Introduction
Hepatocellular carcinoma (HCC) is the most common
primary malignancy of the liver. The prognosis of patients with HCC
is generally poor, as in the majority of cases, HCC is diagnosed at
an intermediate or advanced stage (1), and curative treatments (resection,
transplantation and radiofrequency) are only suitable for early
stage HCC patients (2).
Transarterial radioembolization (TARE) with yttrium-90 microspheres
is an emerging tool for the treatment of primary and metastatic HCC
(3). It produces average disease
control rates >80% and is usually extremely well-tolerated
(4).
Tumor response has been considered to be pivotal for
surrogate assessment of therapy efficacy. Response Evaluation
Criteria in Solid Tumors (RECIST) has been the standard method for
evaluation of solid tumors since its introduction in 2000 (5). A RECIST-defined response depends on
the change in size of target lesions, determined by non-invasive
imaging assessment, while a revised guideline known as the modified
RECIST criteria (mRECIST) takes into consideration changes in the
degree of tumor arterial enhancement (6). TARE may lead to disease stabilization
without actual shrinkage of tumor size, but with a decrease in
hypervascularity and the presentation of necrosis. Therefore,
evaluation based on tumor size alone, as RECIST or mRECIST, may no
longer be adequate for modern tumor therapy follow-up. Choi et
al have developed new criteria for gastrointestinal stromal
tumors (GIST), which assess a change in size or a change in density
of target lesions. Choi criteria appear to be better predictors of
clinical response to imatinib than RECIST (7). However, these criteria have not been
extensively evaluated in HCC patients treated with TARE.
In this study, we compared tumor responses according
to RECIST, mRECIST, Choi and modified Choi criteria in HCC patients
treated with TARE, and investigated their association with time to
progression (TTP) and overall survival (OS).
Patients and methods
Patients
The records of patients who were treated with
yttrium-90 TARE for intermediate or advanced HCC at the University
Hospital of Essen (Essen, Germany) from June, 2008 until December,
2012 were reviewed. A total of 149 patients were identified, of
which 36 were excluded due to either incomplete imaging or a
follow-up period of <12 weeks. All data were analyzed
retrospectively in an anonymous fashion according to the principles
expressed in the Declaration of Helsinki. This study was approved
by the institutional review boards of the University Hospital of
Essen (Essen, Germany).
Treatment
The microspheres used were glass-based (TheraSphere,
Ottawa, ON, Canada) and were composed of 20–25 μm particles.
Pretreatment mesenteric angiography and technetium-99m
macroaggregated albumin scans were performed to assess
gastrointestinal flow and lung shunting (8). For evaluation of TARE efficacy, 12
weeks after the initial treatment a physical examination, abdominal
computed tomography (CT) scan and blood tests were performed.
Thereafter, assessment was performed every 12 weeks.
Radiological assessment of response
Assessment was performed by contrast-enhanced spiral
CT. Treatment responses were evaluated by RECIST, mRECIST, Choi and
modified Choi criteria, in which a response is based on both a
minimum of a 10% reduction in size and a 15% reduction in density
(9,10). The four imaging criteria are shown
in Table I. All criteria
encompassed the following four response categories: Complete
response (CR), partial response (PR), stable disease (SD) and
progressive disease (PD). The CT attenuation of each tumor was
measured in Hounsfield units (HUs) on the portal venous phase. The
HU density value was obtained by delineating a region of interest
(ROI) around the boundary of the entire tumor at baseline and 12
weeks after TARE. The HUs of all lesions were combined and a mean
for each patient was calculated as described previously (7).
 | Table IDefinition of target radiological
responses. |
Table I
Definition of target radiological
responses.
| Response | RECIST 1.1 | mRECIST | Choi criteria | Modified Choi
criteria |
|---|
| CR | Disappearance of all
target lesions | Disappearance of any
intratumoral arterial enhancement in all target lesions | Disappearance of all
target lesions | Disappearance of all
target lesions |
| PR | At least a 30%
decrease in the sum of the greatest unidimensional diameters of
target lesions | At least a 30%
decrease in the sum of unidimensional diameters of viable target
lesions | Decrease in tumor
size ≥10% or decrease in tumor density ≥15% on CT | Decrease in tumor
size ≥10% and decrease in tumor density ≥15% on CT |
| SD | Any cases that do not
qualify for either partial response or progressive disease | Any cases that do not
qualify for either partial response or progressive disease | Does not meet the
criteria for CR, PR or PD | Does not meet the
criteria for CR, PR or PD |
| PD | An increase of at
least 20% in the sum of the diameters of target lesions | An increase of at
least 20% in the sum of the diameters of viable target lesions | Increase in tumor
size ≥10% and does not meet PR criteria by tumor density | Increase in tumor
size ≥10% and does not meet PR criteria by tumor density |
For calculation of the TTP, radiological progression
defined by these four methods was used. TTP was defined as the
number of days from the start of therapy to the day in which
progression was confirmed. Mortality during follow-up without
evidence of radiological progression was censored. Survival time
was evaluated as the time between initial treatment and mortality
or loss to follow-up (11). For TTP
and OS analyses, data collection was terminated on December 31,
2012.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS Inc., Chicago, IL, USA). For the analyses according
to the four different criteria, patients were categorized into
responders (CR + PR) vs. non-responders (SD + PD). Kaplan-Meier
survival analyses and Cox regression were used to explore
differences in TTP and OS between the responders and non-responders
according to RECIST, mRECIST, Choi and modified Choi criteria.
Spearman’s correlation test was performed to detect possible
correlations. The Wilcoxon signed-rank test was used to compare the
changes in size and attenuation at baseline and 12 weeks after
TARE. P<0.05 was considered to indicate a statistically
significant difference.
Results
Baseline demographics
A total of 113 HCC patients treated with TARE were
enrolled in this study. Table I
summarizes the baseline demographics of the cohort. The median age
was 69 years (range, 19–88 years). The majority of the patients
were male (80%) and the predominant etiology of liver disease in
this European cohort was non-alcoholic steatohepatitis. The median
model for end-stage liver disease score was 8 (range, 6–26).
Approximately half the patients (48%) had radiological evidence of
cirrhosis.
Response according to RECIST and
mRECIST
For RECIST response, at 12 weeks post-TARE, all
lesions presented a median change in tumor size of −15% (range,
−100 to +31%). Twenty-five (22%) patients reached PR, 75 (66%)
patients had SD and 13 (12%) patients had PD, resulting in 25
responders and 88 non-responders. For mRECIST response, the median
change in tumor size was −16% (range, −100 to +119%). Thirty (27%)
patients reached PR, 61 (54%) patients had SD and 22 (19%) patients
had PD, hence 30 responders and 83 non-responders.
Response according to Choi and modified
Choi criteria
At baseline, the median tumor size was 77 mm (range,
15–229 mm) for all lesions, with a median attenuation of 37 HUs
(range, 12–80 HUs). At 12 weeks post-TARE, the median size and
attenuation had decreased to 56 mm (range, 0–199 mm; P<0.05) and
29 HUs (range, 10–70 HUs; P<0.05), respectively. Overall, there
was a weak correlation between the percentage change in tumor size
and the percentage change in attenuation (Spearman’s ϱ=0.161,
P=0.044).
On evaluation with Choi criteria at 12 weeks
post-TARE, 88 (78%) patients reached PR, 20 (18%) had SD and 5 (4%)
had PD, resulting in 88 responders and 25 non-responders. According
to modified Choi criteria, 40 (35%) patients reached PR, 68 (60%)
had SD, and 5 (4%) had PD, hence 40 responders and 73
non-responders.
Association with TTP and OS
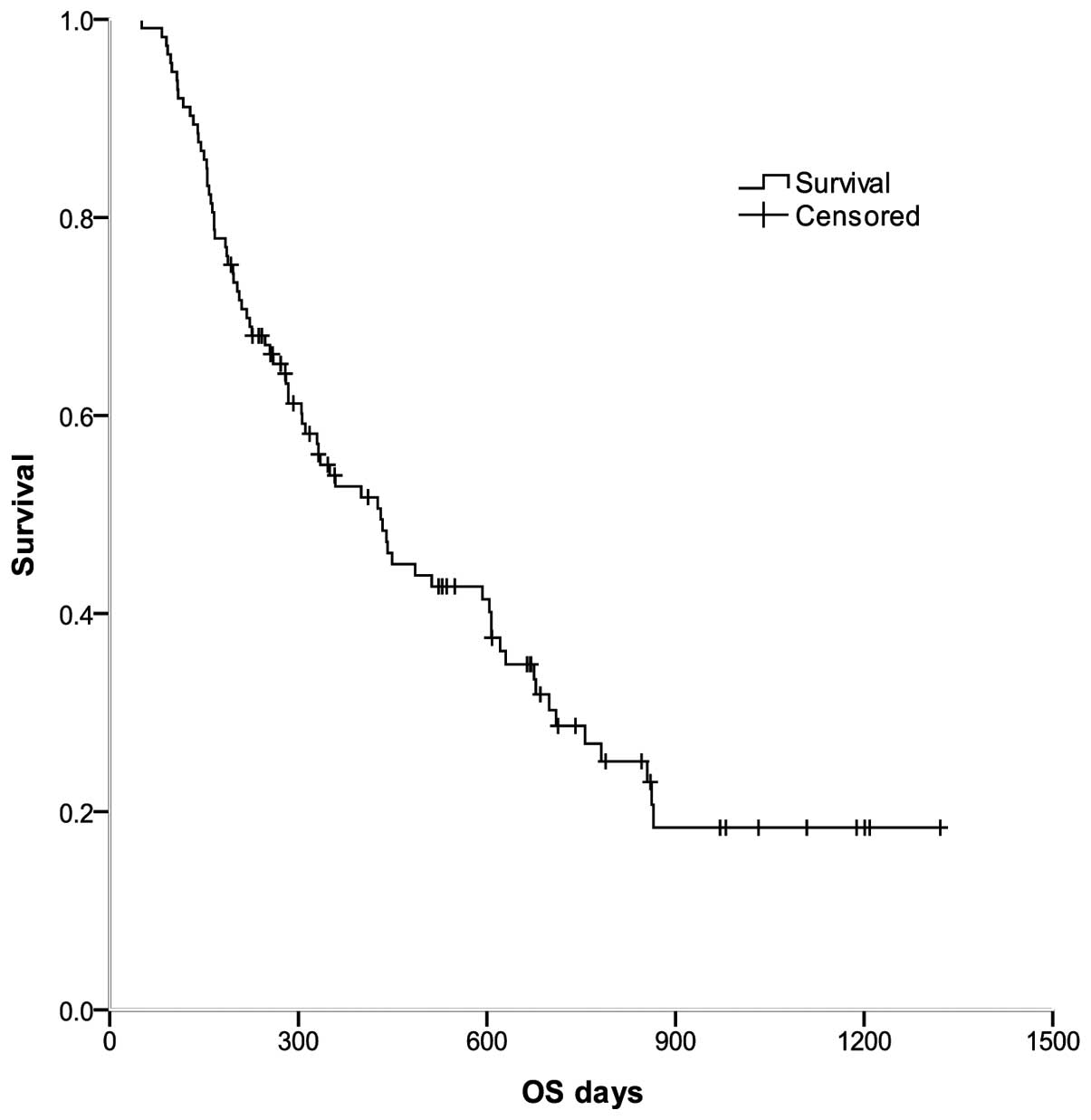
As shown in Fig. 1,
for all 113 patients, the median OS was 431 days [95% confidence
interval (CI), 321–541 days], and the 1- and 2-year survival rates
were 52.8 and 28.7%, respectively.
Kaplan-Meier methods were used to calculate the
median TTP and OS for the responders and non-responders defined by
each of the four criteria (Fig. 2,
Table III). The results
demonstrated that patients who had a response according to Choi
criteria had a significantly longer TTP and OS compared with those
who were non-responders (median, 280 vs. 166 days and 442 vs. 247
days; P=0.002 and 0.003, respectively; Fig. 2, Table
III). The responder and non-responder groups defined by mRECIST
also demonstrated a significant difference in median TTP (P=0.004;
Fig. 2, Table III). Neither RECIST- nor modified
Choi criteria-defined responders exhibited a significant
improvement in TTP and OS compared with the non-responders
(Fig. 2, Table III).
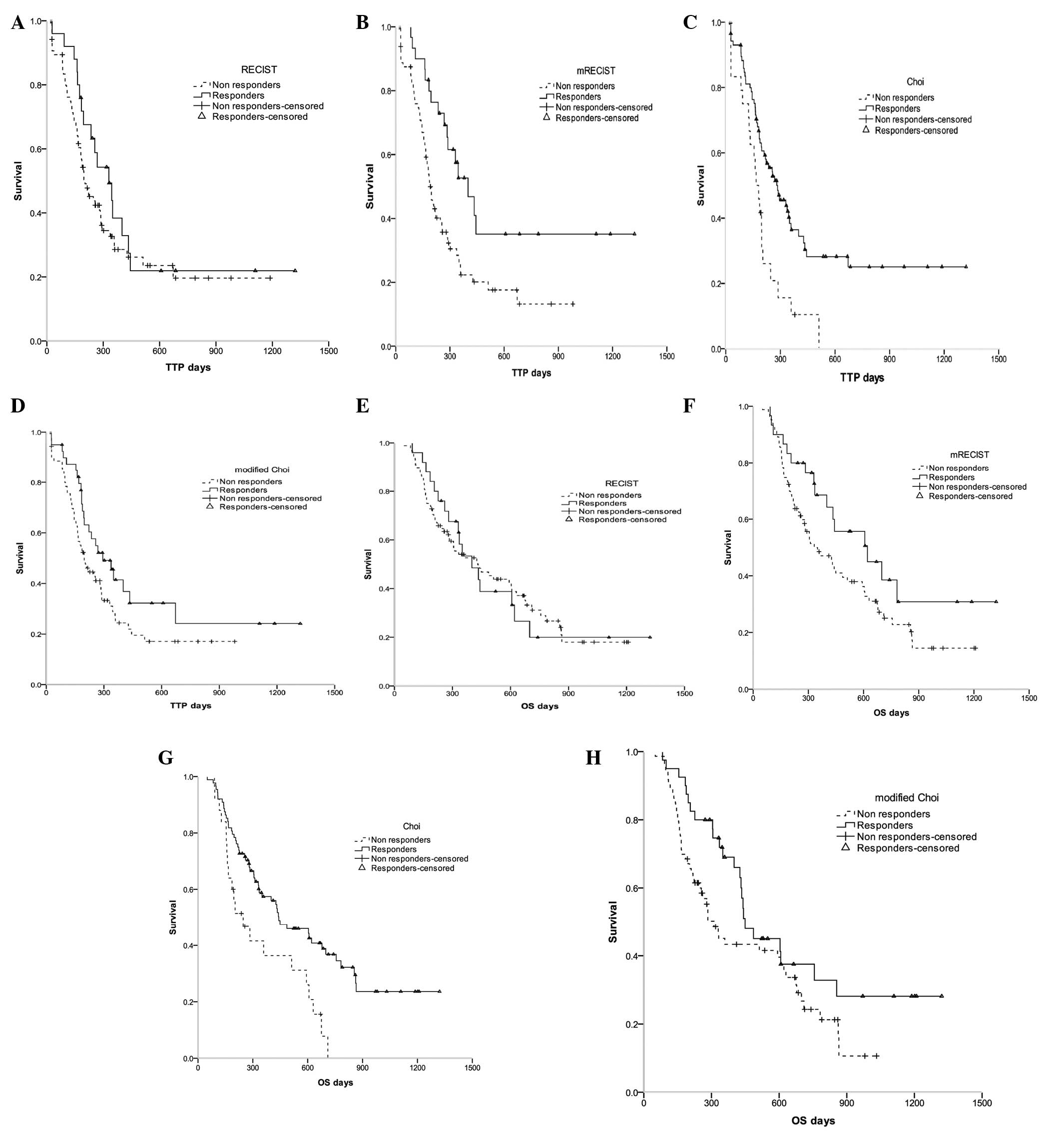 | Figure 2Kaplan-Meier curves were generated to
compare TTP and OS between responders and non-responders according
to four radiological assessment methods. HCC patients undergoing
TARE had radiological responses, as evaluated by four criteria: (A
and E) RECIST, (B and F) mRECIST, (C and G) Choi and (D and H)
modified Choi, performed 12 weeks post-TARE. TTP and OS were
compared between responders and non-responders, according to the
different criteria. TTP, time to progression; OS, overall survival;
HCC, hepatocellular carcinoma; TARE, transarterial
radioembolization; RECIST, Response Evaluation Criteria in Solid
Tumors; mRECIST, modified Response Evaluation Criteria in Solid
Tumors. |
 | Table IIIResponses according to RECIST,
mRECIST, Choi and modified Choi criteria and association with TTP
and OS. |
Table III
Responses according to RECIST,
mRECIST, Choi and modified Choi criteria and association with TTP
and OS.
| TTPa | OSa |
|---|
|
|
|
|---|
| Response
criteria | Responders | Non-responders | P-value | Responders | Non-responders | P-value |
|---|
| RECIST | 330 (207–453) | 203 (146–260) | 0.270 | 400 (259–541) | 431 (241–621) | 0.965 |
| mRECIST | 400 (273–527) | 188 (164–212) | 0.004 | 621 (278–964) | 332 (192–472) | 0.077 |
| Choi | 280 (191–369) | 166 (129–203) | 0.002 | 442 (250–634) | 247 (123–371) | 0.003 |
| Modified Choi | 294 (179–409) | 197 (148–246) | 0.072 | 449 (271–627) | 311 (231–391) | 0.069 |
Cox regression based on either RECIST, mRECIST, Choi
or modified Choi criteria as covariates was generated to compare
TTP and OS between responders and non-responders, according to the
four response criteria (Table IV).
When mRECIST criteria was used, hazard ratios (HRs) for TTP and OS
in responders compared with non-responders were 0.42 (95% CI,
0.24–0.74) and 0.61 (95% CI, 0.35–1.06), respectively (P=0.003 and
0.080, respectively; Table IV).
According to the Choi criteria, the differences in HRs for TTP and
OS between responders and non-responders were significant [0.46 (95
CI, 0.28–0.77) and 0.47 (95% CI, 0.28–0.78); P=0.003 and 0.004,
respectively; Table IV]. Choi
responders had a 53% risk reduction for OS compared with that of
non-responders.
 | Table IVCox regression was generated to
compare TTP and OS between responders and non-responders according
to the four response criteria. |
Table IV
Cox regression was generated to
compare TTP and OS between responders and non-responders according
to the four response criteria.
| | TTP | OS |
|---|
| |
|
|
|---|
| Response
criteria | n | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| RECIST |
| Responders | 25 | 0.74 (0.43–1.27) | 0.274 | 0.99 (0.58–1.70) | 0.965 |
| Non-responders | 88 | 1.0 | | 1.0 | |
| mRECIST |
| Responders | 29 | 0.42
(0.24–0.74) | 0.003 | 0.61
(0.35–1.06) | 0.080 |
|
Non-responders | 84 | 1.0 | | 1.0 | |
| Choi |
| Responders | 88 | 0.46
(0.28–0.77) | 0.003 | 0.47
(0.28–0.78) | 0.004 |
|
Non-responders | 25 | 1.0 | | 1.0 | |
| Modified Choi |
| Responders | 40 | 0.64
(0.40–1.05) | 0.075 | 0.64
(0.39–1.04) | 0.072 |
|
Non-responders | 73 | 1.0 | | 1.0 | |
Discussion
HCC is usually a hypervascular tumor (12) and CT scanning has improved our
ability to detect HCC by allowing acquisition of hepatic arterial
and portal venous dominant sets of images (13). TARE with yttrium-90 microspheres is
an established local-ablative therapy for primary and metastatic
liver cancer, and it has shown promising efficiency (3,4).
Imaging-defined response assessment based solely on change in tumor
size may be appropriate for treatments that result in significant
tumor shrinkage; however, TARE may cause tissue necrosis with no
immediate change in tumor size. Hence the mRECIST, Choi and
modified Choi criteria, which measure hypervascular tumors, have
been proposed as alternatives to RECIST. The Choi criteria define a
partial response by either a 10% reduction in size or a 15%
reduction in density during the portal venous phase of contrast. It
has been suggested that the Choi criteria may be appropriate for
tumor response assessment in GIST cancer (7). However, these four methods have not
been directly compared, nor has their association with survival,
when measured at a single time-point in a series of patients
treated with TARE alone.
In this retrospective study, we focused on the use
of TARE for HCC patients and compared the RECIST, mRECIST, Choi and
modified Choi criteria. The OS and TTP are the major endpoints for
clinical trials in HCC (11). We
evaluated the ability of each criteria-defined response to
correlate with TTP and OS in HCC patients treated with TARE. Our
results demonstrated that for CT assessments, at the 12-week
follow-up of 113 HCC patients, neither RECIST- nor modified Choi
criteria-defined responses correlated with TTP. However mRECIST and
Choi criteria successfully identified that responders had an
extended TTP, while non-responders had a significantly shorter TTP.
Furthermore, the OS between the responders and non-responders
according to RECIST, mRECIST and modified Choi criteria were not
significantly different at the 12-week follow-up. Patients who had
a response according to Choi criteria had a significantly longer OS
compared with the non-responders.
We also investigated which response assessment has
an association with overall survival. Previous studies have shown
that World Health Organization, RECIST and European Society for the
Study of the Liver responses are associated with improved survival
(14,15). Our findings clearly revealed that
overall response according to Choi criteria was a prognostic factor
of survival and associated with a 53% reduction in risk of
mortality. There was no significant association between overal
survival and RECIST, mRECIST and modified Choi responses in this
study. Choi criteria had a significantly better predictive value
compared with the other criteria for TTP and OS in HCC patients
treated with TARE at 12-weeks follow-up and may be valuable for
making early decisions on whether current interventions should be
continued or altered.
However, there are several limitations in the use of
the Choi criteria for evaluation of TARE-induced responses in HCC.
Firstly, the best method to evaluate tumor density using ROI
analysis remains a topic of debate (16). Secondly, measurements of relatively
hypodense lesions at baseline may be less reliable, since a 15%
decrease in HUs of these lesions is less accurate than those in
lesions with higher pretreatment HUs. Therefore, the use of
absolute changes may be more appropriate than the percentage change
in HUs (17). Thirdly, compared
with RECIST, Choi criteria are not able to identify patients with
PD early, possibly due to the ≥10% increase in tumor size compared
with the ≥20% increase used by RECIST.
In conclusion, tumor response assessment according
to Choi criteria at the 12-week follow-up in HCC patients treated
with TARE distinguishes prognostic groups better than RECIST,
mRECIST and modified Choi criteria. This may allow early
discrimination of patients benefiting from further treatment and
those who will not, particularly patients with stable tumor size.
This small sample study requires validation by further, larger
prospective treatment studies to demonstrate the broader
applicability of the Choi criteria.
Acknowledgements
This study was supported by Deutsche
Forschungsgemeinschaft (TRR60).
References
|
1
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma: an update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jelic S and Sotiropoulos GC; ESMO
Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21(Suppl 5): v59–v64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kennedy A, Coldwell D, Sangro B, Wasan H
and Salem R: Integrating radioembolization ((90)Y microspheres)
into current treatment options for liver tumors: introduction to
the international working group report. Am J Clin Oncol. 35:81–90.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sangro B, Iñarrairaegui M and Bilbao JI:
Radioembolization for hepatocellular carcinoma. J Hepatol.
56:464–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi H, Charnsangavej C, Faria SC, et al:
Correlation of computed tomography and positron emission tomography
in patients with metastatic gastrointestinal stromal tumor treated
at a single institution with imatinib mesylate: proposal of new
computed tomography response criteria. J Clin Oncol. 25:1753–1759.
2007. View Article : Google Scholar
|
|
8
|
Hamami ME, Poeppel TD, Müller S, Heusner
T, Bockisch A, Hilgard P and Antoch G: SPECT/CT with 99mTc-MAA in
radioembolization with 90Y microspheres in patients with
hepatocellular cancer. J Nucl Med. 50:688–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nathan PD, Vinayan A, Stott D, Juttla J
and Goh V: CT response assessment combining reduction in both size
and arterial phase density correlates with time to progression in
metastatic renal cancer patients treated with targeted therapies.
Cancer Biol Ther. 9:15–19. 2010. View Article : Google Scholar
|
|
10
|
Smith AD, Lieber ML and Shah SN: Assessing
tumor response and detecting recurrence in metastatic renal cell
carcinoma on targeted therapy: importance of size and attenuation
on contrast-enhanced CT. AJR Am J Roentgenol. 194:157–165. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Llovet JM, Di Bisceglie AM, Bruix J, et
al: Design and endpoints of clinical trials in hepatocellular
carcinoma. J Natl Cancer Inst. 100:698–711. 2008. View Article : Google Scholar
|
|
12
|
Merine D, Takayasu K and Wakao F:
Detection of hepatocellular carcinoma: comparison of CT during
arterial portography with CT after intraarterial injection of
iodized oil. Radiology. 175:707–710. 1990. View Article : Google Scholar
|
|
13
|
Fujita M, Kuroda C, Kumatani T, et al:
Comparison between conventional and spiral CT in patients with
hypervascular hepatocellular carcinoma. Eur J Radiol. 18:134–136.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Llovet JM, Real MI, Montana X, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: a
randomised controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar
|
|
15
|
Riaz A, Miller FH, Kulik LM, et al:
Imaging response in the primary index lesion and clinical outcomes
following transarterial locoregional therapy for hepatocellular
carcinoma. JAMA. 303:1062–1069. 2010. View Article : Google Scholar
|
|
16
|
Goh V, Halligan S, Gharpuray A, Wellsted
D, Sundin J and Bartram CI: Quantitative assessment of colorectal
cancer tumor vascular parameters by using perfusion CT: influence
of tumor region of interest. Radiology. 247:726–732. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Veldt AA, Meijerink MR, van den
Eertwegh AJ, Haanen JB and Boven E: Choi response criteria for
early prediction of clinical outcome in patients with metastatic
renal cell cancer treated with sunitinib. Br J Cancer. 102:803–809.
2010.PubMed/NCBI
|