Introduction
Colorectal cancer (CRC) is one of the most
significant worldwide public health problems (1). During the natural course of the
disease, ~50% of patients may develop metastasis and up to 25% of
these present with metastatic disease at the moment of diagnosis.
Advances in the treatment of metastatic colorectal cancer (mCRC)
include the development of new antitumoral agents, including
epidermal growth factor receptor-targeted monoclonal antibodies
(EGFR-mAbs) and tyrosine kinase inhibitors, and the use of
biomarkers. The KRAS mutational status is currently the only
biomarker that is predictive of the response to therapy using
EGFR-mAbs that have been validated for clinical practice in mCRC
and recommended by the National Comprehensive Cancer Network (NCCN)
2012 Guidelines (version 1, 2012) (2,3).
However, not all mCRC patients with wild-type KRAS respond
to EGFR-mAb treatment, which may be due to alterations in other
genes, including BRAF, PIK3CA and
PTEN(4,5). Furthermore, numerous studies have
shown discordance in the KRAS mutational status between the
primary tumor and the metastatic lesions (6–8). Thus,
the study of KRAS in metastases may have clinical relevance
and may, at least partly, explain the resistance to EGFR-mAbs in
patients with KRAS wild-type primary tumors. Metastasis
involves the concept of circulating tumor cells (CTCs) that are
associated with the colonization of distant organs. Since the study
by Ashworth in 1869, in which malignant cells that were similar to
the primary tumor were identified to circulate in the peripheral
blood (9), increased efforts,
particularly in recent years, have focused on the development of
reliable methods for the enrichment and identification of CTCs
(10–12). The present study describes an easy,
affordable procedure using magnetic labeling that allows the
isolation of an enriched CTC fraction from peripheral blood and,
furthermore, the analysis of the KRAS mutational status of
the CTCs. A comparison between the KRAS mutational status in
CTCs and the corresponding tumor tissue was performed.
Materials and methods
Patients
A total of 23 mCRC patients who were in remission or
whose primary tumor KRAS mutational status was available in
the patient clinical records were selected for this study.
Ethylenediaminetetraacetic acid (EDTA)-anticoagulated peripheral
blood (10 ml) was obtained from each patient, all of who exhibited
disease progression. This study was approved by the ethics
committee of the University Clinic of Navarra. All the participants
provided their informed consent prior to blood sample
extraction.
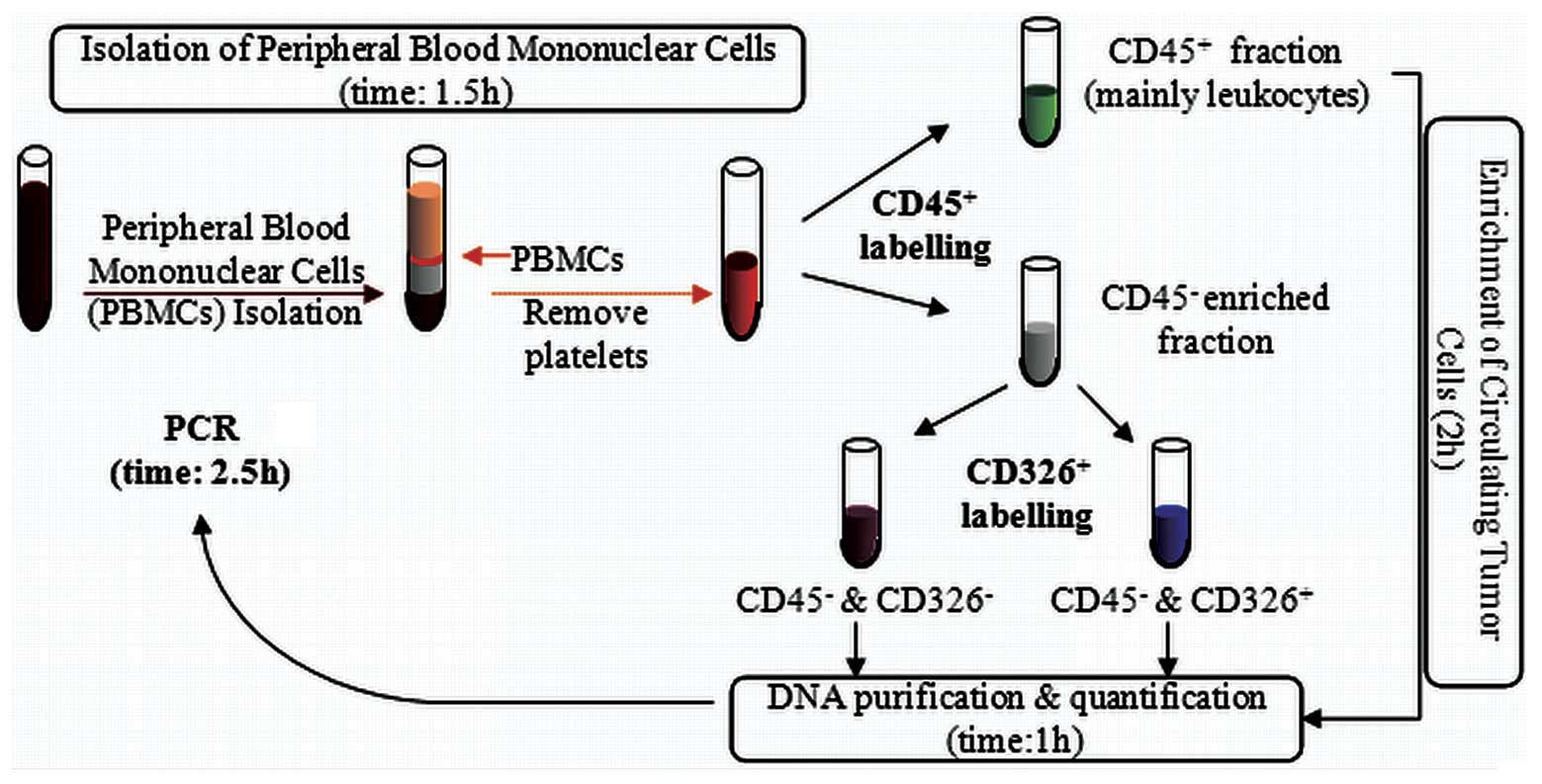
Isolation of peripheral blood mononuclear
cells (PBMCs; time, 1.5 h)
When working with anticoagulated peripheral blood,
PBMCs should be isolated using density gradient centrifugation.
Ficoll-Paque™ Plus was used for this purpose (17-1440-03; GE
Healthcare, Buckinghamshire, UK). The blood samples were diluted
with saline serum (ratio, 1:1) and gently added on the top of the
Ficoll-Paque Plus medium (ratio, 2/3 blood:1/3 Ficoll) taking care
not to mix the two layers. Following centrifugation for 20 min at
400 × g, the PBMCs that were located at the interface layer were
collected by inserting the pipette directly through the plasma
(alternatively, the upper plasma layer may be removed and the PBMCs
may be collected). The PBMCs were washed twice with saline serum
and centrifuged for 8 min at 300 × g. Buffer A was added to the
PBMCs, which were then centrifuged at 200 × g for 10–15 min. Buffer
A consisted of phosphate-buffered saline (PBS; pH 7.2), 0.5% bovine
serum albumin (BSA) and 2 mM EDTA). This step was repeated
twice.
Enrichment of circulating tumor cells
(time, 2 h)
CD45 Human MicroBeads (130-045-801; Miltenyi Biotec
GmbH, Bergisch Gladbach, Germany) were used for the enrichment of
the epithelial tumor cells from the peripheral blood by the
depletion of the CD45+ cells. The cell number from the
previous step was measured and following centrifugation at 300 × g
for 10 min and complete aspiration of the supernatant, 80 μl buffer
A and 20 μl CD45 MicroBeads per 107 total cells were
added, mixed and refrigerated (4–8°C) for 15 min. Following a wash
step with 2 ml buffer A per 107 cells (300 × g for 10
min), 500 μl buffer A was added (up to 108 cells). The
cell suspension was loaded onto a separator column that was placed
in a magnet (MiniMACS Starting kit; Miltenyi Biotec). The
magnetically-labeled CD45+ cells were retained within
the column. The unlabeled cell fraction (CD45− fraction)
that was enriched in the epithelial tumor cells ran through.
CD326 epithelial cell adhesion molecule
(EpCAM) Human MicroBeads are used for the enrichment of epithelial
tumor cells
The cell number from the CD45− fraction
that was obtained in the previous step was measured and following
centrifugation at 300 × g for 10 min and complete aspiration of the
supernatant, 300 μl buffer A and 100 μl FcR reagent (120-000-442;
Miltenyi Biotec) per 5×107 total cells were added and
mixed. A total of 100 μl CD326 Microbeads were added per
5×107 total cells, mixed well and refrigerated at 4–8°C
for 30 min. Following a wash step with 5–10 ml buffer A per
5×107 cells (300 × g for 10 min), 500 μl buffer A was
added up to 108 cells). The cell suspension was loaded
onto a separator column placed in a magnet (MiniMACS Starting kit;
Miltenyi Biotec). Subsequent to the labeling and separating
procedures, three cell fractions were obtained, CD45+,
CD45−/CD326− and
CD45−/CD326+. A schematic of the procedure is
shown in Fig. 1.
DNA purification and quantification
(time, 1 h)
The QIAamp® Mini kit (51101; Qiagen GmbH,
Hilden, Germany) was used for DNA purification from the cell
fractions that were obtained previously. The procedure was
performed as described in the QIAamp Mini kit instructions. DNA
quantification was performed using the NaNoDrop-2000/2000c
Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA,
USA), following the manufacturer’s instructions.
KRAS mutational status analysis (time,
2.5 h)
All DNA was analyzed for a set of seven KRAS
point mutations, 12-Ala, Asp, Arg, Cys, Ser, Val and 13-Asp, using
the Therascreen KRAS kit (870011; Qiagen GmbH). The analysis was
performed on a quantitative PCR instrument (Rotor-Gen Q; Qiagen
GmbH).
Results and discussion
Blood samples were studied from 23 mCRC patients
whose primary tumor KRAS mutational status was available.
Following the enrichment of the CTCs, three fractions were
obtained, CD45+, CD45−/CD326− and
CD45−/CD326+. The KRAS Mutation kit
detected seven specific KRAS mutations using a region of the
KRAS exon-4 as a control. The CD45+ cell fraction
contained mainly leukocytes, thus, a signal appeared in the control
assay that corresponds to the KRAS exon-4, but no
KRAS mutation was detectable, since somatic mutations were
only present in the fraction that contains the CTCs, if detectable.
The CD45−/CD326− and
CD45−/CD326+ cell fractions exhibited a
signal of the KRAS exon-4 control due possibly to the
residual mononuclear cells; however, the amplification signal
appeared at a higher cycle compared with the corresponding
CD45+ since the expected mononuclear cells amount was
low. The CTCs were identified in the
CD45−/CD326+ cell fraction and, therefore,
the KRAS mutations, if detectable by the kit, appear in this
fraction. The present study identified a correspondence between the
primary tumor-KRAS status and the CTC-KRAS status in
17 patients (15 wild-type and two mutated). In five cases, the
tumor-KRAS mutation was not detected in the CTCs. In those
patients, a low concentration of CTCs explained the results as
their DNA concentration was below the kit detection limits.
Notably, another discrepancy was identified in one patient (case
CR6), who demonstrated a KRAS wild-type in the primary tumor
and a KRAS-12Ala mutation in the CTC enriched cell fraction
(Fig. 2). In this case, another
scenario should be proposed. It is known that in a minority of
cases (5–10%) the KRAS mutational status is heterogeneous
and may vary between the primary tumor and the metastasis (13). In a recent study, Bossard et
al defined a KRAS mutational mosaicism, with 4 out of 18
patients showing KRAS status discordances between the
primary colorectal cancer and the metastasis (14). The biological significance of this
mosaicism is unclear, but indicates diverse metastatic potentials
in various populations of tumor cells and/or the acquirement of
mutations during or following metastasis. Bouchahda et al
proposed that KRAS mutations may be acquired late during the
metastatic phase of colorectal cancer more frequently than
currently recognized (15).
Furthermore, since CTCs may be considered as an intermediate step
in colonization, their metastatic potential depends on a number of
genetic abnormalities that may include the acquisition of a
KRAS mutation. Therefore, the CTC-KRAS-Ala mutation
that was identified in a patient from the present study may lead to
an improved adaptation to adverse conditions, migration and
colonization of distant tissues. By the end of the present study,
the CR6 patient developed hepatic metastasis (tissue from
metastases not available) and a change from anti-EGFR to
Irinotecan-based treatment was considered. Future studies examining
large series and examining KRAS, BRAF, PTEN
and PI3K may provide valuable insight into the
carcinogenesis and metastasizing patterns of mCRC, thus guiding
treatment options for patients.
In summary, the present study indicated that the
isolation and analysis of CTCs from the peripheral blood of mCRC
patients, using a minimally invasive, relatively economical and
optimized method, may have high clinical relevance. There are
limitations to the procedure, since during epithelial to
mesenchymal transition, the expression of epithelial markers on
CTCs, such as EpCAM may be downregulated and become undetectable
(16). CTC studies represent an
alternative to invasive procedures and their analysis may become a
prognostic factor, acting as a ‘liquid biopsy’. CTCs are able to
survive chemotherapy and may indicate a lack of therapeutic
efficacy, allowing the early end of ineffective treatments and
providing a representation of the current disease status of the
patient.
Acknowledgements
The authors would like to thank all the patients
that participated in this study.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Allegra CJ, Jessup JM, Somerfield MR, et
al: American Society of Clinical Oncology provisional clinical
opinion: Testing for KRAS gene mutations in patients with
metastatic colorectal carcinoma to predict response to
anti-epidermal growth factor receptor monoclonal antibody therapy.
J Clin Oncol. 27:2091–2096. 2009. View Article : Google Scholar
|
|
3
|
Jimeno A, Messersmith WA, Hirsch FR,
Franklin WA and Eckhardt SG: KRAS mutations and sensitivity to
epidermal growth factor receptor inhibitors in colorectal cancer:
practical application of patient selection. J Clin Oncol.
27:1130–1136. 2009. View Article : Google Scholar
|
|
4
|
Sartore-Bianchi A, Bencardino K,
Cassingena A, et al: Therapeutic implications of resistance to
molecular therapies in metastatic colorectal cancer. Cancer Treat
Rev. 36(Suppl 3): S1–S5. 2010. View Article : Google Scholar
|
|
5
|
De Roock W, De Vriendt V, Normanno N,
Ciardiello F and Tejpar S: KRAS, BRAF, PIK3CA, and PTEN mutations:
Implications for targeted therapies in metastatic colorectal
cancer. Lancet Oncol. 12:594–603. 2011.PubMed/NCBI
|
|
6
|
Artale S, Sartore-Bianchi A, Veronese SM,
et al: Mutations of KRAS and BRAF in primary and matched metastatic
sites of colorectal cancer. J Clin Oncol. 26:4217–4219. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kosakowska EA, Stec R, Charkiewicz R,
Skoczek M and Chyczewski L: Molecular differences in the KRAS gene
mutation between a primary tumor and related metastatic sites -
case report and a literature review. Folia Histochem Cytobiol.
48:597–602. 2010.
|
|
8
|
Baldus SE, Schaefer KL, Engers R, Hartleb
D, Stoecklein NH and Gabbert HE: Prevalence and heterogeneity of
KRAS, BRAF, and PIK3CA mutations in primary colorectal
adenocarcinomas and their corresponding metastases. Clin Cancer
Res. 16:790–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ashworth T: A case of cancer in which
cells similar to those in the tumours were seen in blood after
death. Aust Med J. 14:146–149. 1869.
|
|
10
|
Gerges N, Rak J and Jabado N: New
technologies for the detection of circulating tumour cells. Br Med
Bull. 94:49–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alunni-Fabbroni M and Sandri MT:
Circulating tumour cells in clinical practice: Methods of detection
and possible characterization. Methods. 50:289–297. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lustberg M, Jatana KR, Zborowski M and
Chalmers JJ: Emerging technologies for CTC detection based on
depletion of normal cells. Recent Results Cancer Res. 195:97–110.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baas JM, Krens LL, Guchelaar HJ, Morreau H
and Gelderblom H: Concordance of predictive markers for EGFR
inhibitors in primary tumors and metastases in colorectal cancer: A
review. Oncologist. 16:1239–1249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bossard C, Küry S, Jamet P, et al:
Delineation of the infrequent mosaicism of KRAS mutational status
in metastatic colorectal adenocarcinomas. J Clin Pathol.
65:466–469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bouchahda M, Karaboué A, Saffroy R, et al:
Acquired KRAS mutations during progression of colorectal cancer
metastases: Possible implications for therapy and prognosis. Cancer
Chemother Pharmacol. 66:605–609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wicha MS and Hayes DF: Circulating tumor
cells: not all detected cells are bad and not all bad cells are
detected. J Clin Oncol. 29:1508–1511. 2011. View Article : Google Scholar : PubMed/NCBI
|