Introduction
Gastric carcinoma is the fourth most common type of
cancer worldwide and is the world’s second leading cause of
cancer-related mortality (1). A
combination of traditional treatments, such as curative surgery,
radiotherapy and perioperative chemotherapy, may improve the
survival rate of operable gastric carcinoma patients; up to 40–50%
of patients who undergo potentially curative surgery alone
ultimately relapse and die of metastatic disease (2). Therefore, surgery combined with
chemotherapeutic agents may currently be the optimum strategy for
gastric cancer therapy (3). Over
the past 40 years, 5-fluorouracil (5-FU) has become the first-line
chemotherapeutic agent for treating gastric carcinoma (4). However, low response rates and cell
toxicity present obstacles to 5-FU-based chemotherapy (5,6). Thus,
evaluation of the effect of new drugs or the effect of new
combinations with established drugs is required. In addition,
identification of novel agents that may be combined with 5-FU to
achieve improved therapeutic effects and decreased host toxicity is
a promising method.
Advances in the study of traditional Chinese
medicine have led to the discovery of numerous novel
chemotherapeutic agents. Sinomenine
(7,8-didehydro-4-hydroxyl-3,7-dimethoxy-17-methylmorphinan-6-one;
SIN), a bioactive alkaloid derived from a Chinese medicinal plant,
has been demonstrated to be an effective therapy for rheumatic and
arthritic diseases. Previous studies have demonstrated that the
pharmacological profiles of SIN include immunosuppression,
anti-inflammation and cytoprotection to exert anti-inflammatory and
immunosuppressive activities (7,8). SIN
has been reported to have an antitumor effect in several types of
cancer cells, such as synovial sarcoma, lung cancer, hepatocellular
carcinoma and gastric cancer (9–13).
However, whether it sensitizes human gastric cancer cells to 5-FU
has not yet been investigated. Thus far, there is little
information available regarding the antitumor effects of SIN
combined with 5-FU in human gastric cancer cells. The present study
was designed to evaluate the efficacy of SIN when used in
combination with 5-FU, and to explore the mechanisms underlying the
effects of SIN and 5-FU. In this study, the in vitro
inhibitory effects of SIN on the growth of several human gastric
carcinoma cell lines were evaluated and cell apoptosis was detected
in vitro. The in vitro inhibitory effect was verified
using mouse xenograft models. The findings, particularly following
in vivo verification, provide scientific evidence that a
combination of SIN and 5-FU may be a promising anticancer
therapeutic method, should the results be reproduced in clinical
trials. The results of the present study may provide a novel
perspective on gastric cancer therapy.
Materials and methods
Cell culture and reagents
Human gastric carcinoma cell lines, MKN-28, SGC-709,
BGC-823 and HGC-27, were purchased from the Cell Bank of Type
Culture Collection of Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in RPMI-1640 medium (Sigma-Aldrich,
St. Louis, MO, USA), supplemented with 10% fetal bovine serum
(Gibco-BRL, Gaithersburg, MD, USA), 50 mg/ml streptomycin, 50 IU/ml
penicillin and 2 mM glutamine (Sigma-Aldrich), and the cell
cultures were maintained in a 5% CO2 humidified
atmosphere at 37°C. SIN and 5-FU were obtained from Sigma-Aldrich
and dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich), and stock
solutions (100 mM) were stored at −20°C.
MTT assay and evaluation of the combined
effects of SIN and 5-FU
Cells were seeded at a density of 4×103
cells/well into a 96-well plate and allowed to attach overnight.
The cells were treated with different drug groups (with or without
the combination). For the control group, 0.1% DMSO was applied,
which was the same concentration as that applied to the drug
treatment groups. Upon termination of drug treatment, MTT
(Sigma-Aldrich) was applied to each well at a final concentration
of 0.5 g/l. Following incubation for 4 h at 37°C, the supernatant
was discarded, 100 μl DMSO was applied and the MTT-formazan
products were extracted. The absorbance was read at 570 nm using a
96-well microplate reader (Perkin-Elmer, Waltham, MA, USA). Each
data point is the average of the results from five wells.
Triplicate experiments with triplicate samples were performed. The
results are expressed as inhibition rates (IRs), which were
calculated using the following equation: IR = [(A−B)/A] × 100,
where A and B represent the absorbance of the control and sample
groups, respectively.
The combination index (CI) and isobologram methods
of Chou and Talalay (14) and Chou
et al(15) were used to
evaluate the natural interaction between SIN and 5-FU. Assessment
of the synergy, using a fixed constant ratio of the combination
agents, was accomplished by calculating the CI and isobologram. The
CI values were obtained using Biosoft CalcuSyn software (Biosoft,
Cambridge, UK). CI<1, CI=1 and CI>1 indicate synergism,
summation and antagonism, respectively.
Detection of apoptotic cells by Hoechst
33258 staining and flow cytometry
The morphological features of apoptotic cells
(chromatin condensation and fragmentation) were detected by Hoechst
33258 staining as follows: MKN-28 cells were treated with 100 mg/l
5-FU, 40 μM SIN or 50mg/l 5-FU + 20 μM SIN for 24 h, followed by
incubation with 20 μM Hoechst 33258 (Sigma-Aldrich) for 10 min at
room temperature. The cells were washed twice with
phosphate-buffered saline (PBS) and examined under a Nikon 80i
fluorescence microscope (Nikon Corporation, Tokyo, Japan). In each
case, 10 random visual fields and >500 cells per field were
counted.
The number of apoptotic cells was analyzed by flow
cytometry using the MEBCYTO Apoptosis kit (MBL Co. Ltd., Nagoya,
Japan). Briefly, 2×106 cells were cultured in a 100-mm
culture dish and harvested after a 24-h incubation period with 100
mg/l 5-FU, 40 μM SIN or 50 mg/l 5-FU + 20 μM SIN. The cells were
then gently washed with PBS and resuspended in 100 μl of binding
buffer. Annexin V-FITC (10 μl) and propidium iodide (5 μl) were
applied to the resuspended cells. Following incubation at room
temperature for 15 min in the dark, the stained cells were analyzed
by flow cytometry using a single laser emitting excitation light at
488 nm (Bio-Rad Laboratories, Hercules, CA, USA).
Western blot analysis
Cells were lysed in RIPA lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China) for 20 min on ice,
followed by centrifugation at 13,000 × g for 5 min. The extracted
protein samples were separated on sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gels (40 μg/lane) and
transferred to nitrocellulose membranes (Millipore, Bedford, MA,
USA). The membranes were blocked with 5% skimmed milk in
Tris-buffered saline and Tween 20 (TBST) buffer, and then incubated
with primary antibodies overnight at 4°C. The primary antibodies
and concentrations were as follows: Cytochrome c (1:500;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA); β-actin
(1:1,000; Santa Cruz Biotechnology, Inc.); and caspase-3 and
caspase-9 (1:500; Cell Signaling Technology, Inc., Beverly, MA,
USA). Following extensive rinsing with TBST buffer, the membranes
were incubated with horseradish peroxidase-conjugated secondary
antibodies (1:5,000; Pierce Biotechnology, Inc., Rockford, IL,
USA). The bound antibodies were visualized using an enhanced
chemiluminescence reagent (Amersham Pharmacia Biotech, Piscataway,
NJ, USA) and quantified by densitometry using a Bio-Electrophoresis
image analysis system (SF9-FR-980; Shanghai Furi Science and
Technology Co., Ltd., Shanghai, China). Data are expressed as the
relative density of the protein normalized to that of β-actin. The
rates of inhibition were estimated by comparison with the untreated
control (100%). Triplicate experiments with triplicate samples were
performed.
RT-PCR
Total RNA was extracted from the MKN-28 cells after
a 24-h incubation period with 100 mg/l 5-FU, 40 μM SIN or 50 mg/l
5-FU + 20 μM SIN, using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). Reverse transcription was
performed using the First Strand cDNA Synthesis kit (Toyobo Co.,
Ltd., Osaka, Japan) according to the manufacturer’s instructions.
Primer sequences were as follows: F: 5′-ACCAACCCTGACGACAGAAGA-3′
and R: 5′-AGCGC CATCAGAGGAAGATCT-3′ for thymidylate synthase (TS);
and F: 5′-CCATCGTCCACCGCAAAT-3′ and R: 5′-TGCTC GCTCCAACCGACT-3′
for β-actin. β-actin was used as an internal control (housekeeping
gene) in all experiments. PCR was performed using a Gene Cycler
(Bio-Rad, Hercules, CA, USA). PCR products were confirmed by
agarose gel electrophoresis. Gels were visualized and photographed
under UV light, and the optical densities of the bands were
analyzed using BandScan software, version 5.0 (Glyko, Inc., San
Leandro, USA).
Antitumor effects of SIN and 5-FU in
vivo
Male outbred BALB/c-nu/nu mice (4 weeks of age) were
purchased from the Animal Laboratory of Hubei Provincial Center of
Disease Control (Wuhan, China), and maintained under specific
pathogen-free conditions. The study was approved by the ethics
committee of the Animal Care and Use Committee at Wuhan University
(Wuhan, China). To establish human gastric xenografts, a density of
5.0×106 MKN-28 cells in 0.2 ml PBS were inoculated into
the lower right flank of each nude mouse (n=6 in each group) using
a 24-gauge needle. Following growth for six days, the tumor
xenografts reached a mean size of 100 mm3. Eighteen mice
with tumor xenografts of ~100 mm3 in size were chosen
and randomly divided into four groups: i) control (equal volume of
physiological saline); ii) 40 mg/kg 5-FU; iii) 20 mg/kg SIN; and
iv) 20 mg/kg 5-FU + 10 mg/kg SIN. All mice were administered the
aforementioned drugs via intratumoral injection, once every three
days. Following the last injection, all mice were sacrificed on day
30. During the autopsy procedure, the tumor was completely excised
and weighed. Tumor diameters were measured at regular intervals
with digital calipers, and the tumor volume in mm3 was
calculated using the following formula: Volume = 0.5 × a ×
b2 (a, largest diameter; b, smallest diameter).
TUNEL assay
For histological examination, tumor tissues were
fixed in 10% buffered formalin and embedded in paraffin, and tissue
sections (4-μm) were prepared. A TUNEL assay for apoptosis was
conducted using an In Situ Cell Death Detection kit (Roche
Diagnostics, Branchburg, NJ, USA) according to the manufacturer’s
instructions. Positive cells were counted as the number of
TUNEL-labeled cells per 100 epithelial cancer cells in 10 fields of
the most affected tumor areas, with ×400 magnification, and
analyzed using light microscopy (Carl Zeiss, Thornwood, NY,
USA).
Hematological side effects of SIN and
5-FU in vivo
To assess the hematological side effects of the
chemotherapy in vivo, blood samples were collected before
mice were sacrificed by cardiac puncture using heparin-rinsed 1-ml
syringes (with 20-gauge needles; Shinva Medical Instrument Co.,
Ltd., Zibo, China), and were then centrifuged and maintained at
20°C until analyses. Standard techniques were adopted using an
Olympus AU2700 analyzer (Olympus Optical Co., Ltd., Tokyo, Japan)
to detect the activity of alanine aminotransferase (ALT), aspartate
aminotransferase (AST), blood urea nitrogen (BUN) and serum
creatinine (Cr); the biomarkers of liver and renal injury.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 18.0 (SPSS Inc., Chicago, IL, USA). Data are
expressed as the means ± SD, and comparisons between different
groups were conducted by one-way analysis of variance. P<0.05
was considered to indicate a statistically significant
difference.
Results
Growth inhibitory effect of SIN and/or
5-FU
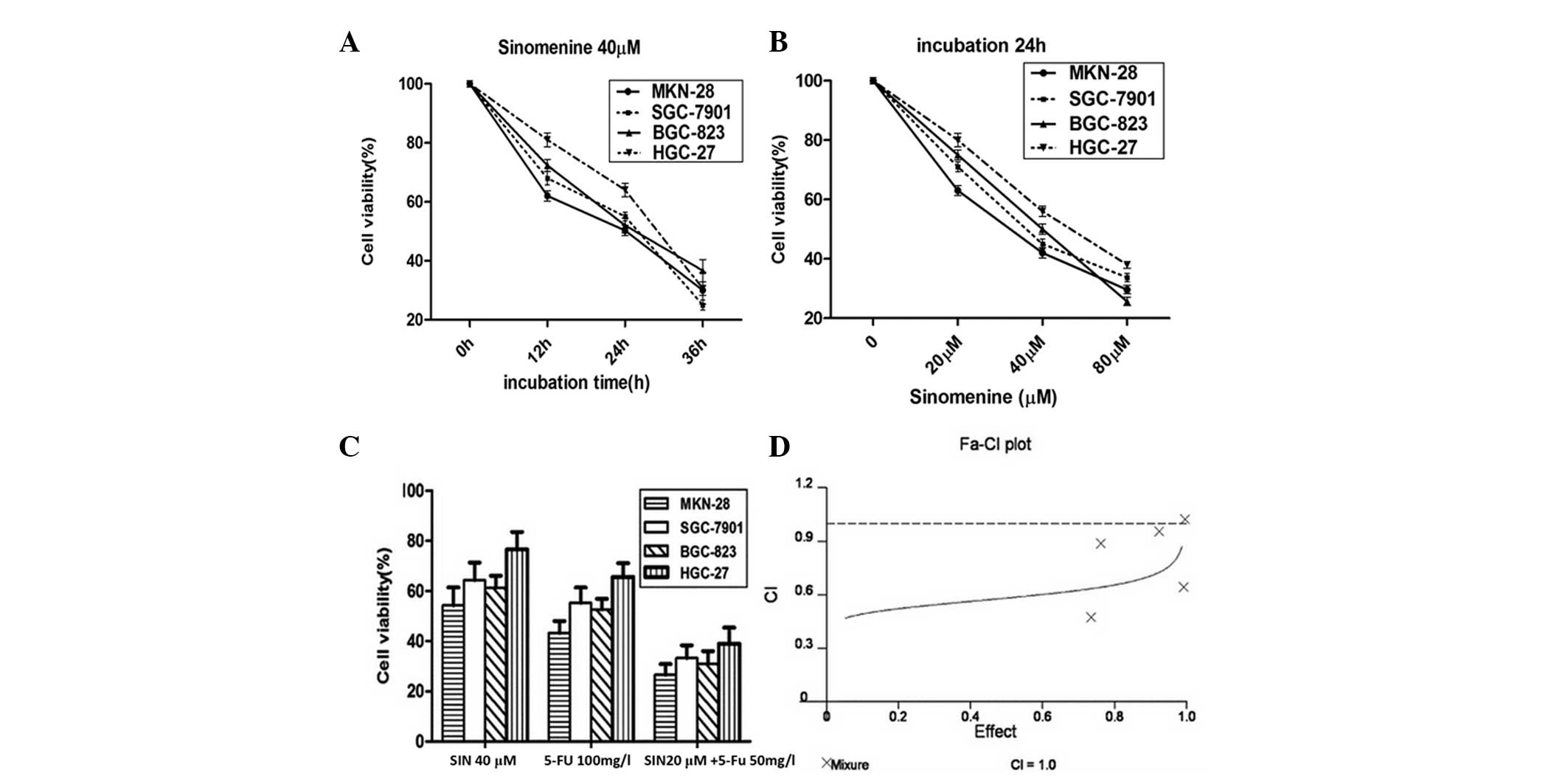
The four gastric cancer cell lines were treated with
SIN at various concentrations (20, 40 and 80 μM) for 24 h, or
treated with 40 μM SIN for different time periods (12, 24 or 36 h)
(Fig. 1A and B). As predicted, the
cell viability was significantly inhibited by SIN treatment in a
dose- and time-dependent manner.
Moreover, when half of the effective doses (20 μM
SIN plus 50 mg/l 5-FU) of two drugs were combined together, the
inhibitory effect was significantly higher than that of either of
the full effective doses of drugs used individually (40 μM SIN or
100 mg/l 5-FU) (Fig. 1C).
The combinational inhibition rate was analyzed using
the CI and isobologram methods of Chou and Talalay (14) and Chou et al(15). The experiments were repeated at
least three times. The mean of the CI values was <1, indicating
that SIN and 5-FU had a synergistic effect on proliferation
inhibition of the gastric cancer cells (Fig. 1D).
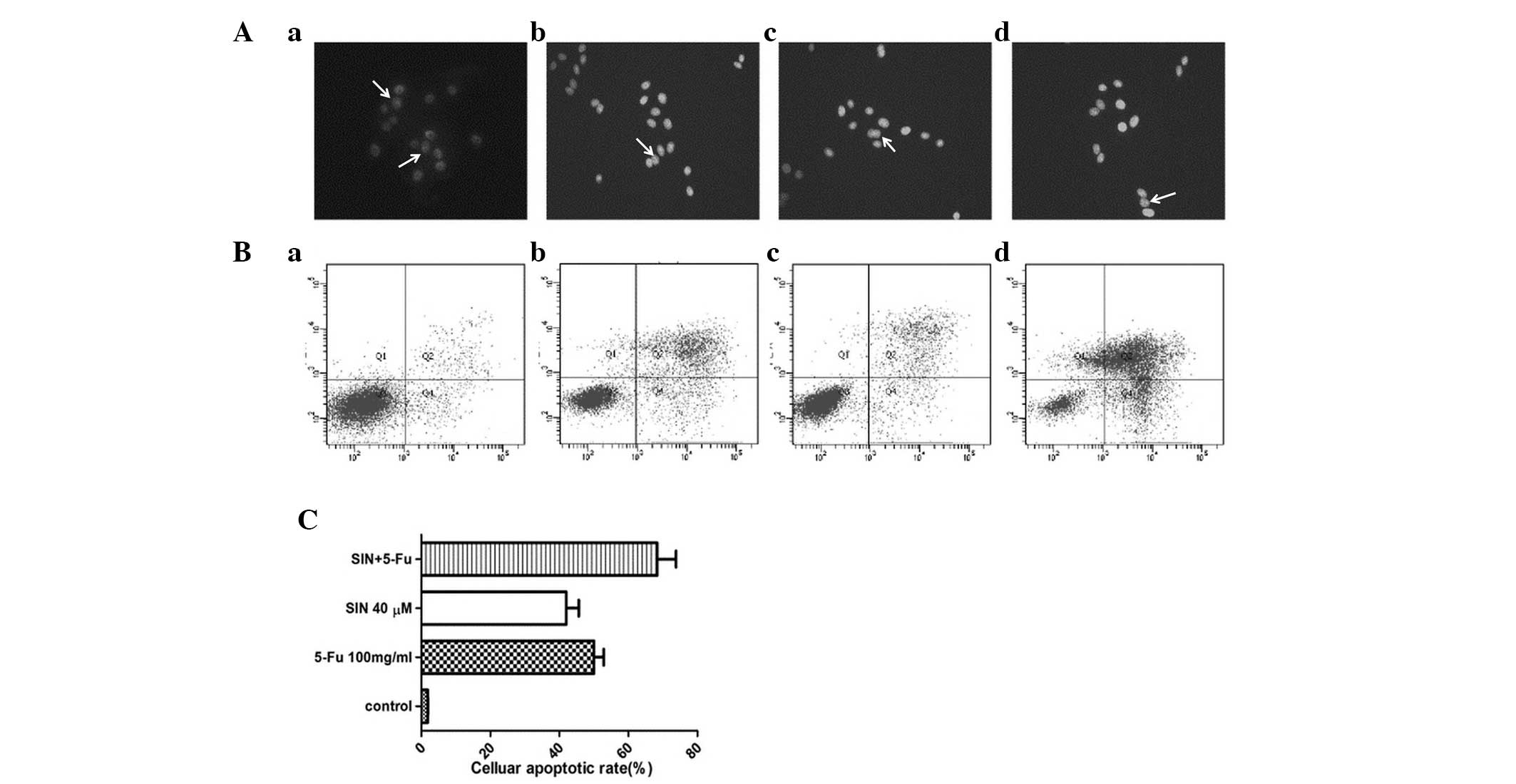
Apoptosis induced by SIN and 5-FU
The MKN-28 cells were exposed to 100 mg/l 5-FU, 40
μM SIN or 20 μM SIN + 50 mg/l 5-FU for 24 h. Morphological changes
characteristic of apoptotic cells (chromatin condensation and
fragmentation) were detected by Hoechst 33258 staining. Typical
apoptotic nuclei are indicated by white arrows in Fig. 2A. The mean apoptotic rate in the SIN
(40 μM), 5-FU (100 mg/l) and combination treatment (20 μM SIN + 50
mg/l 5-FU) groups were 40.37, 50.44 and 68.37%, respectively
(Fig. 2C), demonstrating that SIN
sensitized the gastric cancer cells to 5-FU-induced apoptosis. In
addition, flow cytometry was performed to confirm that addition of
SIN enhances 5-FU-induced early apoptosis (Fig. 2B and Table I).
 | Table IEarly cellular apoptotic rate (%). |
Table I
Early cellular apoptotic rate (%).
| Group | Early cellular
apoptotic rate (%) |
|---|
| Control | 1.1±0.09 |
| SIN (40 μM) | 15.2±1.35a |
| 5-FU (100 mg/l) | 20.8±17.20a |
| SIN (20 μM) + 5-FU
(50 mg/l) | 43.2±5.05a |
To further clarify the potential mechanisms by which
SIN enhances 5-FU-induced apoptosis, the protein expression levels
of cytochrome c, caspase-9 and caspase-3 were examined by
western blot analysis. 5-FU treatment led to the release of
cytochrome c from the mitochondria into the cytosol, and the
activation of caspase-3 and caspase-9, and addition of SIN enhanced
these changes (Fig. 3A).
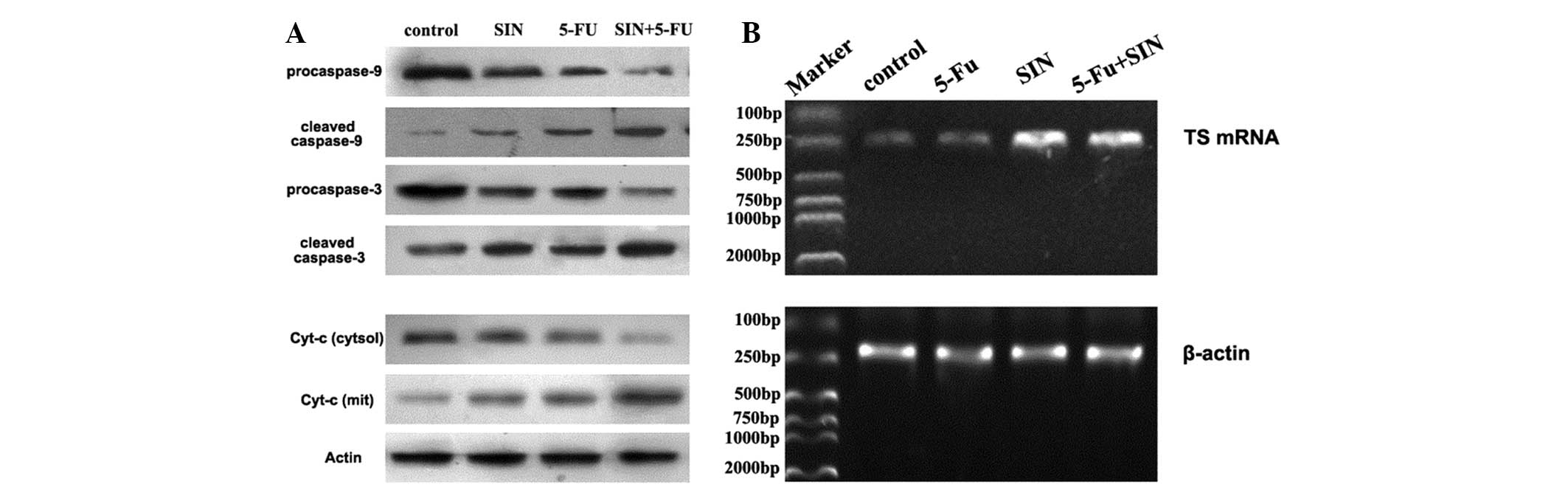 | Figure 3(A) Effect of 24 h of SIN and/or 5-FU
treatment on the expression of apoptosis-related proteins in MKN-28
gastric cancer cells was assessed by immunoblotting analysis. Actin
was used as an internal control. The lanes, from left to right, are
as follows: Lane 1, control; lane 2, 40 μM SIN; lane 3, 100 mg/l
5-FU; and lane 4, 20 μM SIN + 50 mg/l 5-FU. (B) Effect of 24 h of
SIN and/or 5-FU treatment on TS mRNA expression in MKN-28 cells.
β-actin was used as an internal control. The lanes, from left to
right, are as follows: Lane 1, marker; lane 2, control; lane 3, 100
mg/l 5-FU; lane 4, 40 μM SIN; and lane 5, 20 μM SIN + 50 mg/l 5-FU.
SIN, sinomenine; 5-FU, 5-fluorouracil; Cyt-c, cytochrome
c; TS, thymidylate synthase. |
Expression of TS mRNA in 5-FU- and
SIN-treated cells
To understand the molecular basis of the increased
antitumor effects elicited by SIN, RT-PCR was performed to measure
the expression of the 5-FU-associated gene TS, which is widely used
to predict patients’ outcomes after chemotherapy. As shown in
Fig. 3B, 5-FU treatment led to a
decrease in the mRNA levels of TS in the MKN-28 cells, and SIN
treatment potentiated this effect.
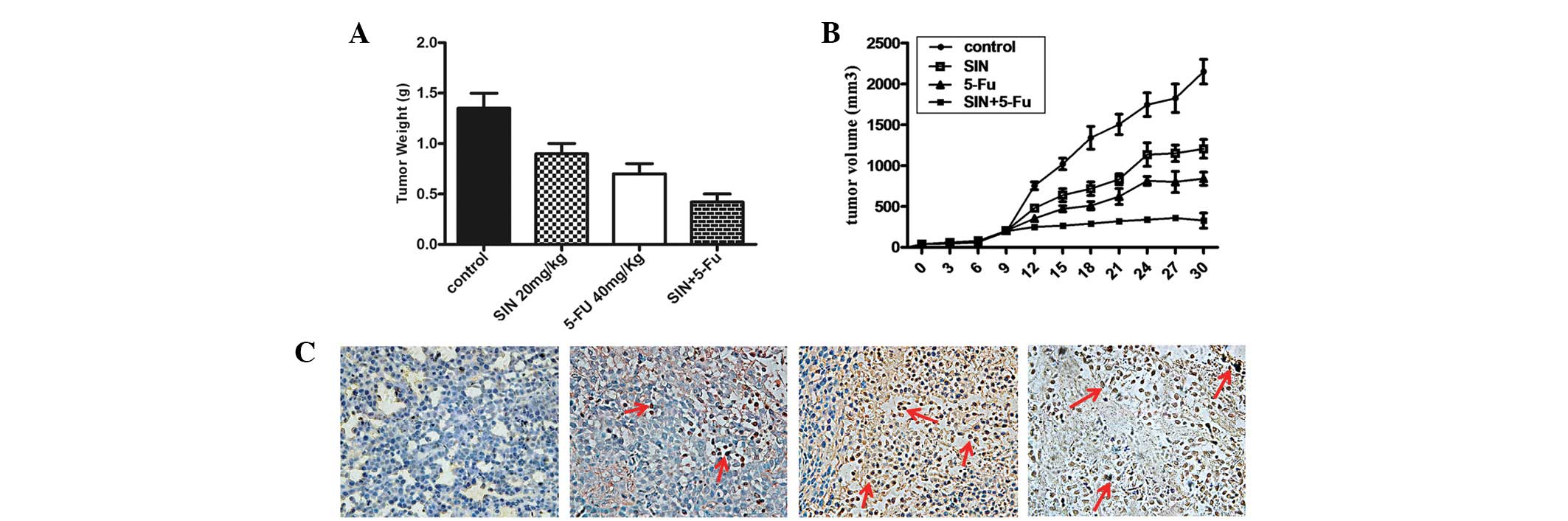
Antitumor effects of SIN, 5-FU and
combination treatment in vivo
An in vivo study was also designed to
evaluate the antitumor efficacy of SIN and/or 5-FU treatment in a
gastric cancer xenograft model. Tumor volumes and weights were
reduced sharply in the drug-treated group compared with those in
the control group, though the degree of tumor suppression varied
(Fig. 4A and B). The tumor volumes
and weights of the combination group (20 mg/kg 5-FU + 10 mg/kg SIN)
were lower than those of the SIN (20 mg/kg) and 5-FU (40 mg/kg)
groups. These results demonstrate that the antitumor effect of SIN
combined with 5-FU was superior to the effect of either drug used
individually.
As previously described, SIN may render cells
sensitive to 5-FU treatment by increasing the induction of
apoptosis in vitro. To further examine this effect in
vivo, an in situ TUNEL assay was used to detect apoptotic cells
in subcutaneous tumor sections. The results demonstrate that
apoptosis occurred in the SIN group, the 5-FU group and the
combined group, whereas few apoptotic cells were found in the
control group (Fig. 4C). Of the
three drug-treated groups, the combined group exhibited a higher
number of apoptotic bodies compared with that of the other two
groups. The 10 mg/kg SIN combined with 20 mg/kg 5-FU treatment was
generally well-tolerated by the mice during the long-term
treatment.
Evaluation of side effects in vivo
On completion of the experiment, the nude mice were
sacrificed, and hepatic and renal toxicity were monitored by
quantitative analysis of the serum ALT, AST, BUN and Cr levels.
Notably, although the mice subjected to 5-FU showed increased
levels of ALT, AST, BUN and Cr in the serum compared with those of
the saline chloride control group (P>0.05), the addition of SIN
did not induce any marked increases in the levels of ALT, AST, BUN
and Cr in the serum (Table II).
The blood cell count of the nude mice, including white blood count
and platelet count were detected. The results indicated that SIN
combined with 5-FU did not enhance the hematological side effects
and no significant reduction in body weight was observed in the SIN
or SIN + 5-FU groups (data not shown).
 | Table IIAnalysis of the hematological index of
SIN- and/or 5-FU-treated groups in vivo. |
Table II
Analysis of the hematological index of
SIN- and/or 5-FU-treated groups in vivo.
| Group | n | ALT (U/l) | AST (U/l) | BUN (μmol/l) | Cr (μmol/l) | PLT
(×109/l) | WBC
(×109/l) |
|---|
| Control | 6 | 37.50 (10.37) | 125.00 (21.37) | 7.23 (0.81) | 17.27 (2.98) | 105.7 (20.4) | 7.3 (1.6) |
| SIN | 6 | 42.33 (11.55) | 135.83 (26.66) | 8.02 (1.88) | 20.26 (1.86) | 103.9 (11.9) | 7.6 (1.5) |
| 5-FU | 6 | 49.50 (16.50) | 140.33 (42.65) | 8.62 (1.18) | 21.25 (3.00) | 109.4 (18.0) | 7.7 (2.0) |
| SIN+5-FU | 6 | 39.00 (10.22) | 131.17 (25.99) | 7.42 (1.31) | 19.89 (1.57) | 110.5 (21.5) | 7.9 (1.4) |
Discussion
This study demonstrated that administration of SIN
leads to an inhibitory effect on gastric cancer cells, and enhances
the antitumor effects of 5-FU in vitro and in vivo.
The key findings of this study include: i) SIN treatment may reduce
cell viability and prominently increase tumor cell apoptosis; ii)
addition of SIN may reduce the effective dose of 5-FU for gastric
cancer treatment; iii) the inhibitory effect of 5-FU was notably
elevated when combined with SIN, as evidenced by the detection of
cell proliferation (tumor growth), apoptosis-related protein and
the 5-FU-associated gene TS; and iv) the data obtained in
vivo indicate that SIN has potential as a novel agent that
sensitizes gastric cancer cells to 5-FU.
Gastric cancer usually has a poor prognosis and most
patients are either diagnosed at an advanced stage or are subject
to relapse following curative surgery (3,16). For
advanced cancer patients, the currently available treatments are
limited to systemic administration of conventional chemotherapy
drugs, 5-FU and cisplatin, or their analogs, with or without an
anthracycline. However, relying solely on these individual drugs
does not improve the five-year survival rate of patients due to
their severe side effects and associated drug resistance (17,18).
Plant-derived compounds have attracted great interest due to their
potential anticancer properties and low toxicity levels.
SIN is a bioactive alkaloid isolated from the
Chinese herbal plant Sinomenium acutum Rehd. et Wils
(Menispermaceae family). It has been utilized to treat rheumatic
and arthritic diseases in China for >1,000 years (19,20).
Increasing evidence has indicated that SIN exhibits antitumor
actions in various types of cancer cells (9–12).
However, its effect on gastric cancer remains unknown. The only
study to date that has addressed the association between SIN and
gastric cancer was that by Lv et al(13) in the USA. The authors indicated that
SIN inhibits the proliferation of SGC-7901 gastric adenocarcinoma
cells via suppression of cyclooxygenase-2 expression. Yet, whether
SIN is able to sensitize gastric cancer cells to the effect of 5-FU
is still not clear. The current study further confirmed that SIN
inhibited the proliferation of several types of gastric cancer
cells. It also demonstrated a synergistic antiproliferative effect
of SIN with 5-FU, by inducing apoptosis in a time- and
concentration-dependent manner.
Apoptosis is a highly regulated process that is
activated by various stimuli that converge via different pathways.
The mitochondrial pathway is considered to be pivotal in cell
apoptosis. In the process, a number of stimuli cause the disruption
of mitochondrial function and ultimately lead to the release of
cytochrome c from the mitochondria into the cytosol
(21). Cytochrome c then
binds to Apaf-1, which further complexes with caspase-9 to form the
apoptosome and promotes cleavage of downstream effector caspases
(such as caspase-3) to trigger apoptosis (22–24).
To elucidate the mechanisms underlying synergistic apoptosis
induction by SIN and 5-FU, the present study investigated the
expression of key apoptosis-related molecules. The data show that
combining the 5-FU treatment with SIN increases cytochrome c
release from the mitochondria into the cytosol, and increases the
activation of caspase-3 and caspase-9, compared with that of 5-FU
treatment alone. Therefore, our findings imply that the
mitochondrial pathway is a key factor in enabling SIN to enhance
5-FU-induced apoptosis.
Another predominant finding of the present study was
that SIN treatment significantly lowers the levels of TS mRNA.
Previous studies have confirmed that TS is not only a key gene
involved in 5-FU metabolism; it is closely associated with the
resistance to 5-FU chemotherapy that is observed in numerous cancer
patients. Three separate studies have identified that increased TS
expression is clearly associated with resistance to 5-FU in murine
colon adenocarcinoma and human gastrointestinal cancer cell lines
(25–27). Conversely, several studies have
revealed that decreased TS expression levels in tumors are closely
associated with enhanced efficacy of 5-FU treatment (28–31).
Consistent with these studies, the results of the present study
showed that SIN treatment significantly inhibited TS mRNA
expression; this effect may be responsible for SIN’s enhancement of
sensitivity to 5-FU.
Collectively, the data presented in this study
suggest that SIN may serve as a drug sensitizer for 5-FU in gastric
cancer cells, and that the mechanisms underlying this effect may be
associated with increases in apoptosis via the mitochondrial
pathway and downregulation of TS mRNA expression. This indicated
that a combination of SIN and 5-FU may result in an improved
response to therapy in patients with gastric cancer compared with
that in patients treated with 5-FU alone. These findings reveal a
promising strategy to improve chemotherapeutic sensitivity in
gastric cancer patients.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 30871147). The
authors sincerely thank Mr. Hong Xia from the Institute of
Gastroenterology and Hepatology, Renmin Hospital of Wuhan
University (Wuhan, China).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Jamal A, Murray T, Samuels A, Ghafoor A,
Ward E and Thun M: Cancer statistics, 2003. CA Cancer J Clin.
53:5–26. 2003. View Article : Google Scholar
|
|
3
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Engl
J Med. 345:725–730. 2001. View Article : Google Scholar
|
|
4
|
Mackenzie M, Spithoff K and Jonker D:
Systemic therapy for advanced gastric cancer: a clinical practice
guideline. Curr Oncol. 18:e202–e209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shekhar MP: Drug resistance: challenges to
effective therapy. Curr Cancer Drug Targets. 11:613–623. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pasini F, Fraccon AP and DE Manzoni G: The
role of chemotherapy in metastatic gastric cancer. Anticancer Res.
31:3543–3554. 2011.PubMed/NCBI
|
|
7
|
Qian L, Xu Z, Zhang W, Wilson B, Hong JS
and Flood PM: Sinomenine, a natural dextrorotatory morphinan
analog, is anti-inflammatory and neuroprotective through inhibition
of microglial NADPH oxidase. J Neuroinflammation. 4:23–37. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q and Li XK: Immunosuppressive and
anti-inflammatory activities of sinomenine. Int Immunopharmacol.
11:373–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Yue PY, Ha WY, et al: Effect of
sinomenine on gene expression of the IL-1 beta-activated human
synovial sarcoma. Life Sci. 79:665–673. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang T, Zhou L, Zhang W, Qu D, Xu X, Yang
Y and Li S: Effects of sinomenine on proliferation and apoptosis in
human lung cancer cell line NCI-H460 in vitro. Mol Med Report.
3:51–56. 2010.PubMed/NCBI
|
|
11
|
Zhou L, Luan H, Liu Q, Jiang T, Liang H,
Dong X and Shang H: Activation of PI3K/Akt and ERK signaling
pathways antagonized sinomenine-induced lung cancer cell apoptosis.
Mol Med Report. 5:1256–1260. 2012.PubMed/NCBI
|
|
12
|
Hong Y, Yang J, Shen X, et al: Sinomenine
hydrochloride enhancement of the inhibitory effects of
anti-transferrin receptor antibody-dependent on the COX-2 pathway
in human hepatoma cells. Cancer Immunol Immunother. 62:447–454.
2013. View Article : Google Scholar
|
|
13
|
Lv Y, Li C, Li S and Hao Z: Sinomenine
inhibits proliferation of SGC-7901 gastric adenocarcinoma cells via
suppression of cyclooxygenase-2 expression. Oncol Lett. 2:741–745.
2011.PubMed/NCBI
|
|
14
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chou TC, Motzer RJ, Tong Y and Bosl GJ:
Computerized quantitation of synergism and antagonism of taxol,
topotecan, and cisplatin against human teratocarcinoma cell growth:
a rational approach to clinical protocol design. J Natl Cancer
Inst. 86:1517–1524. 1994. View Article : Google Scholar
|
|
16
|
Dikken JL, van de Velde CJ, Coit DG, Shah
MA, Verheij M and Cats A: Treatment of resectable gastric cancer.
Therap Adv Gastroenterol. 5:49–69. 2012. View Article : Google Scholar
|
|
17
|
Tsutani Y, Yoshida K, Sanada Y, et al:
Decreased orotate phosphoribosyltransferase activity produces
5-fluorouracil resistance in a human gastric cancer cell line.
Oncol Rep. 20:1545–1551. 2008.
|
|
18
|
Meyer HJ and Wilke H: Treatment strategies
in gastric cancer. Dtsch Arztebl Int. 108:698–705. 2011.PubMed/NCBI
|
|
19
|
Liu L, Buchner E, Beitze D, Schmidt-Weber
CB, et al: Amelioration of rat experimental arthritides by
treatment with the alkaloid sinomenine. Int J Immunopharmacol.
18:529–543. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu B, Zeng Y, Yin C, Wang H, Yang X, Wang
S and Ji X: Sinomenine reduces iNOS expression via inhibiting the
T-bet IFN-γ pathway in experimental autoimmune encephalomyelitis in
rats. J Biomed Res. 26:448–455. 2012.PubMed/NCBI
|
|
21
|
Boatright KM and Salvesen GS: Mechanisms
of caspase activation. Curr Opin Cell Biol. 15:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J, Liu X, Bhalla K, et al: Prevention
of apoptosis by Bcl-2: release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Slee EA, Harte MT, Kluck RM, et al:
Ordering the cytochrome c-initiated caspase cascade: hierarchical
activation of caspases-2,-3,-6,-7,-8, and-10 in a
caspase-9-dependent manner. J Cell Biol. 144:281–292. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spears CP, Shahinian AH, Moran RG,
Heidelberger C and Corbett TH: In vivo kinetics of
thymidylatesynthetase inhibition of 5-fluorouracil-sensitive and
-resistant murine gastric adenocarcinomas. Cancer Res. 42:450–456.
1982.PubMed/NCBI
|
|
26
|
Kitchens ME, Forsthoefel AM, Barbour KW,
Spencer HT and Berger FG: Mechanisms of acquired resistance to
thymidylate synthase inhibitors: the role of enzyme stability. Mol
Pharmacol. 56:1063–1070. 1999.PubMed/NCBI
|
|
27
|
Kirihara Y, Yamamoto W, Toge T and
Nishiyama M: Dihydropyrimidine dehydrogenase, multidrug
resistance-associated protein, and thymidylate synthase gene
expression levels can predict 5-fluorouracil resistance in human
gastrointestinal cancer cells. Int J Oncol. 14:551–556. 1999.
|
|
28
|
Peters G, Van der Wilt C, Van Triest B, et
al: Thymidylate synthase and drug resistance. Eur J Cancer.
31:1299–1305. 1995. View Article : Google Scholar
|
|
29
|
Goekkurt E, Hoehn S, Wolschke C, Wittmer
C, Stueber C, Hossfeld DK and Stoehlmacher J: Polymorphisms of
glutathione S-transferases (GST) and thymidylate synthase (TS) -
novel predictors for response and survival in gastric cancer
patients. Br J Cancer. 94:281–286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnston PG, Lenz HJ, Leichman CG,
Danenberg KD, Allegra CJ, Danenberg PV and Leichman L: Thymidylate
synthase gene and protein expression correlate and are associated
with response to 5-fluorouracil in human colorectal and gastric
tumors. Cancer Res. 55:1407–1412. 1995.PubMed/NCBI
|
|
31
|
Lenz HJ, Leichman CG, Danenberg KD, et al:
Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a
predictor for primary tumor response and overall survival. J Clin
Oncol. 14:176–182. 1996.PubMed/NCBI
|