBP50, a beta-catenin-associating protein,
enhances Wnt signaling and is over-expressed in hepatocellular
carcinoma. Hepatology. 38:178–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song J, Bai J, Yang W, Gabrielson EW, Chan
DW and Zhang Z: Expression and clinicopathological significance of
oestrogen-responsive ezrin-radixin-moesin-binding phosphoprotein 50
in breast cancer. Histopathology. 51:40–53. 2007. View Article : Google Scholar
|
|
12
|
Zheng JF, Sun LC, Liu H, Huang Y, Li Y and
He J: EBP50 exerts tumor suppressor activity by promoting cell
apoptosis and retarding extracellular signal-regulated kinase
activity. Amino Acids. 38:1261–1268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bellizzi A, Mangia A, Malfettone A,
Cardone RA, Simone G, Reshkin SJ and Paradiso A: Na+/H+ exchanger
regulatory factor 1 expression levels in blood and tissue predict
breast tumour clinical behaviour. Histopathology. 58:1086–1095.
2011.
|
|
14
|
Ingraffea J, Reczek D and Bretscher A:
Distinct cell type-specific expression of scaffolding proteins
EBP50 and E3KARP: EBP50 is generally expressed with ezrin in
specific epithelia, whereas E3KARP is not. Eur J Cell Biol.
81:61–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sobin LH and Fleming ID: TNM
Classification of Malignant Tumors, fifth edition (1997). Union
Internationale Contre le Cancer and the American Joint Committee on
Cancer. Cancer. 80:1803–1804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Y, Wu Q, Liu S, Wei L, Chen X, Yan Z,
et al: Study of rice pollen grains by multispectral imaging
microscopy. Microsc Res Tech. 68:335–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo N, Zeng L and Wu Q: A method based on
multispectral imaging technique for white blood cell segmentation.
Comput Biol Med. 37:70–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dickinson ME, Bearman G, Tille S, Lansford
R and Fraser SE: Multi-spectral imaging and linear unmixing add a
whole new dimension to laser scanning fluorescence microscopy.
Biotechniques. 31:12721274–1276. 12782001.PubMed/NCBI
|
|
19
|
Chen H, Xue J, Zhang Y, Zhu X, Gao J and
Yu B: Comparison of quantum dots immunofluorescence histochemistry
and conventional immunohistochemistry for the detection of
caveolin-1 and PCNA in the lung cancer tissue microarray. J Mol
Histol. 40:261–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao W, Feng D, Bian W, et al: EBP50
inhibits EGF-induced breast cancer cell proliferation by blocking
EGFR phosphorylation. Amino Acids. 43:2027–2035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kislin KL, McDonough WS, Eschbacher JM, et
al: NHERF-1: modulator of glioblastoma cell migration and invasion.
Neoplasia. 11:377–387. 2009.PubMed/NCBI
|
|
22
|
Malfettone A, Saponaro C, Paradiso A, et
al: Peritumoral vascular invasion and NHERF1 expression define an
immunophenotype of grade 2 invasive breast cancer associated with
poor prognosis. BMC Cancer. 12:1062012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bornschein J and Malfertheiner P: Gastric
carcinogenesis. Langenbecks Arch Surg. 396:729–742. 2011.
View Article : Google Scholar
|
|
24
|
Kawachi T, Kurisu M, Numanyu N, Sasajima K
and Sano T: Precancerous changes in the stomach. Cancer Res.
36:2673–2677. 1976.PubMed/NCBI
|
|
25
|
Muthu MS, Kulkarni SA, Raju A and Feng SS:
Theranostic liposomes of TPGS coating for targeted co-delivery of
docetaxel and quantum dots. Biomaterials. 33:3494–3501. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shao L, Gao Y and Yan F: Semiconductor
quantum dots for biomedicial applications. Sensors (Basel).
11:11736–11751. 2011. View Article : Google Scholar : PubMed/NCBI
|