Introduction
Taxanes, including paclitaxel (PTX) and docetaxel
(DOC), are among the most active and widely used classes of
cytotoxic agents for breast cancer treatment (1,2).
Taxanes are poorly soluble in water due to their hydrophobic
properties and thus, require solvents, including polyoxyethylated
castor oil and ethanol (3). The use
of these solvents has been associated with toxic responses,
including hypersensitivity reactions (HSRs) and prolonged sensory
neuropathy (3). Nanoparticle
albumin-bound PTX (nab-PTX) is a novel formulation of PTX that
allows reconstitution of this agent with a saline solution instead
of solvents and administration without premedication for HSRs
(4,5). The aim of the present study was to
report the safety of nab-PTX, as a result of the administration of
the formulation to 4 breast cancer patients with contraindications
to PTX or DOC. Written informed consent was obtained from the
patients.
Case reports
Case 1
In May 2004, a 43-year-old female with right-sided
breast cancer underwent a partial resection of the breast (Bp) and
axillary lymph node dissection (Ax). The patient was of
pathological stage IIB [T2N1M0; estrogen receptor (ER)-positive,
progesterone receptor (PR)-positive and human epidermal growth
factor receptor 2 (HER2)-negative]. The patient was treated with
adjuvant therapy consisting of 4 cycles of oral cyclophosphamide
(100 mg/m2 daily on days 1–14), doxorubicin (30
mg/m2 on days 1 and 8) and 5-fluorouracil (500
mg/m2 on days 1 and 8) every 4 weeks and sequential
endocrine therapy with tamoxifen (20 mg/day). At 4-years
post-surgery, multiple bone metastases were detected. The patient
was initially treated with endocrine therapy (2.5 mg/day letrozole
followed by 25 mg/day exemestane) and zoledronic acid (4 mg every
3–4 weeks). After 21 months, the patient exhibited new lesions in
the liver and the endocrine therapy was adjusted to chemotherapy
with PTX (175 mg/m2 on day 1) and gemcitabine (1,250
mg/m2 on days 1 and 8) every three weeks.
PTX was administered via a 180-min infusion
following premedication consisting of corticosteroid
(dexamethasone, 20 mg/body) and antihistamines (ranitidine and
diphenhydramine, both 50 mg/body). However, the patient developed
dyspnea and flushing 10 mins after the initiation of intravenous
administration. The symptoms were considered to be due to an HSR to
PTX and the infusion was terminated. After 30 mins, the symptoms
improved and, subsequently, the regimen was adjusted to
capecitabine. After six months, the individual exhibited
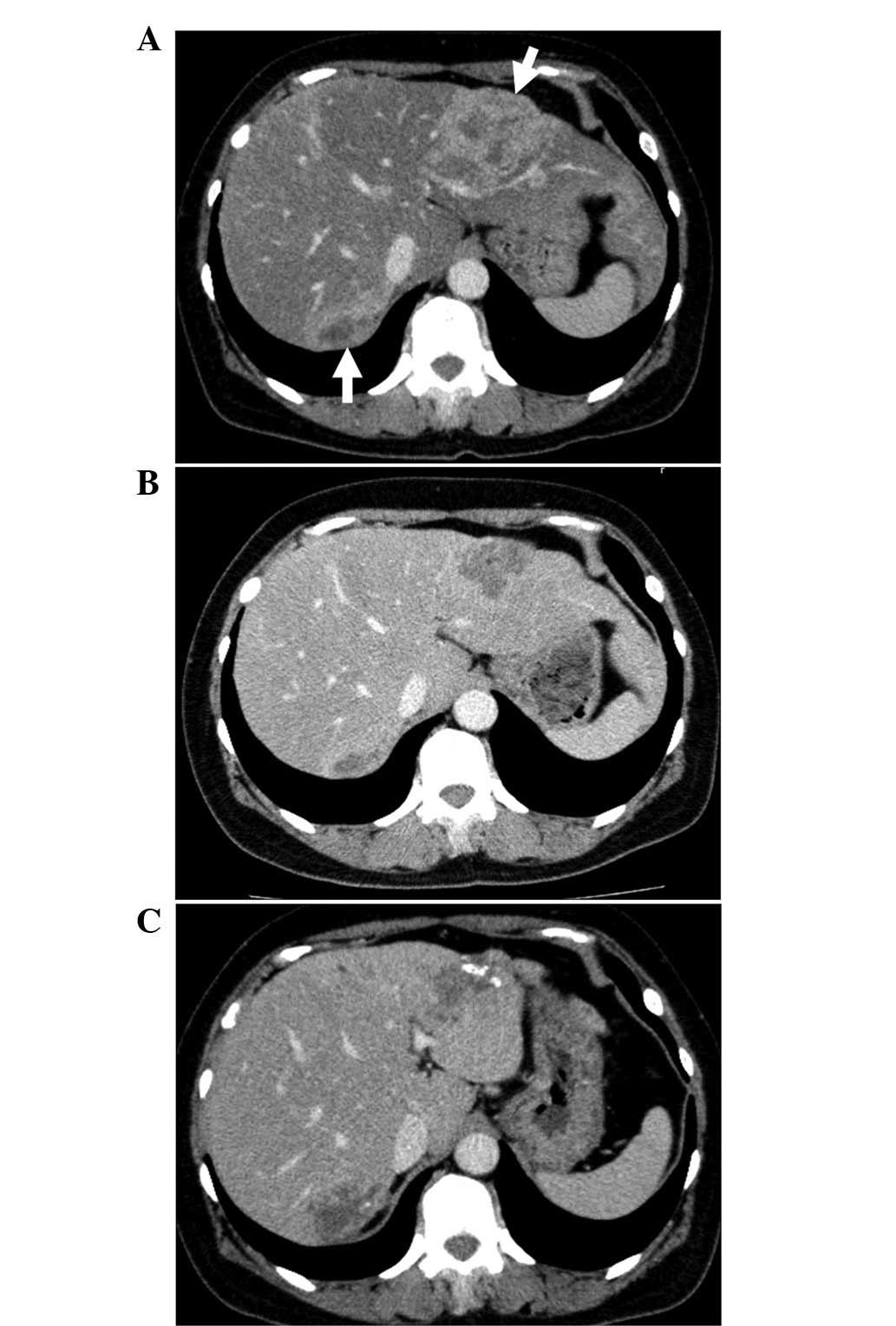
progressive disease in the liver (Fig.
1) and the regimen was adjusted to nab-PTX (260
mg/m2) every three weeks for 30 mins, with premedication
of dexamethasone (8 mg) only. No HSRs were exhibited and the
nab-PTX treatment was continued. Abdominal computed tomography (CT)
was performed following 4 cycles of therapy and the patient
exhibited a stable disease (SD) state. However, following 11 cycles
of treatment, the patient experienced disease progression;
therefore, nab-PTX treatment was discontinued (Fig. 1). During nab-PTX treatment, the
patient exhibited no HSRs and sensory neuropathy and neutropenia
were recorded as grade 1. Following nab-PTX treatment, the patient
had three other chemotherapy treatments for seven months as
follows: i) Six cycles of 1.4 mg/m2 eribulin mesylate on
days 1 and 8 every three weeks; ii) four cycles of FEC (100
mg/m2 epirubicin, 500 mg/m2 5-fluorouracil
and 500 mg/m2 cyclophosphamide) every three weeks; and
iii) one cycle of 1,200 mg/m2 gemcitabine and 25
mg/m2 vinorelbine on days 1 and 8 every three weeks. The
outcome of the patient was good.
Case 2
In May 2012, a 36-year-old female was diagnosed with
left-sided breast cancer (stage IIIC; T3N3bM0; ER-positive,
PR-positive and HER2-negative). The patient began pre-operative
systemic therapy (PST) with 4 cycles of 5-fluorouracil (500
mg/m2), epirubicin (100 mg/m2) and
cyclophosphamide (500 mg/m2) every 3 weeks, followed by
4 cycles of DOC (75 mg/m2) every 3 weeks. Dexamethasone
(20 mg/body) was used as premedication for DOC. Although the
patient received the initial cycle of DOC without developing an
HSR, dyspnea and nausea developed 5 mins after administration of
the second DOC. These symptoms were considered to be due to an HSR
to DOC and the infusion was terminated. After 1 h, the symptoms
improved and, subsequently, the regimen was adjusted to treatment
with nab-PTX. The patient was administered nab-PTX (260
mg/m2) for 30 mins every 3 weeks, with premedication
consisting of dexamethasone (8 mg) only. The patient exhibited no
HSR and continued nab-PTX for 3 cycles. During the nab-PTX
treatment, the patient exhibited no HSRs and sensory neuropathy and
neutropenia were recorded as grade 1. Following PST, the patient
experienced a clinically complete response (cCR; Fig. 2) and underwent a Bp and Ax. The
patient had no recurrence.
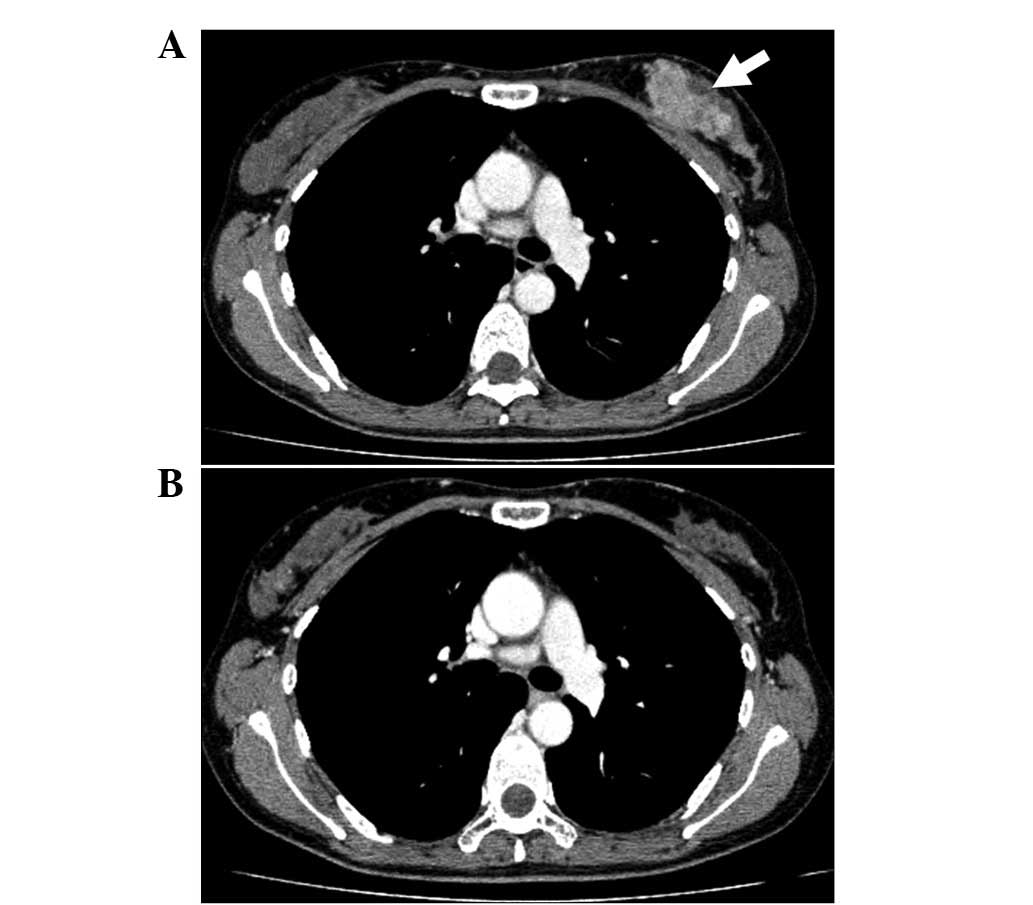 | Figure 2(A) Chest CT scan of a 36-year-old
female showed left-sided breast cancer, as indicated by the arrow.
(B) Following PST with 4 cycles of FEC, 1 cycle of DOC and 3 cycles
of nab-PTX, the patient showed a cCR. CT, computed tomography; PST,
pre-operative systemic therapy; nab-PTX, nanoparticle albumin-bound
paclitaxel; FEC, 5-fluorouracil, epirubicin and cyclophosphamide;
DOC, docetaxel; cCR, clinically complete response. |
Case 3
In December 2000, a 45-year-old female with
right-sided breast cancer underwent a total mastectomy and Ax. The
patient was of pathological stage IIIB (T4bN2M0; ER-positive,
PR-positive and HER2-negative). The patient was treated with 6
cycles of adjuvant chemotherapy with oral cyclophosphamide (100
mg/m2 daily on days 1–14), methotrexate (40
mg/m2 on days 1 and 8) and 5-fluorouracil (500
mg/m2 on days 1 and 8) every 4 weeks. At 9-years
post-surgery, multiple lung metastases were detected and the
patient was initially treated with anastrozole (1.0 mg/day). After
21 months, progressive disease was identified in the lung and FEC
treatment (500 mg/m2 5-fluorouracil, 100
mg/m2 epirubicin and 500 mg/m2
cyclophosphamide) was initiated.
The patient had a past medical history of diabetes
and their HbA1c was controlled to <7% by metformin hydrochloride
(2,250 mg/day). Following FEC initiation, the patient’s fasting
blood sugar increased to >250 mg/dl and insulin glargine (6
U/day) was prescribed. However, the patient’s HbA1c continued to
increase to 8.7% and the individual developed a thirst and general
fatigue. The symptoms appeared to be associated with the
dexamethasone, which was being used as a premedication for FEC.
Therefore, the regimen was adjusted to nab-PTX (260
mg/m2) every 3 weeks. In January 2012, the patient was
administered with nab-PTX for 30 mins without dexamethasone. No
HSRs were exhibited and the nab-PTX treatment was continued for 15
cycles. The patient’s thirst and general fatigue improved, and four
months after the adjustment to nab-PTX, the patient’s HbA1c also
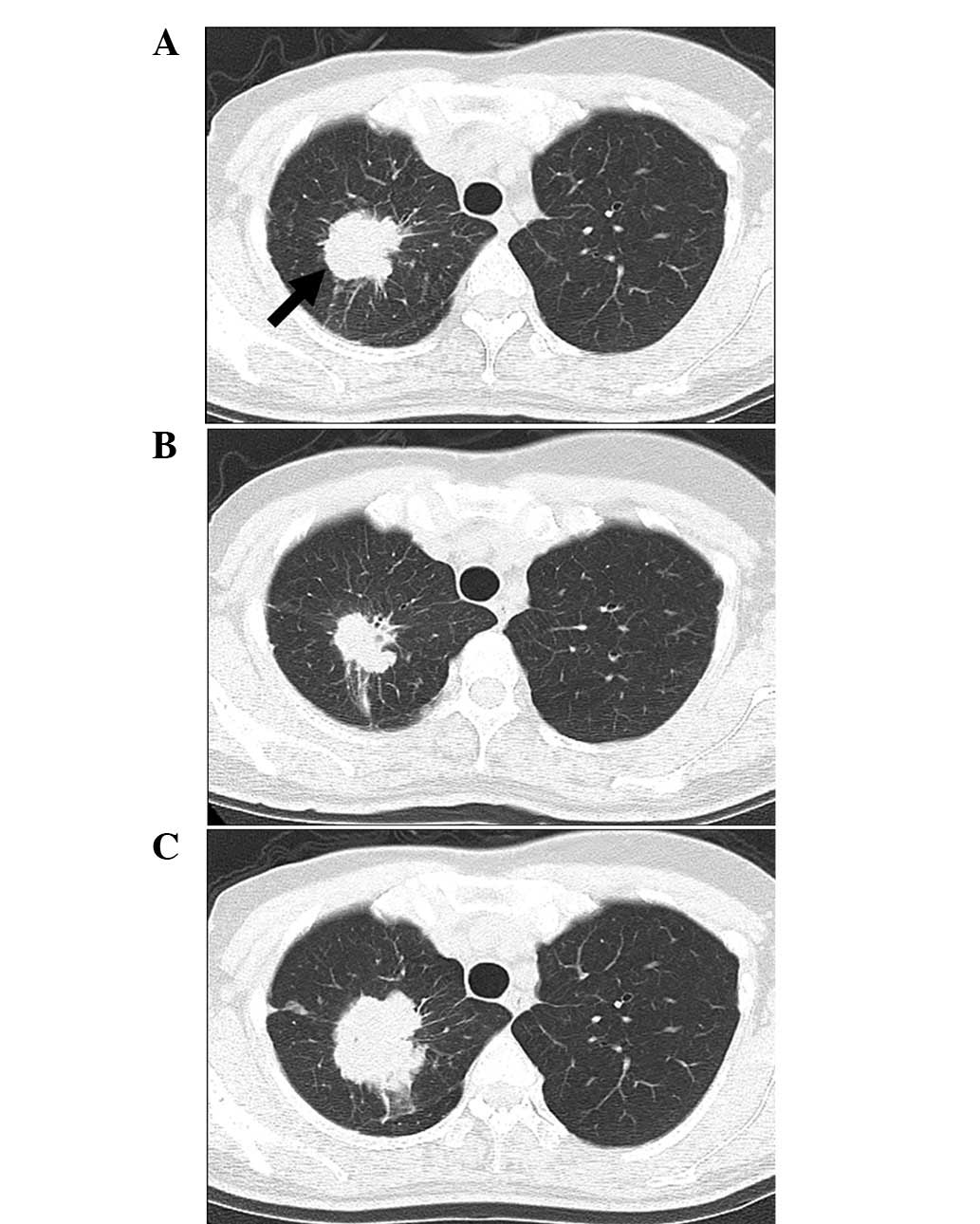
improved. A chest CT following 4 cycles of therapy showed a partial
response of the lung metastases. However, following 15 cycles of
therapy, the patient experienced disease progression; therefore,
an-PTX treatment was discontinued (Fig.
3). During the nab-PTX treatment, the patient exhibited no HSRs
and grade 2 sensory neuropathy was recorded. Following nab-PTX
treatment, the patient was administered with capecitabine (2,400
mg/day for 21 days every 4 weeks) for 7 months until now.
Case 4
In November 2012, a 47-year-old female with
left-sided breast cancer underwent a Bp and Ax. The patient was of
pathological stage IIIA (T2N2M0; ER-positive, PR-positive and
HER2-negative). Although adjuvant chemotherapy with 4 cycles of FEC
(500 mg/m2 5-fluorouracil, 100 mg/m2
epirubicin and 500 mg/m2 cyclophosphamide) followed by 4
cycles of DOC (75 mg/m2) was initially prescribed,
nab-PTX (260 mg/m2) without dexamethasone was selected
since the patient was positive for the hepatitis B virus (HBV)
antigen. Therefore, it was necessary to use entecavir hydrate and
not a steroid as prophylaxis to avoid HBV reactivation. The patient
completed the adjuvant chemotherapy, with no HSRs or HBV
reactivation.
Discussion
To ensure the safety of taxane treatment, PTX and
DOC require solvents, including Cremophor EL and ethanol or
polysorbate 80, and premedication with corticosteroids to reduce
the risk of HSRs associated with these solvents (3,6). By
contrast, nab-PTX may be administered without corticosteroids due
to its novel formulation with albumin that allows reconstitution of
nab-PTX with a saline solution instead of solvents (3).
A phase III trial previously compared the
administration of nab-PTX (260 mg/m2) with PTX (175
mg/m2) every three weeks in patients with metastatic
breast cancer (MBC). Nab-PTX demonstrated a significantly improved
overall response rate and longer time to progression compared with
PTX (5). In addition, the incidence
of grade 4 neutropenia was significantly lower for nab-PTX compared
with PTX. However, nab-PTX was associated with a higher rate of
grade 3 sensory neuropathy compared with PTX. The incidence of HSRs
in the trial was low for the two arms of the study (nab-PTX and
PTX), however, patients receiving PTX were premedicated, whereas
those receiving nab-PTX were not. A phase II trial performed in
2009 reported that patients with MBC administered with weekly
nab-PTX (100 or 150 mg/m2 during the first 3/4 weeks)
demonstrated a higher overall response rate and median
progression-free survival compared with those administered with DOC
(100 mg/m2 every three weeks) (7). In addition, a phase III trial in 2012
compared the administration of weekly nab-PTX (150
mg/m2) to that of weekly PTX (90 mg/m2) with
bevacizumab, as first-line therapies for MBC. However, nab-PTX was
not shown to be superior to PTX for progression-free survival
(8). Therefore, it has been unclear
which patients are likely to benefit from nab-PTX treatment.
The present study reports two patients who
experienced HSRs associated with PTX and DOC and were subsequently
administered nab-PTX, which did not result in an HSR. One patient
(Case 1) was treated with nab-PTX in a metastatic setting and was
able to continue treatment for 11 cycles with an overall response
of a SD state. The other patient (case 2) was treated with PST and
achieved cCR. Adverse reactions of the two patients were recorded
as grade 1. Therefore, nab-PTX appears to be safe and effective for
these patients. When HSRs to taxanes occur, the administration of
the taxane is usually terminated. However, taxanes are among the
most effective cytotoxic agents for breast cancer treatment.
Therefore, we hypothesize that even if a patient has experienced
HSRs to other taxanes, nab-PTX should be selected as a treatment,
rather than withdrawing from the use of taxanes completely.
In addition, the current study reported two cases
with contraindications to steroids. One was a patient with diabetes
(case 3) who needed to avoid the poor glycemic control associated
with steroid administration. Following treatment with nab-PTX, the
patient exhibited no HSRs and good glycemic control. Nab-PTX was
continued for 15 cycles and the highest overall response was a
partial response. The other patient (case 4) was a carrier for HBV
and, therefore, HBV reactivation had to be prevented. The patient
completed adjuvant chemotherapy without any HSRs or HBV
reactivation. The adverse reactions of the two patients were
recorded as grade 1 and 2. Since nab-PTX does not require
concomitant steroid treatment, this agent must be selected for
patients with clinical contraindications to steroids.
In conclusion, nab-PTX administration appears to be
safe and effective for patients who have previously experienced
HSRs to other taxanes or in those who have contraindications to
steroids.
References
|
1
|
De Laurentiis M, Cancello G, D’Agostino D,
et al: Taxane-based combinations as adjuvant chemotherapy of early
breast cancer: a meta-analysis of randomized trials. J Clin Oncol.
26:44–53. 2008.PubMed/NCBI
|
|
2
|
Piccart-Gebhart MJ, Burzykowski T, Buyse
M, et al: Taxanes alone or in combination with anthracyclines as
first-line therapy of patients with metastatic breast cancer. J
Clin Oncol. 26:1980–1986. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gradishar WJ: Albumin-bound paclitaxel: a
next-generation taxane. Expert Opin Pharmacother. 7:1041–1053.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto Y, Kawano I and Iwase H:
Nab-paclitaxel for the treatment of breast cancer: efficacy,
safety, and approval. Onco Targets Ther. 4:123–136. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gradishar WJ, Tjulandin S, Davidson N, et
al: Phase III trial of nanoparticle albumin-bound paclitaxel
compared with polyethylated castor oil-based paclitaxel in women
with breast cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Binder S: Evolution of taxanes in the
treatment of metastatic breast cancer. Clin J Oncol Nurs. 17:9–14.
2013. View Article : Google Scholar
|
|
7
|
Gradishar WJ, Krasnojon D, Cheporov S, et
al: Phase II trial of nab-paclitaxel compared with docetaxel as
first-line chemotherapy in patients with metastatic breast cancer:
final analysis of overall survival. Clin Breast Cancer. 12:313–321.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rugo HS, Barry WT, Moreno-Aspitia A, et
al: CALGB 40502/NCCTG N063H: Randomized phase III trial of weekly
paclitaxel (P) compared to weekly nanoparticle albumin bound
nab-paclitaxel (NP) or ixabepilone (Ix) with or without bevacizumab
(B) as first-line therapy for locally recurrent or metastatic
breast cancer (MBC). J Clin Oncol. 30:CRA10022012.
|