Introduction
Malignant mesothelioma most commonly arises from the
pleura (1), but it may also arise
from the peritoneum (2),
pericardium (3) and tunica
vaginalis testis (4,5). However, primary intrahepatic malignant
mesothelioma (PIHMM) is an extremely rare tumor (6–11).
Malignant mesothelioma is known to originate from transformed
mesothelial cells (12), which are
not present in the hepatic parenchyma under normal physiological
conditions. A possible explanation for the origin of PIHMM was
proposed by Leonardou et al(7), who speculated that the tumor arose
from mesothelial cells derived from an intruded Glisson’s capsule.
However, the clinicopathological characteristics of PIHMM remain to
be elucidated. The current study reports a case of PIHMM with
multiple lymphadenopathies due to non-tuberculous mycobacteria and
also presents the findings of a literature review. Written informed
consent was obtained from the patient.
Case report
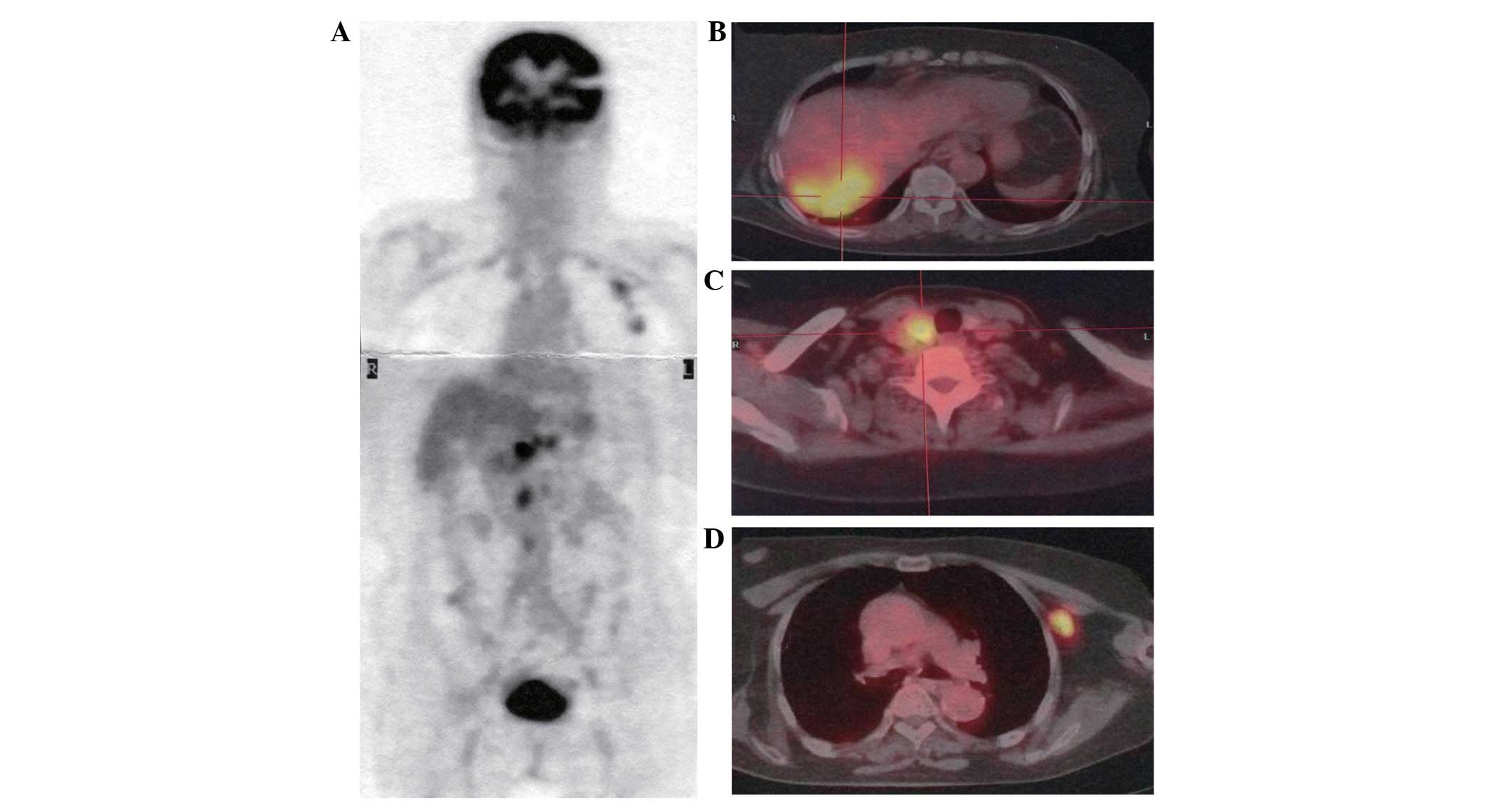
A 68-year-old female presented to Kansai Medical
University Takii Hospital (Osaka, Japan) with an intrahepatic tumor
and multiple lymph node swellings accompanied by a prolonged
low-grade fever. The patient did not have a history of asbestos
exposure or cigarette smoking. A computed tomography (CT) scan
revealed cervical, axillary and abdominal para-aortic lymph node
swellings, in addition to an intrahepatic tumor with a diameter of
70 mm in the right lobe of the liver. The intrahepatic tumor was
heterogeneously enhanced by contrast-enhanced CT. There was no
evidence of pleural effusion, ascites, pleural thickening or a
peritoneal tumor. Subsequently,
2-deoxy-2[18F]-fluoro-D-glucose (FDG)-positron emission
tomography(PET)/CT was performed, which clearly revealed a high FDG
uptake in the lymph nodes and intrahepatic tumor (Fig. 1A–D). In contrast, no significant
accumulation of FDG was noted in the pleura or the peritoneum. A
laboratory examination showed that the C-reactive protein (CRP) and
lactic dehydrogenase (LDH) levels were slightly elevated (CRP,
3.907 mg/dl; LDH, 247 U/l). However, the serum tumor marker levels
were not elevated. The results of all the other laboratory
examinations were within normal limits.
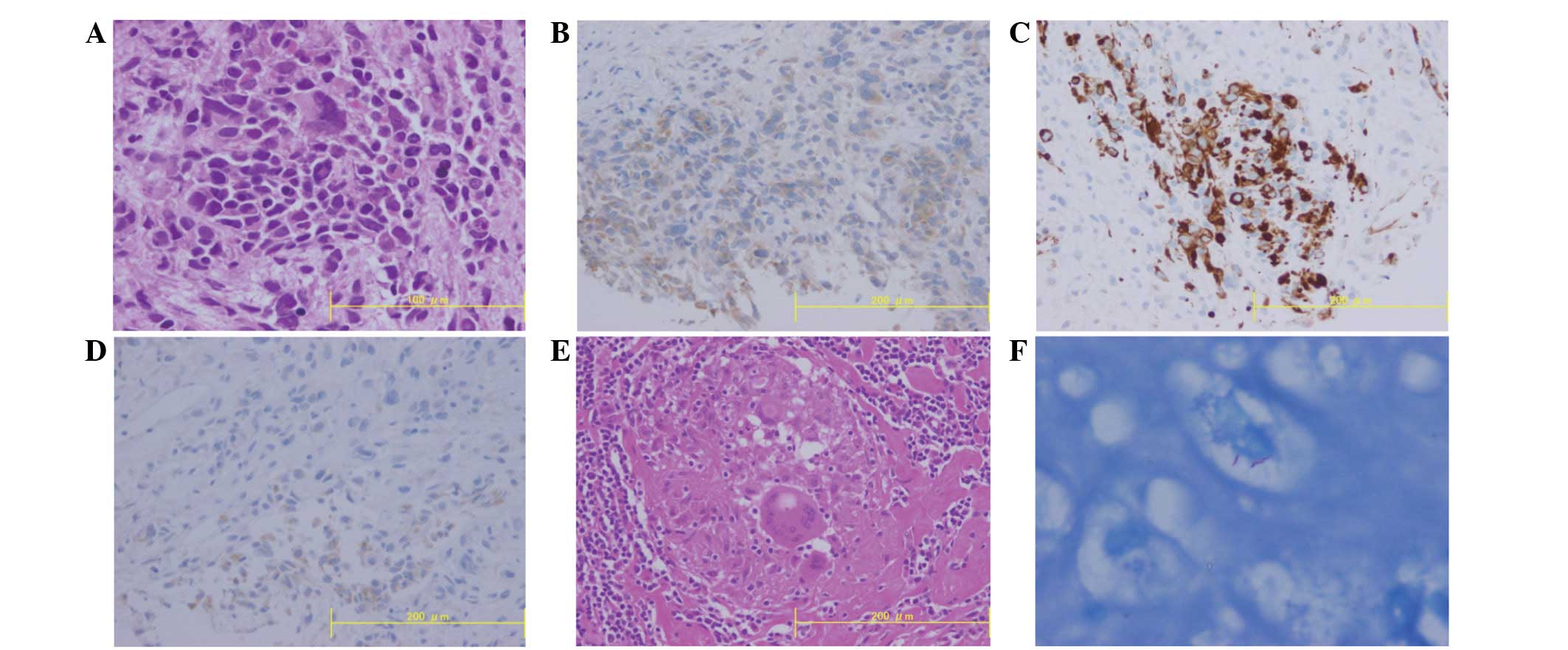
An ultrasound-guided fine-needle aspiration biopsy
of the liver tumor was performed. A histological examination of the
biopsy specimen revealed islands of polygonal tumor cells with a
high nuclear to cytoplasmic ratio and prominent nucleoli (Fig. 2A). Few mitotic figures were noted.
The tumor islands were surrounded by inflammatory infiltrates.
Alcian blue and periodic acid-Schiff staining clearly demonstrated
intracytoplasmic mucopolysaccharides in the tumor cells.
Immunohistochemical examination revealed that the
tumor cells were negative for carcinoembryonic antigen,
carbohydrate antigen 19-9, p53 and CD34. In contrast, the tumor
cells stained positive for epithelial membrane protein, cytokeratin
(CK) 7, CK20, CD10 and vimentin. In addition, immunohistochemical
staining revealed that the tumor cells were positive for
calretinin, Wilms tumor gene-1 (WT-1) and D2–40 (Fig. 2B–D). These findings strongly
suggested that the intrahepatic tumor cells exhibited the
phenotypical features of malignant mesothelioma.
An axillary lymph node biopsy was performed to
determine whether the lymph node swellings were due to intrahepatic
mesothelioma metastasis. However, the histological examination
showed an epithelioid granuloma (Fig.
2E) and Ziehl-Neelsen staining revealed the presence of
acid-fast bacilli (Fig. 2F). Thus,
a final diagnosis of PIHMM accompanied by lymphadenopathies due to
a mycobacterial infection was confirmed. PCR for Mycobacterium
tuberculosis and the avium-intracellulare complex was
negative. The cultivation for acid-fast bacillus failed to yield a
mycobacterium species. Therefore, the pathogenic mycobacterium was
not determined in this case. Since the lymphadenopathy was not due
to mesothelioma metastasis, the PIHMM was considered to be a
localized tumor. Therefore, a surgical resection of the liver tumor
with curative intent was planned. However, a hepatic rupture
occurred due to the rapid growth of the liver tumor and the general
condition of the patient deteriorated. Therefore, no further
investigations or treatments were possible in this case.
Discussion
PIHMM is an extremely rare tumor. To the best of our
knowledge, only six cases of PIHMM have been previously reported in
the published literature (6–11). A
review of these six cases, plus the present study, is summarized in
Table I. The cases consisted of
five male and two female patients (2.5:1), with an age range of
53–68 years (median, 62 years). Previous reviews focusing on
conventional mesothelioma have shown that the male/female ratio and
median age at the initial diagnosis ranged from 2.2:1–12.6:1 and
64–68 years, respectively (13–15).
However, in a subgroup of non-occupational mesothelioma cases, the
male/female ratio and median age at the initial diagnosis were
reported to be 0.8:1–1.4:1 and 57.8–63.0 years, respectively
(13,16). Thus, gender and age distribution did
not differ significantly between PIHMM and non-occupational
mesothelioma. Only one of the seven patients (14.3%) had a history
of asbestos exposure, although it has previously been shown that
conventional mesothelioma is frequently associated with asbestos
exposure (58.9–86.8%) (13–15).
 | Table ICharacteristics of patients with
PIHMM. |
Table I
Characteristics of patients with
PIHMM.
| First author, year
(ref.) | Age, years | Gender | Asbestos
exposure | Histology | OS, months | Location
(segment) | Size, cm | Treatment | Relapse |
|---|
| Imura et al,
2002 (6) | 64 | M | (−) | Ep | 40 | Rt (S7) | 3.2 | Surg | None |
| Leonardou et
al, 2003 (7) | 54 | F | N/E | Ep | 2 | Rt | 16.0 | Surg | None |
| Gütgement et
al, 2006 (8) | 62 | M | (−) | Ep | 5 | Rt | 5.8 | Surg | LNR |
| Kim et al,
2008 (9) | 53 | M | (−) | Bp | N/E | Rt | 13.0 | Surg | DI |
| Sasaki et al,
2009 (10) | 66 | M | (+) | Bp | 6 | Rt (S8) | 4.4 | Surg | None |
| Buchholz et
al, 2009 (11) | 62 | M | (−) | Ep | 36 | Rt (S5, S8) | 5.8 | Surg | LNR |
| Present case | 68 | F | (−) | Ep | 3 | Rt (S7) | 7.0 | BSC | N/E |
The prevalence of distant metastasis at the initial
diagnosis has been recorded as 25.2–55.1% in conventional
mesothelioma (13,16). Therefore, surgical resection is not
always performed. However, all seven of the PIHMM patients reviewed
in the present study had solitary tumors that were localized in the
liver at the time of the initial diagnosis, and surgical resection
had been performed in all cases, with the exception of the present
case. All the tumors arose in the right lobe, were located in the
subcapsular region and were between 3.2 and 16 cm in diameter
(mean, 7.8 cm). Cavity effusion was not associated with PIHMM in
any of the reviewed cases, however malignant serositis is usually
observed in conventional mesothelioma (13). Three of the six patients that
underwent a surgical resection relapsed post-surgery, one of which
received systemic chemotherapy with pemetrexed in combination with
cisplatin. Two of the three relapsed cases showed translymphatic
progression. The prevalence of lymph node metastasis in
conventional mesothelioma has been evaluated. Rahman et al
reported that 18 of 53 patients (34.0%) with malignant pleural
mesothelioma had positive lymph node involvement at the time of
surgery (17). In addition, Edwards
et al reported that 44 of 92 consecutive patients (47.8%)
with malignant mesothelioma who underwent extrapleural
pneumonectomy had positive lymph node involvement (18). Therefore, translymphatic progression
is not an unusual event in malignant mesothelioma. In the present
study, the survival factors were not evaluated due to the small
size of the study population and inadequate survival
information.
The review of the seven cases showed that five were
epithelioid type (71.4%) and two were biphasic (28.6%). A
sarcomatoid type was not noted among the reviewed cases. Among the
conventional mesothelioma cases, the prevalence of epithelioid,
biphasic and sarcomatoid subtypes was 32–67.2, 21.7–34 and 9.8–33%,
respectively (13–16). The distribution of the histological
types among the non-occupational mesotheliomas was not dissimilar
to that among the conventional mesotheliomas (epithelioid, 64.9%;
biphasic, 15.3% and sarcomatoid, 6.1%) (19). Therefore, the distribution of the
histological subtypes in PIHMM was equivalent to that in
conventional mesothelioma. The tumor phenotypes of the reviewed
cases are summarized in Table II.
Previously, several studies had been conducted to clarify the
phenotypical features of malignant mesothelioma using
immunohistochemistry and these results are also listed in Table II. Consequently, this literature
review clearly showed that PIHMM and conventional mesothelioma do
not differ significantly with respect to cellular phenotype
(20–28).
 | Table IIImmunohistochemical phenotypes of
PIHMM and conventional mesothelioma. |
Table II
Immunohistochemical phenotypes of
PIHMM and conventional mesothelioma.
| Items | D2–40 | WT-1 | Calretinin | Vimentin | p53 | CK7 | CK20 |
|---|
| PIHMM, degree of
staining |
| Imura et al,
2002 (6) | N/E | N/E | (+) | N/E | (+) | N/E | N/E |
| Leonardou et
al, 2003 (7) | N/E | N/E | (+) | (+) | N/E | N/E | N/E |
| Gütgement et
al, 2006 (8) | (+) | (+) | (+) | (±) | (+) | N/E | (−) |
| Kim et al,
2008 (9) | N/E | N/E | (+) | N/E | N/E | (+) | (−) |
| Sasaki et al,
2009 (10) | (+) | (+) | (+) | (+) | (+) | (+) | N/E |
| Buchholz et
al, 2009 (11) | (+) | (+) | (+) | (±) | (+) | N/E | (−) |
| Present case | (±) | (+) | (+) | (+) | (−) | (+) | (+) |
| Conventional
mesotheliomaa, % (refs.) | 85 (20) | 55–100 (20,21,23) | 39.8–100 (20–22) | 27.7–96 (21,22,24) | 45–69.6 (22,25) | 65–100 (26–28) | 0 (26–28) |
In the present case, multiple lymphadenopathies were
observed in addition to the liver tumor. FDG-PET/CT did not
demonstrate the difference in metabolic behavior between the
intrahepatic tumor and lymphadenopathies. However, the lymph node
lesions were identified to be non-cancerous granulomas due to the
presence of mycobacteria. FDG-PET/CT examination was insufficient
to differentiate epithelioid granuloma due to mycobacterial
infection from malignant mesothelioma in this case. Studies have
also demonstrated that mycobacteriosis commonly causes increased
18F-FDG uptake (29–32).
Thus, an aggressive biopsy is warranted when multiple lymph node
swellings are associated with a malignant tumor.
The present study describes a case of PIHMM with
multiple lymphadenopathies due to non-tuberculous mycobacteria. A
literature review clearly indicated that the clinicopathological
characteristics of PIHMM are similar to those of non-occupational
mesothelioma. However, the tumors in all the reviewed cases were
solitary tumors that were localized in the liver and none were
accompanied by cavity effusion. Further investigation of the
pathophysiological features of PIHMM is required to develop an
appropriate treatment strategy.
References
|
1
|
Maitra A and Kumar V: The lung and upper
respiratory tract. Robbins Basic Pathology. 7th edition. Saunders;
Philadelphia: pp. 453–509. 2002
|
|
2
|
Battifora H and McCaughey E: Tumors of the
Serosal Membranes. Armed Forces Institute of Pathology; Washington,
DC: 1994
|
|
3
|
Thomason R, Schlegel W, Lucca M, et al:
Primary malignant mesothelioma of the pericardium. Case report and
literature review. Tex Heart Inst J. 21:170–174. 1994.PubMed/NCBI
|
|
4
|
Kasdon EJ: Malignant mesothelioma of the
tunica vaginalis propria testis. Report of two cases Cancer.
23:1144–1150. 1969.PubMed/NCBI
|
|
5
|
Kozlowski H and Zoltowska A: Mesothelioma
of spermatic cord. Neoplasma. 15:97–100. 1968.PubMed/NCBI
|
|
6
|
Imura J, Ichikawa K, Takeda J, Iwasaki Y,
et al: Localized malignant mesothelioma of the epithelial type
occurring as a primary hepatic neoplasm: a case report with review
of the literature. APMIS. 110:789–794. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leonardou P, Semelka RC, Kanematsu M, et
al: Primary malignant mesothelioma of the liver: MR imaging
findings. Magn Reson Imaging. 21:1091–1093. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gütgemann I, Standop J and Fischer HP:
Primary intrahepatic malignant mesothelioma of epithelioid type.
Virchows Arch. 448:655–658. 2006.PubMed/NCBI
|
|
9
|
Kim DS, Lee SG, Jun SY, et al: Primary
malignant mesothelioma developed in liver. Hepatogastroenterology.
55:1081–1084. 2008.PubMed/NCBI
|
|
10
|
Sasaki M, Araki I, Yasui T, et al: Primary
localized malignant biphasic mesothelioma of the liver in a patient
with asbestosis. World J Gastroenterol. 15:615–621. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buchholz BM, Gütgemann I, Fischer HP, et
al: Lymph node dissection in primary intrahepatic malignant
mesothelioma: case report and implications for diagnosis and
therapy. Langenbecks Arch Surg. 394:1123–1130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Husain AN, Colby TV, Ordóñez NG, et al:
Guidelines for pathologic diagnosis of malignant mesothelioma: a
consensus statement from the International Mesothelioma Interest
Group. Arch Pathol Lab Med. 133:1317–1331. 2009.PubMed/NCBI
|
|
13
|
Yates DH, Corrin B, Stidolph PN and Browne
K: Malignant mesothelioma in south east England:
clinicopathological experience of 272 cases. Thorax. 52:507–512.
1997.PubMed/NCBI
|
|
14
|
Borasio P, Berruti A, Billé A, et al:
Malignant pleural mesothelioma: clinicopathologic and survival
characteristics in a consecutive series of 394 patients. Eur J
Cardiothorac Surg. 33:307–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gemba K, Fujimoto N, Kato K, et al:
National survey of malignant mesothelioma and asbestos exposure in
Japan. Cancer Sci. 103:483–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Law MR, Hodson ME and Heard BE: Malignant
mesothelioma of the pleura: relation between histological type and
clinical behaviour. Thorax. 37:810–815. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdel Rahman AR, Gaafar RM, Baki HA, et
al: Prevalence and pattern of lymph node metastasis in malignant
pleural mesothelioma. Ann Thorac Surg. 86:391–395. 2008.PubMed/NCBI
|
|
18
|
Edwards JG, Stewart DJ, Martin-Ucar A, et
al: The pattern of lymph node involvement influences outcome after
extrapleural pneumonectomy for malignant mesothelioma. J Thorac
Cardiovasc Surg. 131:981–987. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Metintas M, Metintas S, Ak G, et al:
Epidemiology of pleural mesothelioma in a population with
non-occupational asbestos exposure. Respirology. 13:117–121. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saad RS, Lindner JL, Lin X, et al: The
diagnostic utility of D2–40 for malignant mesothelioma versus
pulmonary carcinoma with pleural involvement. Diagn Cytopathol.
34:801–806. 2006.
|
|
21
|
Ordóñez NG: The immunohistochemical
diagnosis of mesothelioma: A comparative of epithelioid
mesothelioma and lung adenocarcinoma. Am J Surg Pathol.
27:1031–1051. 2003.PubMed/NCBI
|
|
22
|
Roberts F, Harper CM, Downie I and Burnett
RA: Immunohistochemical analysis still has a limited role in the
diagnosis of malignant mesothelioma. A study of thirteen
antibodies. Am J Clin Pathol. 116:253–262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pu RT, Pang Y and Michael CW: Utility of
WT-1, p63, MOC31, mesothelin, and cytokeratin (K903 and CK5/6)
immunostains in differentiating adenocarcinoma, squamous cell
carcinoma, and malignant mesothelioma in effusions. Diagn
Cytopathol. 36:20–25. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moch H, Oberholzer M, Christen H, et al:
Diagnostic tools for differentiating pleural mesothelioma from lung
adenocarcinoma in paraffin embedded tissue. II Design of an expert
system and its application to the diagnosis of mesothelioma.
Virchows Arch A Pathol Anat Histopathol. 423:493–496. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Attanoos RL and Gibbs AR: Pathology of
malignant mesothelioma. Histopathol. 30:403–418. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tot T: The value of cytokeratins 20 and 7
in discriminating metastatic adenocarcinoma from pleural
mesotheliomas. Cancer. 92:2727–2732. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang NP, Zee S, Zarbo RJ, et al:
Coordinate expression of cytokeratins 7 and 20 defines unique
subsets of carcinomas. Appl Immunohistochem. 3:99–107. 1995.
|
|
28
|
Chu P, Wu E and Weiss LM: Cytokeratin 7
and cytokeratin 20 expression in epithelial neoplasms: a survey of
435 cases. Mod Pathol. 13:962–972. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Treglia G, Taralli S, Calcagni ML, et al:
Is there a role for fluorine 18 fluorodeoxyglucose-positron
emission tomography and positron emission tomography/computed
tomography in evaluating patients with mycobacteriosis? A
systematic review. J Comput Assist Tomogr. 35:387–393. 2011.
View Article : Google Scholar
|
|
30
|
Das CJ, Kumar R, Balakrishnan VB, et al:
Disseminated tuberculosis masquerading as metastatic breast
carcinoma on PET-CT. Clin Nucl Med. 33:359–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li YJ, Cai L, Sun HR, et al: Increased FDG
uptake in bilateral adrenal tuberculosis appearing like malignancy.
Clin Nucl Med. 33:191–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin WY, Hung GU and Tsai SC: A pitfall of
FDG-PET image interpretation: accumulation of FDG in the dependent
area of the urinary bladder after bladder irrigation - the
usefulness of the prone position. Clin Nucl Med. 30:638–639. 2005.
View Article : Google Scholar : PubMed/NCBI
|