Introduction
Colorectal cancer is one of the leading causes of
cancer-associated mortality in the world. According to the linear
model of cancer initiation, proposed by Fearon and Vogelstein,
cancer is a disease that arises from multiple serial somatic
mutations (1). The characteristic
alterations to this model comprise mutations of the tumor
suppressor genes, including APC, TP53 and K-Ras. TP53 mutations
have been identified in >50% of human tumors. Since TP53 is a
tumor suppressor gene, loss of function mutations are the general
cause of abnormality (1).
The TP53 gene is known as the guardian of the genome
or the cellular gatekeeper. The gene contains 11 exons, which
encode 2.8 kb mRNA that is translated into a 53 kDa protein.
Following exposure to stress conditions, including hypoxia,
oncogene activation, DNA damage, nucleotide defects and viral
transformation, p53 is subjected to certain post-translational
modifications that regulate the subcellular localization and
stability of the protein (2–5). Under
these stress conditions, there are three cellular outcomes: i)
Repair mechanisms are prompted (5);
ii) if there is no way to avoid it, the cells undergo apoptosis or
cell cycle arrest (6–17); and iii) the cells defense mechanisms
are affected and the cells become cancerous. p53 may act as a key
downstream regulator for all the processes mentioned previously and
also for telomerase activity. p53 regulates the inactivation of the
catalytic subunit of telomerase, which is a reverse transcriptase
that is activated in cancer cells (18,19).
However, there are questions that require clarification with regard
to cell fate, since there is no clear mechanism or requirement as
to which possible outcome is preferred. In the present study, the
following two issues were examined: i) The preferred mechanism of
cell fate following 5 Gy γ-irradiation, depending on p53 expression
in p53 +/+ and p53 −/− HCT116 colon cancer cells; and ii) whether
the cell response is p53-associated or p53-independent in HCT116
colon cancer cells.
Materials and methods
Cell culture and irradiation
p53+/+ and p53−/− HCT116 colon cancer cell lines
were cultured in complete McCoy's 5A medium, consisting of 10%
fetal bovine serum, 1% penicillin/streptomycin and 1% L-Glutamine
at 37°C, in a humidified incubator containing 5% CO2.
Once 80–90% confluency was reached in T75 culture flasks, the cells
were treated with 5 Gy γ-irradiation (Co60-Dmax) and collected to
evaluate the cell cycle, apoptosis and telomerase activity.
Detection of apoptotic cells and analysis
of the cell cycle
Following irradiation, the trypsinized cells were
washed and collected by centrifugation. The cell numbers were
counted using a hemocytometer. RNase (Sigma, St. Louis, MO, USA)
and propidium iodide (Sigma) were added to the cells and mixed
using a vortex. Following a 20-min incubation period in the dark at
room temperature, the cells were filtered through a nylon mesh (37
μm) and evaluated using flow cytometry (EPICS XL MCL; Beckman
Coulter Inc., Brea, CA, USA). The ratios of the cells in the
G0/G1, S and/or G2/M phases and
the apoptotic cell numbers were evaluated by McCycle software
(Phoenix Flow System, San Diego, CA, USA) using dichotomous
variable DNA histograms.
Telomerase activity
Protein lysates were prepared from a CHAPS lysis
buffer and quantified using the Bradford method. In order to detect
telomerase activity, the TRAPeze XL Telomerase Detection kit
(Chemicon, Temecula, CA, USA) was used.
Statistical analysis
The statistical analyses were performed using SPSS
software version 13 (SPSS, Inc., Chicago, IL, USA). The variables
were investigated using visual (histograms and probability plots)
and analytical (Kolmogorov-Simirnov/Shapiro-Wilk's test) methods to
determine whether or not they were normally distributed. The
χ2 test was used to statistically analyze the cell cycle
and apoptosis. The telomerase activity was evaluated using a
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Apoptosis
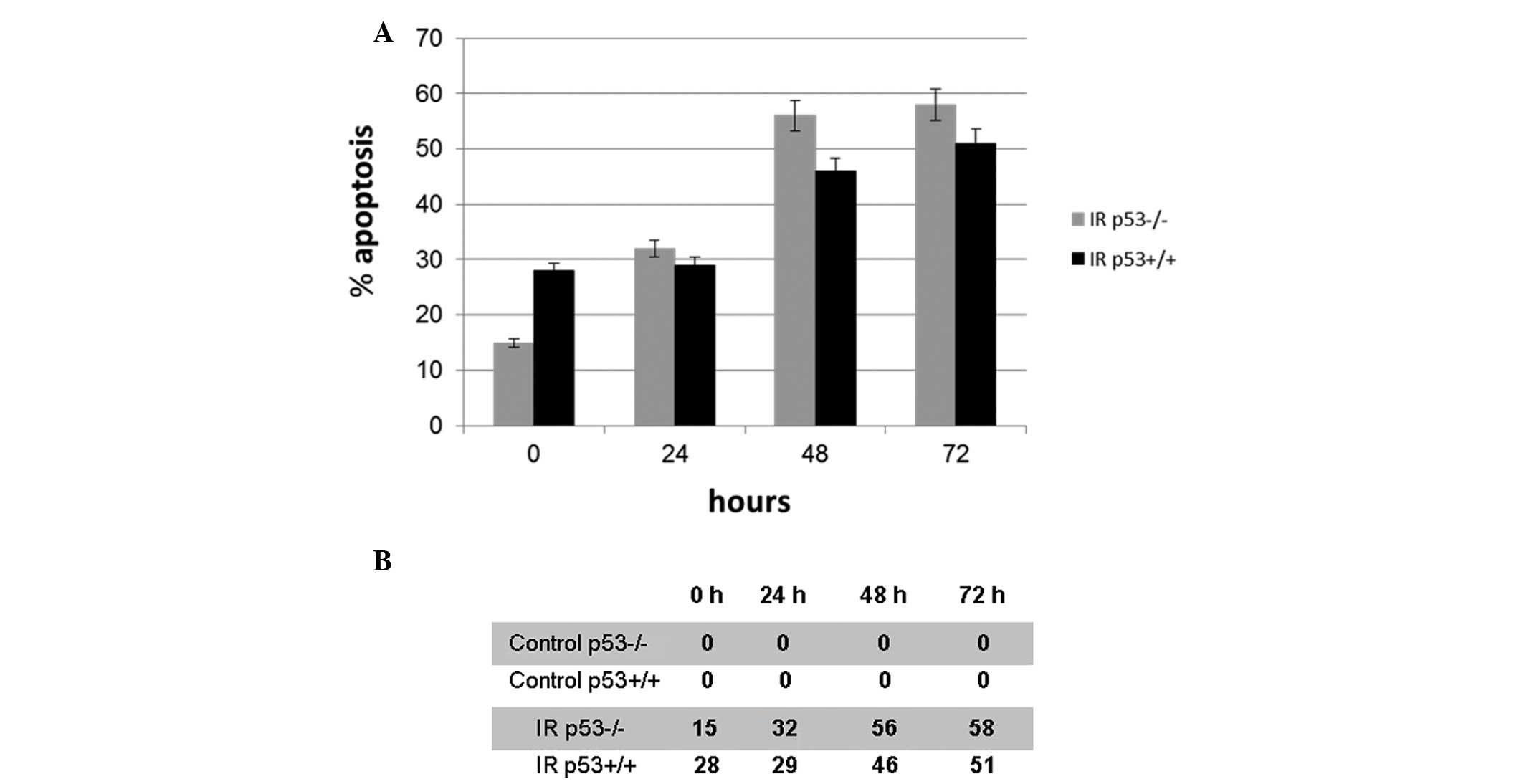
The apoptotic ratios of the cells following exposure
to irradiation were evaluated using the sub-G0 DNA
content. Following treatment with 5 Gy γ-irradiation, the average
apoptotic percentages of cell number significantly increased in a
time-dependent manner in the p53+/+ and p53−/− cells (P<0.05),
whereas there was no change in the apoptotic cell number in the
non-irradiated control cells (Fig.
1A). The average apoptotic cell numbers of the irradiated and
non-irradiated control cells are demonstrated in Fig. 1B. Values of <10% were considered
to be the threshold in the non-irradiated control p53−/− and p53+/+
cells.
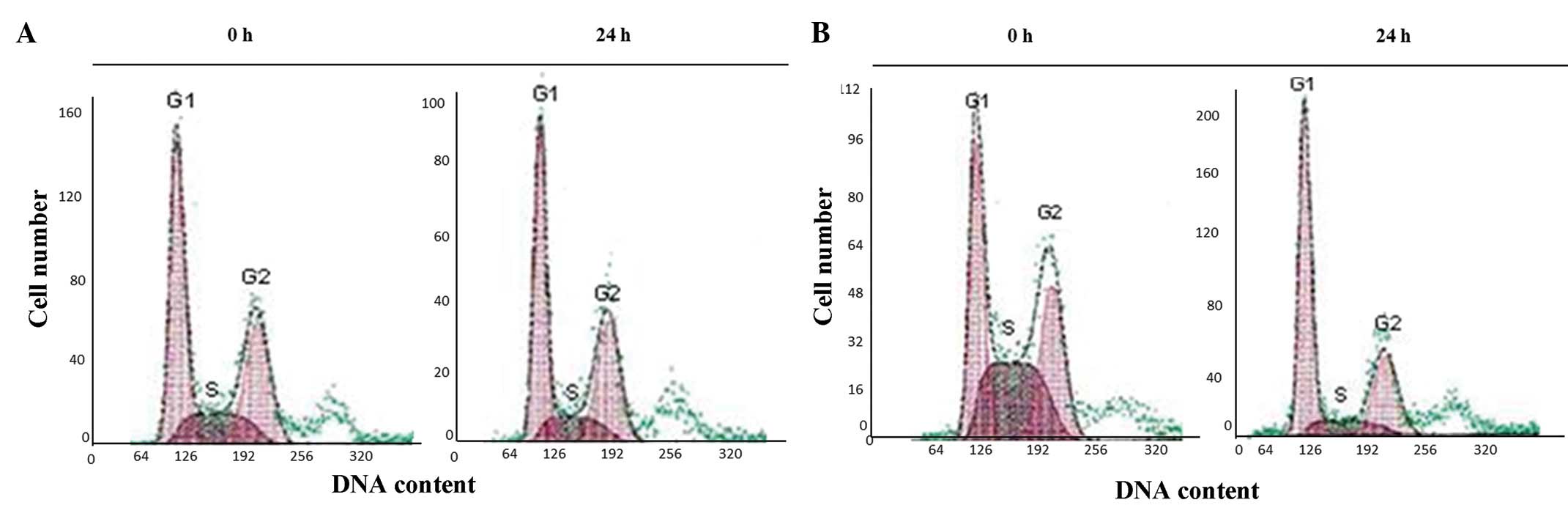
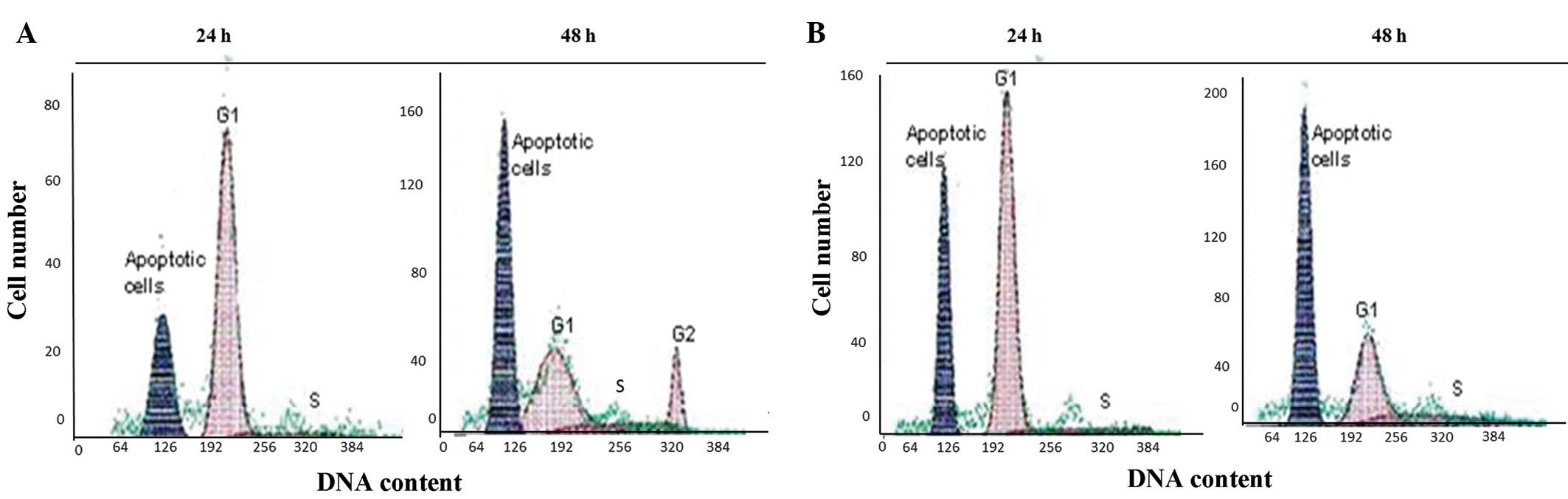
Cell cycle analysis
The G1, S and G2 phases of the
cell cycle showed a normal distribution in the non-irradiated
p53+/+ and p53−/− control cells (Fig.
2). However, following exposure to irradiation, the p53+/+
cells became permanently accumulated in the G1 phase
within 24 h. At 48 h post-irradiation, the cells passed to the S
phase and arrested there. The apoptotic cell numbers, according to
the sub-G0 DNA contents, were increased at the indicated
time-points in the p53+/+ cells following irradiation. (Fig. 3). The irradiated p53−/− cells also
showed a similar pattern to the p53+/+ cells within 24 h. However,
the cells that escaped from the G1 phase accumulated in
the G2 phase at 48 h (Fig.
3). Overall, the apoptotic cell numbers of the irradiated
p53−/−cells showed similar patterns to the irradiated p53+/+
cells.
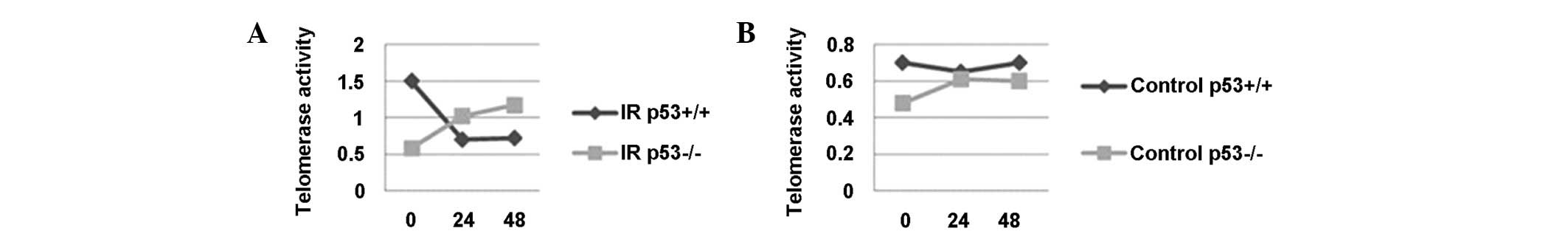
Telomerase activity
The telomerase activity of the non-irradiated p53+/+
cells was nearly uniform at 0, 24 and 48 h. The telomerase activity
of the non-irradiated p53−/− cells was marginally different to the
p53+/+ cells at the designated time-points. In the irradiated
p53+/+ cells, the telomerase activity was low at 0 h and continued
to decrease at 24 and 48 h. In contrast to this, the telomerase
activity was similar to the non-irradiated p53−/− cells at 0 h.
However, at 24 h post-irradiation, the telomerase activity
increased and remained at a higher level at 48 h (Fig. 4).
Discussion
γ-irradiation causes double or single strand breaks
depending on the application dose, for example 5 or 7.5 Gy, in
cells (20). Following single or
double strand DNA breaks, time protective processes should be
activated (16). The four
checkpoints during the cell cycle are at G1/S, S,
G2/M and M. If there is a problem in any of the
checkpoints, the cycle is stopped and allowed sufficient time for
repair. However, in certain cases, the repair pathways themselves
may be defective due to abnormal enzyme activity in the signaling
cascade. p53 prevents cell proliferation via telomere shortening
and telomerase activity and by activating cellular senescence. In
this case, an alternative conserving mechanism, which is generally
termed apoptosis, is activated. Therefore, cells are protected from
transferring the wrong copies to their daughter cells. The cellular
gatekeeper p53 protein is situated in the nucleus and activates
genes that are responsible for repair, apoptosis and telomere
regulation (6–7,11,21).
The present study aimed to clarify the p53 dependency of
G1 and G2 arrest, apoptosis and telomerase
activity following 5 Gy γ-irradiation in p53+/+ and p53−/− HCT116
colon carcinoma cells.
In the present study, the HCT116 cells expressing
p53+/+ were arrested in the G1 phase of the cell cycle
at 24 h and at 48 h, then escaped from G1 arrest and
accumulated in the S phase, driving apoptosis at an additional 48 h
after 5 Gy γ-irradiation. Attardi et al showed that p53+/+
MEF (mouse embryonic fibroblast) cells accumulated in G1
phase arrest following 5 Gy irradiation. Following irradiation, the
p21 promoter is triggered depending on the increased expression of
p53 in the MEFs. However, p53−/− MEFs underwent G2 phase
arrest subsequent to irradiation. G2 arrest in the MEFs
occurred independently of p53 (12). There is evidence from certain
studies that suggests that the CDC25A molecule is significant in
p53-independent G2 phase arrest (22). CDC25A is one of the key molecules
involved in the cell cycle, and following treatment with
γ-irradiation, CDC25A is decreased in cells. Thus, the cells are
prevented from entering the M phase by a p53-independent mechanism.
p21+/+ and p53−/− HCT116 cells undergo G2 phase arrest
instead of G1 phase arrest following irradiation. In the
p53−/− cells of the present study, p21 and CDK may have regulated
p53-independent G2 arrest. The G1 and
G2 arrests were accompanied by cell death with an
increasing rate of occurrence of ≤2 h after exposure in the p53+/+
and p53−/− cells. According to a study using Drosophila
melanogaster as a model, following ionizing radiation
treatment, apoptosis occurred in a p53-independent manner (23).
While telomerase activity was decreased in the
p53−/− cells of the present study, increased telomerase activity
was identified in the p53+/+ cells following γ-irradiation. The
activity of TERT, which is a catalytic subunit of telomerase,
depends on whether p53 is expressed. Following irradiation, TERT
activity is decreased depending upon the level of p53 expression.
However, in p53−/− cells, TERT activity is increased due to the
absence of p53 (24). There are
various studies with regard to the association between p53 and
telomerase activity, and a number of conclusions have been made. In
a study using pituitary adenoma cells with no p53 expression,
telomerase activity was increased during malignant transformation
(25). While 10 Gy γ-irradiation
has not been shown to alter telomerase activity, accelerated
senescence has been observed in p53+/+ MCF7 breast cancer cells
(26). Subsequent to 0.1–1 Gy doses
of X-rays, telomerase activity and telomere lengthening were
induced in TK6-expressing p53+/+ and p53 mutant WTK1 human
lymphoblast cell lines. However, the triggering of telomerase
activity following radiation was not believed to be associated with
the p53 pathway (21).
In summary, the exposure to 5 Gy γ-irradiation,
telomerase activity and G1 cell cycle arrest were
regulated depending on the p53 status in the HCT116 colon cancer
cells. However, G2 arrest and the apoptotic response
were promoted in a p53-independent pathway.
Acknowledgements
The p53+/+ and p53−/− HCT116 colon cancer cell lines
were provided by Professor Bert Vogelstein.
References
|
1
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caspari T: How to activate p53. Curr Biol.
10:R315–R317. 2000. View Article : Google Scholar
|
|
3
|
Brooks CL and Gu W: Ubiquitination,
phosphorylation and acetylation: the molecular basis for p53
regulation. Curr Opin Cell Biol. 15:164–171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang SH and Clarke MF: Regulation of p53
localization. Eur J Biochem. 268:2779–2783. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adimoolam S and Ford JM: p53 regulation of
DNA damage recognition during nucleotide excision repair. DNA
Repair (Amst). 2:947–954. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Appella E and Anderson CW:
Post-translational modifications and activation of p53 by genotoxic
stresses. Eur J Biochem. 268:2764–2772. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Attardi LD, de Vries A and Jacks T:
Activation of the p53-dependent G1 checkpoint response in mouse
embryo fibroblasts depends on the specific DNA damage inducer.
Oncogene. 23:973–980. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Donzelly M and Draetta GF: Regulating
mammalian checkpoints through Cdc25 inactivation. EMBO Rep.
4:671–677. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hermeking H, Lengauer C, Polyak K, He TC,
Zhang L, Thiagalingam S, Kinzler KW and Vogelstein B: 14-3-3 sigma
is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1:3–11.
1997. View Article : Google Scholar
|
|
10
|
Dou PQ, An B and Will PL: Induction of a
retinoblastoma phosphatase activity by anticancer drugs accompanies
p53-independent G1 arrest and apoptosis. Proc Natl Acad Sci USA.
92:9019–9023. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fei P and El-Deiry WS: P53 and radiation
responses. Oncogene. 22:5774–5783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Attardi LD: The role of p53-mediated
apoptosis as a crucial anti-tumor response to genomic instability:
lessons from mouse models. Mutat Res. 569:145–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Enns L, Bogen KT, Wizniak J, Murtha AD and
Weinfeld M: Low-dose radiation hypersensitivity is associated with
p53-dependent apoptosis. Mol Cancer Res. 2:557–566. 2004.PubMed/NCBI
|
|
14
|
Hussain SP and Harris CC: p53 biological
network: at the crossroads of the cellular-stress response pathway
and molecular carcinogenesis. J Nippon Med Sch. 73:54–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Speidel D, Helmbold H and Deppert W:
Dissection of transcriptional and non-transcriptional p53
activities in the response to genotoxic stress. Oncogene.
25:940–953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vávrová J, Rezácová M, Vokurková D and
Psutka J: Cell cycle alteration, apoptosis and response of leukemic
cell lines to gamma radiation with high- and low-dose rate. Physiol
Res. 53:335–342. 2004.PubMed/NCBI
|
|
17
|
Vousden KH: p53: death star. Cell.
103:691–694. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kusumoto M, Ogawa T, Mizumoto K, Ueno H,
Niiyama H, Sato N, Nakamura M and Tanaka M: Adenovirus-mediated p53
gene transduction inhibits telomerase activity independent of its
effects on cell cycle arrest and apoptosis in human pancreatic
cancer cells. Clin Cancer Res. 5:2140–2147. 1999.
|
|
19
|
Shats I, Milyavsky M, Tang X, Stambolsky
P, Erez N, Brosh R, Kogan I, Braunstein I, Tzukerman M, Ginsberg D
and Rotter V: p53-dependent down-regulation of telomerase is
mediated by p21waf1. J Biol Chem. 279:50976–50985. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Myllyperkiö MH, Koski TR, Vilpo LM and
Vilpo JA: Gammairradiation-induced DNA single- and double-strand
breaks and their repair in chronic lymphocytic leukemia cells of
variable radiosensitivity. Hematol Cell Ther. 41:95–103.
1999.PubMed/NCBI
|
|
21
|
Neuhof D, Ruess A, Wenz F and Weber KJ:
Induction of telomerase activity by irradiation in human
lymphoblasts. Radiat Res. 155:693–697. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mailand N, Podtelejnikov AV, Groth A, Mann
M, Bartek J and Lukas J: Regulation of G(2)/M events by Cdc25A
through phosphorylation-dependent modulation of its stability. EMBO
J. 21:5911–5920. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wichmann A, Jaklevic B and Su TT: Ionising
radiation induces caspase-dependent but Chk2-and p53-independent
cell death in Drosophila melanogaster. Proc Natl Acad Sci
USA. 103:9952–9957. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao Y, Li H, Deb S and Liu JP: TERT
regulates cell survival independent of telomerase enzymatic
activity. Oncogene. 21:3130–3138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harada K, Arita K, Kurisu K and Tahara H:
Telomerase activity and the expression of telomerase components in
pituitary adenoma with malignant transformation. Surg Neurol.
53:267–274. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jones KR, Elmore LW, Jackson-Cook C,
Demasters G, Povirk LF, Holt SE and Gewirtz DA: p53-Dependent
accelerated senescence induced by ionising radiation in breast
tumour cells. Int J Radiat Biol. 81:445–458. 2005. View Article : Google Scholar : PubMed/NCBI
|