Introduction
Colon cancer is the third most common cancer in the
world. The primary treatment of colon cancer is to surgically
remove part of or the whole of the colon. Chemotherapy is an
important supplementary approach that is capable of prolonging
survival for individuals whose cancer has spread to other organs
(1,2). Targeted chemotherapy specifically
kills tumor cells with minimal toxicity to normal cells.
Tissue factor (TF) is a transmembrane receptor that
is overexpressed in angiogenic tumor vascular endothelial cells and
numerous types of cancer cells, including solid tumors and leukemia
(3). The TF levels of cancer cells
are ~1,000-fold greater than those of their normal counterparts.
This overexpression is also observed in clinical samples of
numerous types of human cancer, with only a few exceptions (e.g.
renal cancer) (4). Therefore, TF is
an ideal target for cancer therapy. Factor VII (FVII) is the
natural ligand of TF and binds TF with exceptionally high
specificity and affinity (~10−12 M) (5). Therefore, FVII may be used as a
targeting vehicle to develop a targeted therapeutic agent.
Several TF-targeting therapeutic agents have been
tested for the treatment of cancer and non-cancerous diseases. Hu
et al developed the first TF-targeting agent, the
antibody-like FVII-targeted Icon (FVII/IgG1 Fc), for cancer
immunotherapy in 1999 (6). In the
following years, Icon immunotherapy was tested for the eradication
of the pathological neovasculature in other tumors (7–9). Hu
et al also developed two FVII-targeted photodynamic
therapeutics for breast cancer by conjugating Sn(IV) chlorine e6
(SnCe6) (10) or verteporfin
(3) to FVII. Similarly, Shoji et
al reported the use of recombinant human FVII as a carrier for
the targeted delivery of a potent synthetic curcumin analog (EF24)
to TF-expressing tumor cells (11).
In order to reduce the coagulation activity of
FVII-targeted therapeutics, the serine protease activity of FVII
was inactivated by biological or chemical methods in the previous
studies. These methods have the following drawbacks: i) The risk of
the coagulation cascade reaction initiated by FVII-targeted
therapeutics may not be completely excluded; ii) the production
cost of the therapeutics is expensive; and iii) the high molecular
weight of the therapeutics negatively affects their ability to
penetrate into tumors.
Activated FVII is composed of a 20-kDa
amino-terminal light chain and a 30-kDa carboxy-terminal heavy
chain, which are linked by a disulfide bond (12). The light chain binds to TF and the
heavy chain initiates the blood coagulation pathway (13,14).
We previously demonstrated that the light chain of FVII was able to
effectively bind to TF (15). In
the present study, the light chain of mouse FVII (mlFVII) was
selected as the targeting vehicle. The single-chain mlFVII molecule
is significantly smaller than the parental two-chain FVII molecule,
which should provide two therapeutic advantages: i) Facilitating
access of the mlFVII-targeting therapeutics to solid tumors
(16); and ii) completely
preventing blood clots that may otherwise occur when the
two-chained FVII molecule binds to TF (14).
The cytotoxic molecule selected was lidamycin (LDM),
a member of the enediyne antibiotic family derived from
Streptomyces globisporus C1027 (17). The LDM molecule is composed of an
843-Da enediyne chromophore (AE), which binds DNA in the minor
groove and causes double-stranded DNA breaks and tumor cell death,
and a 10.5-kDa apoprotein of LDM (LDP) that forms a hydrophobic
pocket protecting AE (18). LDM is
cytotoxic to cultured tumor cells (19).
In the present study, an energized fusion protein,
mlFVII-LDP-AE, was constructed containing mlFVII as the targeting
domain and LDM (LDP-AE) as the effector domain. The results of the
study showed that the energized fusion protein was able to inhibit
not only the growth, but also the metastasis of mouse colon
cancer.
Materials and methods
Cell culture
The human liver cancer lines HepG2 and 7721, human
lung cancer lines A549 and NCI-H292, human colorectal cancer lines
HCT-116, human skin fibroblast BJ and human embryonic kidney
(HEK)293 cells were cultured in Dulbecco’s modified Eagle’s medium.
Human breast cancer MCF-7 cells were cultured in Eagle’s minimum
essential medium. Human breast cancer MDA-MB-231 cells were
cultured in Leibovitz’s L-15 medium. Cells from mouse colon cancer
line C26, mouse melanoma line B16F10 and mouse prostate cancer line
RM-1 were cultured in RPMI-1640 medium. All media were supplemented
with 10% fetal bovine serum (FBS) when used. All media and FBS were
purchased from GIBCO (Carlsbad, CA, USA).
Construction of expression plasmid
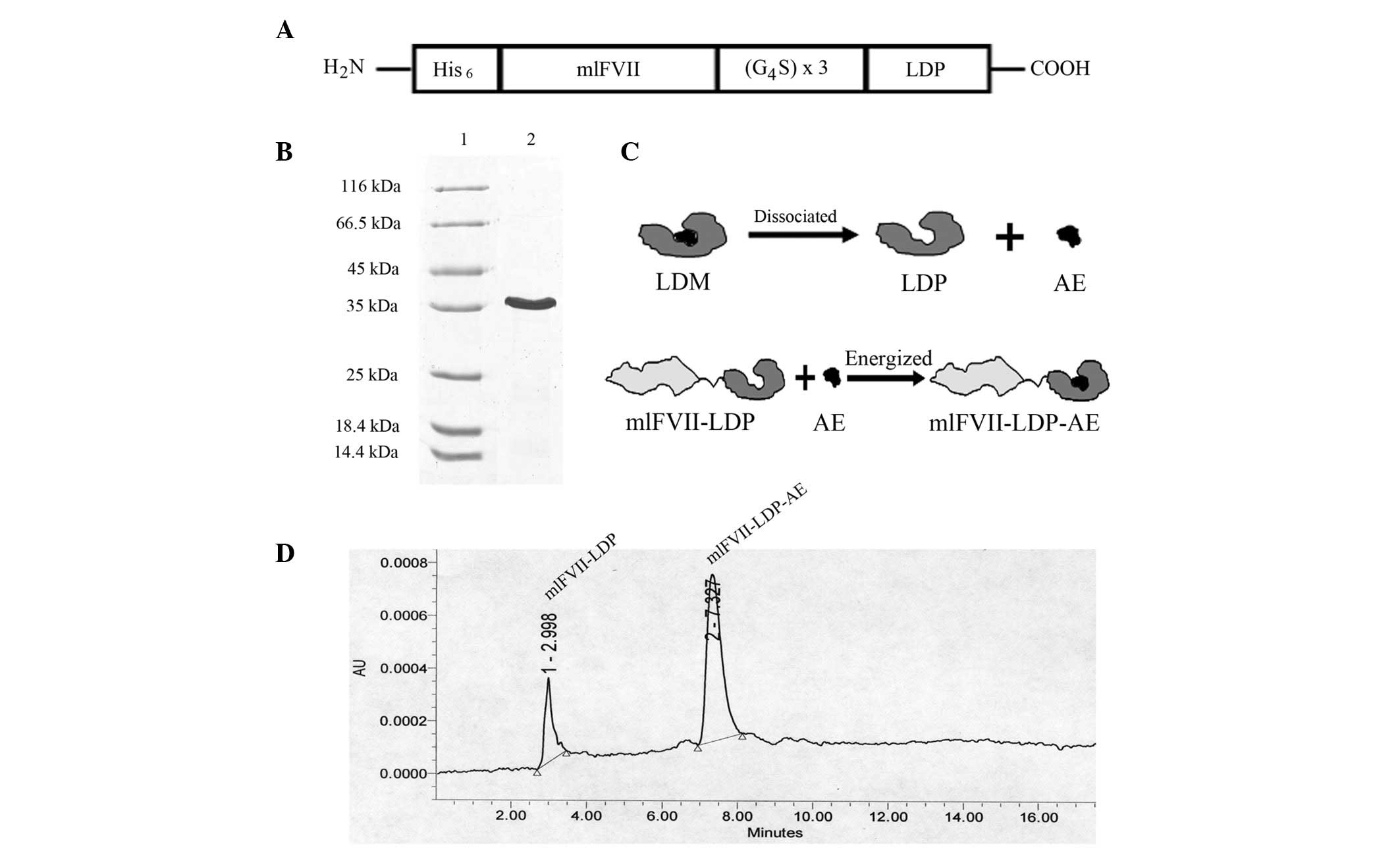
A diagram of the recombinant protein, mlFVII-LDP, is
shown in Fig. 1A. The expression
plasmid of mlFVII-LDP was constructed by conventional molecular
cloning techniques. The DNA fragment encoding mlFVII (152 amino
acids) was cloned from the plasmid pcDNA3.1-mFVII/hFc (6). The coding sequence for LDP was cloned
from the plasmid pET-VH-LDP (18).
The recombinant DNA fragment encoding the fusion protein was
spliced by overlap extension PCR. mlFVII and LDP were linked by a
flexible peptide. A 6xHis tag sequence was added to the N-terminal
of the recombinant protein. The recombinant DNA fragment was
inserted into the expression vector pET-19b (Novagen, Darmstadt,
Germany).
Expression and purification of
recombinant proteins
The recombinant protein, mlFVII-LDP, was expressed
and purified as described previously (15,19).
The recombinant plasmid was transformed into Rosetta-gami B (DE3)
pLysS competent cells (Novagen). A single colony was inoculated
into 5 ml LB medium (pH 7.5) containing 100 μg/ml ampicillin, 34
μg/ml chloramphenicol, 15 μg/ml kanamycin and 12.5 μg/ml
tetracycline and cultured overnight at 37ºC. The next day, 1 liter
of culture medium was inoculated with the overnight culture and
incubated with agitation at 37ºC until A600 = 0.6–1.
Isopropyl-β-D-1-thiogalactopyranosid (IPTG) was added to the medium
to a final concentration of 0.8 mM. Subsequent to being induced for
6 h at 37ºC, the bacteria were lysed using pulse sonication
followed by 60 min centrifugation at 48,400 × g. His-tagged
recombinant protein was purified by affinity chromatography
(HisTrap HP; GE Healthcare, Little Chalfont, UK) and ion exchange
chromatography (HiTrap Q HP; GE Healthcare) according to the
manufacturer’s instructions.
Preparation of energized fusion protein
hlFVII-LDP-AE
The energized fusion protein hlFVII-LDP-AE was
prepared as described previously (15). In brief, the active AE of LDM was
separated using a C4 column (GE Healthcare) with a 22% acetonitrile
in 0.05% trifluoroacetic acid mobile phase. The AE-containing
solution was added to mlFVII-LDP/phosphate-buffered saline (PBS; 10
mM, pH 7.0) with a molecular ratio of 3:1 and was incubated at 4ºC
for 12 h with rocking. Free AE was removed with a Sephadex G-75
column (GE Healthcare). The assembled energized fusion protein was
confirmed by reverse-phase high-performance liquid chromatography
(HPLC) using a Vydac C4 300A column (Grace, Deerfield, IL, USA).
Absorbance was measured at 350 nm.
Western blot analysis
The cells were cultured in six-well plates. When the
cells were 80–90% confluent, they were washed twice with PBS.
Subsequently, 1 ml ice-cold PBS was added to the wells. The cells
were scraped down and collected by centrifugation at 800 × g for 5
min, then 300 μl ice-cold lysis buffer (20 mM Tris-HCl, 150 mM
NaCl, 10 mM KCl, 0.5 mM EDTA, 1.5 mM MgCl2, 0.5 mM PMSF,
2 mM DTT, 2.5 mM CaCl2, 0.5% NP-40 and 10% glycerol; pH
7.9) was added and the cells were incubated on ice. The cell
suspensions were vortexed five times with 2 min intervals. Lysates
were centrifuged at 16,000 × g at 4ºC for 5 min. The lysate
supernatants were resolved with a 12% SDS-PAGE gel and detected
using a mouse anti-human TF antibody (MAB23391, R&D Systems,
Minneapolis, MN, USA). β-actin was also detected as a control.
Fluorescence-activated cell sorting
(FACS) analysis
The cells (1×106) were incubated with a
goat anti-mouse TF antibody (AF3178; R&D Systems) in 1 ml PBS
for 30 min at room temperature. The cells were washed twice with
PBS and incubated with a mouse anti-goat IgG antibody conjugated to
FITC (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA;
sc-53800) for 30 min at room temperature. The cells were then
washed, resuspended and evaluated with a FACS machine (BD
Biosciences, San Diego, CA, USA). Matched isotype control antibody
was used in all analyses. For analysis, the relative log
fluorescence of live cells was determined using a FACScan flow
cytometer with CellQuest software (BD Biosciences).
Tumor xenograft growth inhibition in
vivo
Mouse colon cancer C26 cells in the exponential
growth phase were dissociated with 5 mM EDTA in PBS, centrifuged,
washed and resuspended in PBS. Subsequently, six-week-old female
BALB/c mice (Institute of Laboratory Animal Sciences, Chinese
Academy of Medical Sciences, Shanghai, China) were injected
subcutaneously (s.c.) into the left armpit with 5×106
C26 cells per mouse in 100 μl of PBS. This study was approved by
the ethics committee of Sichuan University (Chengdu, China). At
three weeks post-injection, tumors were aseptically dissected and
sections of tumor tissue (2–6 mm3 in size) were
transplanted s.c. into the left armpit of the mice using a trocar.
After 10 days, the tumors were removed and the tumor cells were
resuspended with PBS (W:V=1:5).
On day 0, the tumor cell suspension was injected
s.c. into the left armpit of the mice at 200 μl per mouse. On day
3, the mice were divided into five groups (n=10). Four groups were
injected i.v. into the tail vein twice at days 3 and 10 with LDM
(0.05 mg/kg, tolerated dose) or mlFVII-LDP-AE (0.2, 0.4 and 0.8
mg/kg) in 200 μl PBS. The control group was injected with 200 μl
PBS. On day 14, the tumors were removed and weighed. The tumor
growth inhibition rate (TGIR) = 1 − tumor weight (treated)/tumor
weight (control) × 100. The mice were weighed on days 3 and 14.
Tumor metastasis inhibition in vivo
A mouse colon cancer C26 cell suspension was
prepared, as described previously. On day 0, the cell suspension
was inoculated into the spleens (caudal end) of the mice at 20 μl
per mouse. Any bleeding was stopped using thrombin. On day 3, the
mice were divided into five groups (n=10). Four of the groups were
injected i.v. into the tail vein twice, at days 3 and 10, with LDM
(0.05 mg/kg) or mlFVII-LDP-AE (0.2, 0.4 and 0.6 mg/kg) in 200 μl
PBS. The control group was injected with 200 μl PBS. On day 14, the
mice were dissected and the metastatic nodes on the liver surfaces
were counted. The tumor metastatic inhibition rate (TMIR) = 1 −
tumor weight (treated)/tumor weight (control) × 100. The mice were
weighed on days 3 and 14.
Statistical analysis
Data are expressed as the mean ± SD in all cases.
Comparisons of the mean values between the different treatments
were performed using the two-way Independent-Sample t-test with
Excel 2003 software (Microsoft, Redmond, WA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Construction and preparation of
mlFVII-LDP-AE
Recombinant DNA encoding the protein mlFVII-LDP was
cloned and inserted into pET-19b expression vectors. After
induction and purification by Ni2+ affinity
chromatography and ion exchange chromatography, the fusion protein
was detected by SDS-PAGE (Fig. 1B).
The energized fusion protein, mlFVII-LDP-AE, was prepared by
molecular reconstitution with AE and mlFVII-LDP (Fig. 1C). Data from reverse-phase HPLC
showed that the AE molecule was integrated into mlFVII-LDP
successfully and the purity of hlFVII-LDP-AE was 78.6% (Fig. 1D).
Expression of TF in human and mouse cell
lines
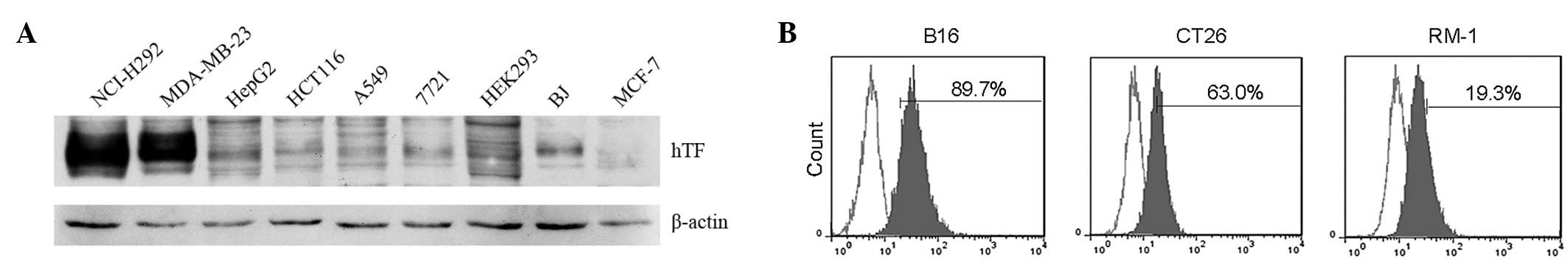
The expression of human TF (hTF) in human cell lines
was detected by western blotting. As shown in Fig. 2A, hTF has a high level of expression
in the MDA-MB-231 breast cancer and NCI-H292 lung cancer cells and
almost no expression in the HEK293 and MCF-7 breast cancer cells.
The expression of mouse TF (mTF) was then detected in mouse cancer
cell lines by FACS analysis. As shown in Fig. 2B, mTF has evidently high expression
in melanoma B16F10 and colon cancer C26 cells. The mouse colon
cancer C26 cell line was selected as the model cell line to study
the therapeutic efficacy of mlFVII-LDP-AE for colon cancer in the
present study.
Tumor growth inhibition of mlFVII-LDP-AE
in vivo
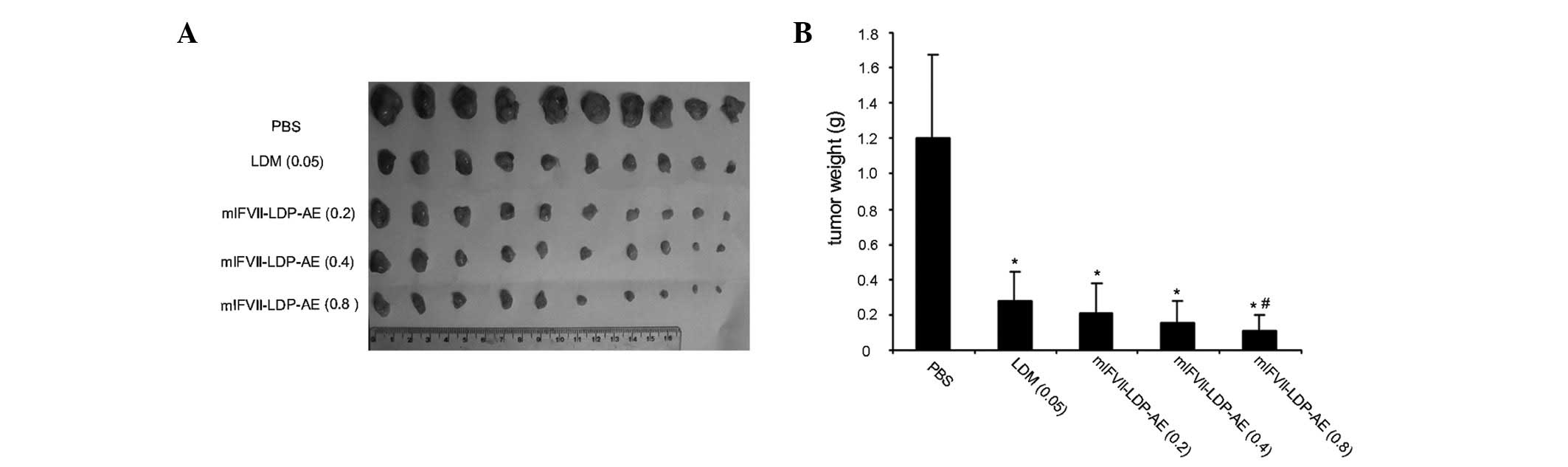
The tumor growth inhibition effects of mlFVII-LDP-AE
in vivo were tested in a BALB/c mouse xenograft model using
mouse colon cancer C26 cells. At the end of the experiment, the
tumors were excised (Fig. 3A) and
weighed. As shown in Fig. 3B, free
LDM (0.05 mg/kg, tolerated dose) showed a 77% TGIR, while
mlFVII-LDP-AE at 0.2, 0.4 and 0.8 mg/kg increased the antitumor
effects to a TGIR of 82.5, 87.4 and 91.2%, respectively. The TGIRs
of mlFVII-LDP-AE at 0.2 and 0.4 mg/kg were not observed to be
significantly different compared with that of LDM at 0.05 mg/kg
(P>0.05). However, the TGIR of mlFVII-LDP-AE at 0.8 mg/kg
exhibited a significant difference compared with that of LDM
(P<0.05).
Tumor metastasis inhibition of
mlFVII-LDP-AE in vivo
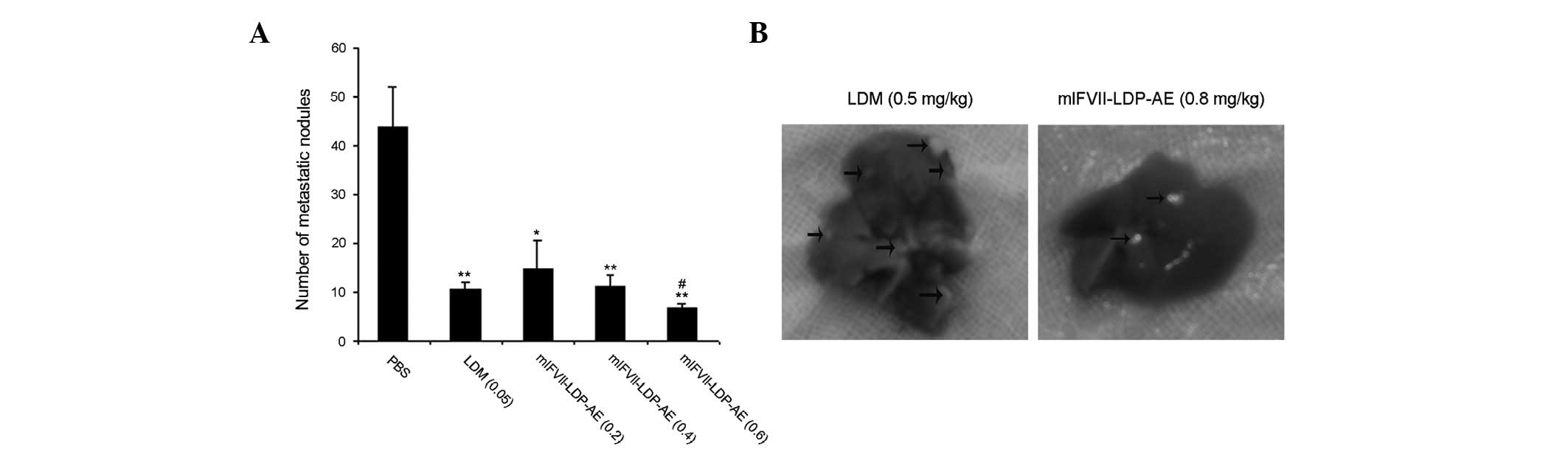
The tumor metastasis inhibition effects of
mlFVII-LDP-AE in vivo were tested with a liver metastasis
C26 mouse colon cancer model in BALB/c mice. At the end of the
experiment, the mice were dissected and the liver surface
metastatic nodes of the mice were counted (Fig. 4B). As shown in Fig. 4A, the TMIR of free LDM (0.05 mg/kg,
tolerated dose) was 76%, while that of mlFVII-LDP-AE at 0.2, 0.4
and 0.6 mg/kg was 66.9, 74.2 and 84.7%, respectively. The results
of the statistical analysis showed that the TMIR of mlFVII-LDP-AE
at 0.8 mg/kg was significantly different from that of LDM
(P<0.05).
Safety of mlFVII-LDP-AE for application
in vivo
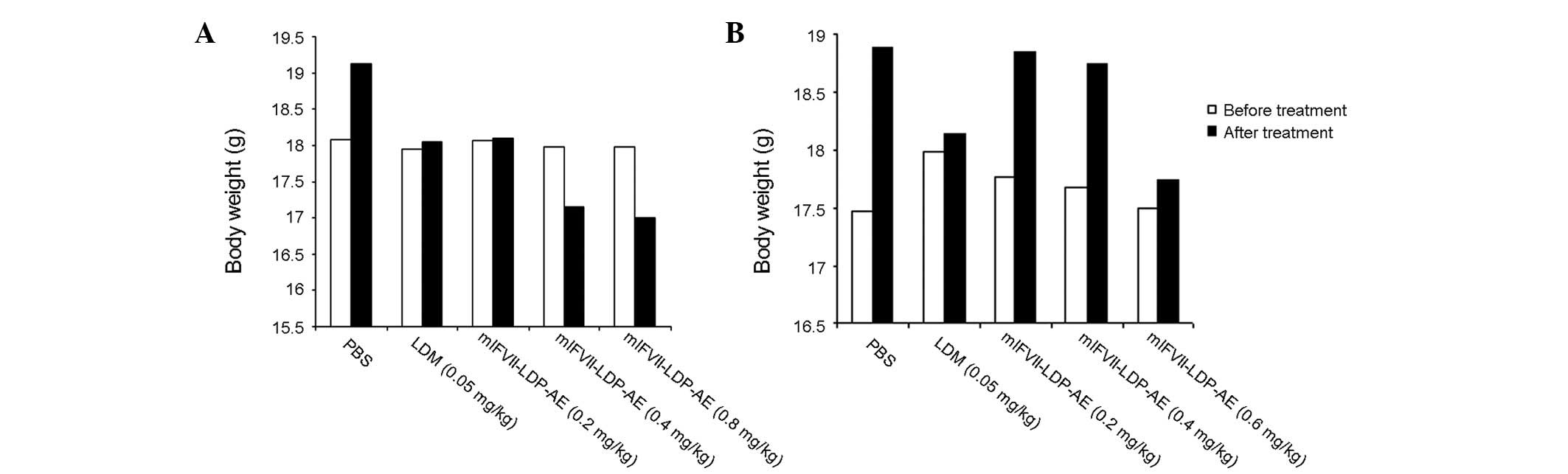
The body weight of the mice was monitored as a
systemic toxicity indicator of the drug administered. In the tumor
growth inhibition experiment, as shown in Fig. 5A, the body weights of the mice
treated with PBS, LDM and mlFVII-LDP-AE (0.2 mg/kg) increased by
5.4, 0.6 and 2.0%, respectively at the end of the experiment. The
body weights of the mice treated with mlFVII-LDP-AE at 0.4 and 0.8
mg/kg were decreased by 4.9 and 5.7%, respectively, at the end of
the experiment. In the tumor metastasis inhibition experiment, the
body weights of all the mice increased. The mice treated with
mlFVII-LDP-AE at 0.6 mg/kg showed a 1.5% increase, while the mice
treated with LDM only showed a 0.8% increase.
To summarize, the body weight loss caused by
mlFVII-LDP-AE did not exceed 10%. Therefore, the dosages of
mlFVII-LDP-AE used in the present study were tolerated (18).
Discussion
Under normal conditions, TF is expressed on the
extravascular cells of several normal tissues, as well as in the
adventitial layer of large blood vessel walls (20,21).
TF is anatomically sequestered from its natural ligand, FVII, and
TF-targeting therapeutic agents circulating in blood by the intact
semipermeable endothelial layer of normal blood vessels (22). Therefore, TF-targeting therapeutic
agents do not damage normal tissues. In cancer patients, TF is
overexpressed on angiogenic tumor vascular endothelial cells and
numerous types of cancer cells. TF-targeting therapeutic agents are
able to destroy tumor vessels by attacking tumor vascular
endothelial cells. Systemically administered TF-targeting
therapeutic agents also penetrate into tumor tissue and kill the
tumor cells through leaks in the tumor vasculature (23). Therefore, TF is a safe and effective
target for cancer therapy.
LDM is a member of the enediyne antibiotic family
derived from Streptomyces globisporus C1027. LDM exhibits
extremely potent cytotoxicity, antitumor activity and marked growth
inhibition against transplantable tumors in mice. In terms of the
half maximal inhibitory concentration (IC50) values, the
cytotoxicity of LDM has been shown to be 10,000-fold more potent
than those of mitomycin and doxorubicin (24). The maximal dose (tolerated dose) of
free LDM for mice is 0.05 mg/kg. In the present study, LDM was
linked with mlFVII to target TF for colon cancer therapy. The
therapeutic dose of mlFVII-LDP-AE was increased to 0.8 mg/kg. The
therapeutic efficacy was superior to that of LDM (0.05 mg/kg).
Therefore, mlFVII-LDP-AE is an efficient cancer targeting
therapeutic agent.
In conclusion, the present study reports for the
first time that an mlFVII-targeted LDM effectively inhibited the
growth and metastasis of mouse colon cancer. As human TF and FVII
have features similar to those of mice, hlFVII-LDP-AE may be
expected to have therapeutic potential for human colon cancers.
Acknowledgements
The present study was supported by the ‘Significant
new drugs development’ Science and Technology Major Projects of
China (No. 2009ZX09103-698), the Natural Science Foundation of
Jiangsu Province (BK2012146) and the Jiangsu Provincial Office of
Education Foundation (JHB2012-34).
References
|
1
|
Hong SW, Jung KH, Choi MJ, et al:
Anticancer effects of KI-10F: a novel compound affecting apoptosis,
angiogenesis and cell growth in colon cancer. Int J Oncol.
41:1715–1722. 2012.PubMed/NCBI
|
|
2
|
Amsterdam A, Raanan C, Schreiber L,
Freyhan O, Fabrikant Y and Melzer E: Use of multiple biomarkers for
the localization and characterization of colon cancer stem cells by
indirect immunocytochemistry. Int J Oncol. 41:285–291. 2012.
|
|
3
|
Hu Z, Rao B, Chen S and Duanmu J:
Targeting tissue factor on tumour cells and angiogenic vascular
endothelial cells by factor VII-targeted verteporfin photodynamic
therapy for breast cancer in vitro and in vivo in mice. BMC Cancer.
10:2352010. View Article : Google Scholar
|
|
4
|
Rak J, Milsom C, Magnus N and Yu J: Tissue
factor in tumour progression. Best Pract Res Clin Haematol.
22:71–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waxman E, Ross JB, Laue TM, et al: Tissue
factor and its extracellular soluble domain: the relationship
between intermolecular association with factor VIIa and enzymatic
activity of the complex. Biochemistry. 31:3998–4003. 1992.
View Article : Google Scholar
|
|
6
|
Hu Z, Sun Y and Garen A: Targeting tumor
vasculature endothelial cells and tumor cells for immunotherapy of
human melanoma in a mouse xenograft model. Proc Natl Acad Sci USA.
96:8161–8166. 1999. View Article : Google Scholar
|
|
7
|
Tang Y, Borgstrom P, Maynard J, et al:
Mapping of angiogenic markers for targeting of vectors to tumor
vascular endothelial cells. Cancer Gene Ther. 14:346–353. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu Z and Garen A: Targeting tissue factor
on tumor vascular endothelial cells and tumor cells for
immunotherapy in mouse models of prostatic cancer. Proc Natl Acad
Sci USA. 98:12180–12185. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Z and Garen A: Intratumoral injection
of adenoviral vectors encoding tumor-targeted immunoconjugates for
cancer immunotherapy. Proc Natl Acad Sci USA. 97:9221–9225. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu Z, Rao B, Chen S and Duanmu J:
Selective and effective killing of angiogenic vascular endothelial
cells and cancer cells by targeting tissue factor using a factor
VII-targeted photodynamic therapy for breast cancer. Breast Cancer
Res Treat. 126:589–600. 2011. View Article : Google Scholar
|
|
11
|
Shoji M, Sun A, Kisiel W, et al: Targeting
tissue factor-expressing tumor angiogenesis and tumors with EF24
conjugated to factor VIIa. J Drug Target. 16:185–197. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nemerson Y: Tissue factor and hemostasis.
Blood. 71:1–8. 1988.PubMed/NCBI
|
|
13
|
Ruf W, Kalnik MW, Lund-Hansen T and
Edgington TS: Characterization of factor VII association with
tissue factor in solution. High and low affinity calcium binding
sites in factor VII contribute to functionally distinct
interactions. J Biol Chem. 266:15719–15725. 1991.PubMed/NCBI
|
|
14
|
Toomey JR, Smith KJ and Stafford DW:
Localization of the human tissue factor recognition determinant of
human factor VIIa. J Biol Chem. 266:19198–19202. 1991.PubMed/NCBI
|
|
15
|
Zhang Q, Liu XJ, Hu L, et al: Factor VII
light chain-targeted lidamycin targets tissue factor-overexpressing
tumor cells for cancer therapy. Int J Mol Med. 29:409–415.
2012.PubMed/NCBI
|
|
16
|
Allen TM: Ligand-targeted therapeutics in
anticancer therapy. Nat Rev Cancer. 2:750–763. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He QY, Liang YY, Wang DS and Li DD:
Characteristics of mitotic cell death induced by enediyne
antibiotic lidamycin in human epithelial tumor cells. Int J Oncol.
20:261–266. 2002.PubMed/NCBI
|
|
18
|
Guo XF, Zhu XF, Shang Y, Zhang SH and Zhen
YS: A bispecific enediyne-energized fusion protein containing
ligand-based and antibody-based oligopeptides against epidermal
growth factor receptor and human epidermal growth factor receptor 2
shows potent antitumor activity. Clin Cancer Res. 16:2085–2094.
2010. View Article : Google Scholar
|
|
19
|
Zhang Q, Liu X, Xu S, et al: Factor VII
light chain-targeted lidamycin shows intensified therapeutic
efficacy for liver cancer. Cancer Biother Radiopharm. 27:384–391.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Drake TA, Morrissey JH and Edgington TS:
Selective cellular expression of tissue factor in human tissues.
Implications for disorders of hemostasis and thrombosis. Am J
Pathol. 134:1087–1097. 1989.PubMed/NCBI
|
|
21
|
Wilcox JN, Smith KM, Schwartz SM and
Gordon D: Localization of tissue factor in the normal vessel wall
and in the atherosclerotic plaque. Proc Natl Acad Sci USA.
86:2839–2843. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Z, Rao B, Chen S and Duanmu J:
Selective and effective killing of angiogenic vascular endothelial
cells and cancer cells by targeting tissue factor using a factor
VII-targeted photodynamic therapy for breast cancer. Breast Cancer
Res Treat. 126:589–600. 2011. View Article : Google Scholar
|
|
23
|
Senger DR, Galli SJ, Dvorak AM, Perruzzi
CA, Harvey VS and Dvorak HF: Tumor cells secrete a vascular
permeability factor that promotes accumulation of ascites fluid.
Science. 219:983–985. 1983. View Article : Google Scholar
|
|
24
|
Xin C, Ye S, Ming Y, et al: Efficient
inhibition of B-cell lymphoma xenografts with a novel recombinant
fusion protein: anti-CD20Fab-LDM. Gene Ther. 17:1234–1243. 2010.
View Article : Google Scholar : PubMed/NCBI
|