Introduction
Breast carcinoma is the most commonly diagnosed
cancer in females of all ethnic groups and its incidence and
mortality rank second in China (1).
According to Cancer Statistics 2012 (2), breast cancer was the most common form
of cancer among females in USA. In the USA, there are ~40,000
female breast cancer mortalities and 230,480 new cases of breast
cancer each year. Current therapeutic approaches for human breast
cancer include hormonal therapy with anti-estrogenic compounds, as
well as surgery, radiotherapy, hyperthermia and chemotherapy
(3). At present, patients with
breast cancer have certain clinical responses to these strategies,
although they remain limited in the clinic. Novel and effective
treatments for breast cancer are urgently required.
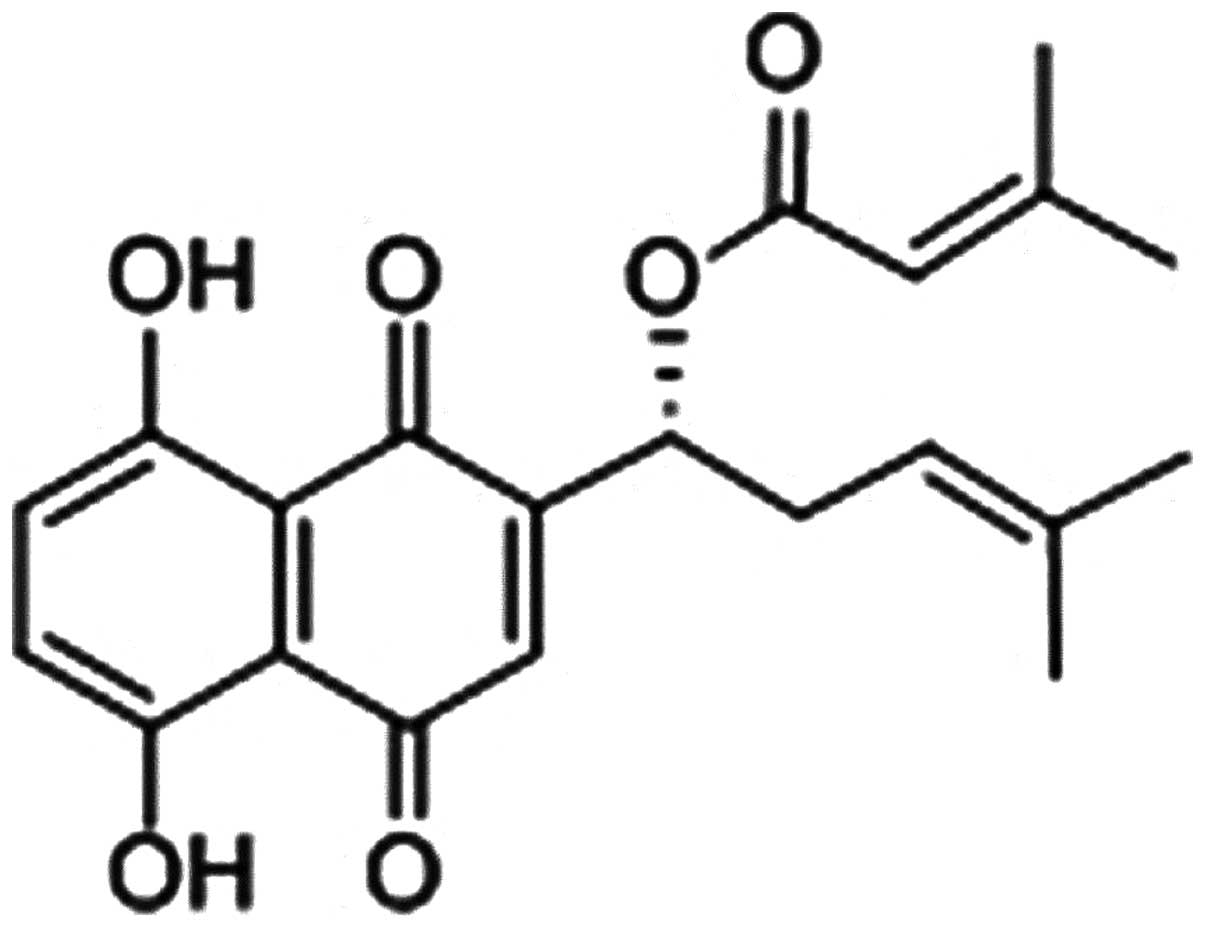
β,β-dimethylacrylshikonin (DA; Fig. 1) is a natural naphthoquinone
derivative compound from the root tissues of Lithospermum
erythrorhizon (L. erythrorhizon) a famous Chinese
medical herb called ‘Zicao’ (4).
L. erythrorhizon has been widely used as a traditional
Chinese medicine for thousands of years to treat burns or promote
wound healing through its antibacterial and anti-inflammatory
activities (5). Shikonin and its
derivatives have been demonstrated to exert anticancer and
apoptotic activities against tumor cells, such as sarcoma 180
(S-180) ascites cells, gastric cancer, hepatocellular carcinoma,
colon adenocarcinoma, epidermoid carcinoma, leukemia and prostate
cancer (6–10). However, little is known regarding
the effects and mechanisms of DA in breast cancer cells.
The present study aimed to evaluate the antitumor
effects of DA on the human breast cancer MCF-7 cell line and
investigate its molecular mechanisms.
Materials and methods
Reagents
DA was obtained from Huakang Pharmaceutical Company
(Deyang, China) and the purity was demonstrated to be >98% by
high performance liquid chromatography. Primary rabbit anti-human
p65, rabbit anti-human Iκb and phosphorylated Iκb antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
All secondary antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture
MCF-7 cells were obtained from the Shanghai
Institute of Cell Biology (Chinese Academy of Sciences, Shanghai,
China) and maintained in RPMI-1640 (Hali, Chengdu, China)
supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA),
100 U/ml penicillin and 100 μg/ml streptomycin (Huabei
Pharmaceuticals Ltd., Shijiazhuang, China), in a humidified
atmosphere with 5% CO2 at 37°C.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The inhibitory effects of DA on the proliferation of
MCF-7 cells were measured using the MTT assay (7). The cells were seeded in 96-well plates
at a density of 5×103/well. Following incubation for 24
h, the cells were treated with the indicated concentrations of DA
(0.27, 0.135, 0.0675, 0.0337, 0.0169, 0.0084 and 0.0042 mM) for 24,
48 and 72 h. Subsequently, 20 μl MTT (Sigma, St. Louis, MO, USA)
solution (5 mg/ml) in phosphate-buffered saline (PBS) was added at
24, 48 and 72 h after treatment, followed by incubation for a
further 4 h. After the medium was removed, dark blue formazan was
dissolved in 150 μl DMSO (Sigma). Following agitation for 15 min,
the optical density of each well was measured with a 680c
microplate reader (Bio-Rad, Hercules, CA, USA) at a wavelength of
570 nm. The 50% inhibitory concentration (IC50) was
determined via the Bliss method (11).
4,6-Diamidino-2-phenylindole
dihydrochloride hydrate (DAPI) staining
Apoptotic cells were detected using DAPI (Sigma)
staining (5), which identifies
typical apoptotic nuclear changes, including condensed and
fragmented nuclei. The cells were plated in 6-well plates at a
density of 2×104/well. After treatment with DA for 48 h,
the cells were fixed using PBS containing 4% paraformaldehyde for
30 min and incubated with DAPI (1 mg/ml) for a further 30 min. The
cells were then visualized under a fluorescence microscope
(DMI4000B; Olympus, Tokyo, Japan) with a 360–370 nm excitation
light and a 420–460 nm emission filter.
Flow cytometry
Annexin V/propidium iodide (PI) staining was
employed to detect the morphological changes of the cells (12). After DA treatment, cells were
harvested and washed with ice-cold PBS three times. The cells were
then stained with Annexin V-FITC and PI, and monitored for
apoptosis using flow cytometry according to the manufacturer’s
instructions (Boster, Wuhan, China). Non-stained cells were
indicated to be viable. PI-positive staining indicated necrosis,
while Annexin V-FITC-positive staining showed cells in the early
stages of apoptosis. PI- and Annexin V-FITC-positive cells were
considered to be in late stage apoptosis. Additional exposure to PI
made it possible to differentiate the early apoptotic cells from
the late apoptotic ones.
Reverse-transcription polymerase chain
reaction (RT-PCR)
The expression levels of Bcl-2, Bax and caspase-3
mRNA were measured using RT-PCR as previously described (13). MCF-7 cells were plated at a density
of 5×104/well into six-well plates for 24 h. The cells
were then treated with the indicated concentrations of DA (0.0125,
0.025 and 0.05 mM) for 48 h. Total mRNA was extracted from
DA-treated or control cells using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) and reverse transcribed using the RevertAid™
First Strand cDNA Synthesis kit (Fermentas, Burlington, ON,
Canada), following the manufacturer’s instructions. The primer
sequences used in this study were as follows: Bcl-2,
5′-TGTGGCCTTCTTT GAGTTCG-3′ and 5′-TCACTTGTGGCTCAGATAGG-3′; Bax,
5′-GCGTCCACCCAAGAAGCTGAG-3′ and 5′-ACCAC CCTGGTCTTGGATCC-3′;
caspase-3, 5′-CAAACTTTT CAGAGGGGATCG-3′ and
5′-GCATACTGTTTCAGCATGGCAC-3′; β-actin, 5′-TCACCCACACTGTGCCCATC
TACGA-3′ and 5′-CAGCGGAACCGCTCATTGCCAA TGG-3′. β-actin was used in
each experiment as an internal control. The PCR products were
electrophoresed on 1% agarose gels, stained with ethidium bromide
and observed under ultraviolet light. The relative mRNA levels were
expressed as the ratio of the signal intensity of the target gene
to that of β-actin. Analysis was performed with Quantity One V4.62
software (Bio-Rad).
Western blotting
Proteins from the cell lysates of DA-treated MCF-7
cells were obtained using lysis buffer [50 mmol/l Tris (pH 7.5),
100 mmol/l NaCl, 1 mmol/l EDTA, 0.5% NP40, 0.5% Triton X-100 and 1
mmol/l PMSF] and protein concentrations were measured with a
Bio-Rad Protein Assay kit (Bio-Rad) based on the Bradford method,
according to the manufacturer’s instructions. Proteins were
incubated for 3 min at 100°C prior to electrophoresis, then
separated using 12% SDS-PAGE at 120 V for 3–4 h. The proteins were
transferred onto polyvinylidene difluoride (PVDF) membranes
(Bio-Rad). After blocking with 5% non-fat milk at 4°C overnight,
the membranes were incubated in fresh 5% Tris-buffered saline with
Tween-20 (TBST)-Bovine lacto transfer technique optimizer with
1:500 primary antibodies for 2 h at room temperature. After being
washed with TBST for 10 min, the PVDF membranes were incubated with
secondary antibodies for 1 h. Proteins were then detected with the
Superstar Enhanced Chemiluminescent kit (AR1111; Boster). The
expression levels of the proteins were compared with those of the
β-actin control, based on the relative intensities of the bands.
Band density was quantified using Quantity One V4.62 software
(Bio-Rad).
Statistical analysis
Data are expressed as the mean ± SD of three
independent experiments. The Statistical Package for Social
Sciences version 13.0 (SPSS Inc., Chicago, IL, USA) was used for
standard statistical analysis by one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
DA inhibits the proliferation of MCF-7
cells
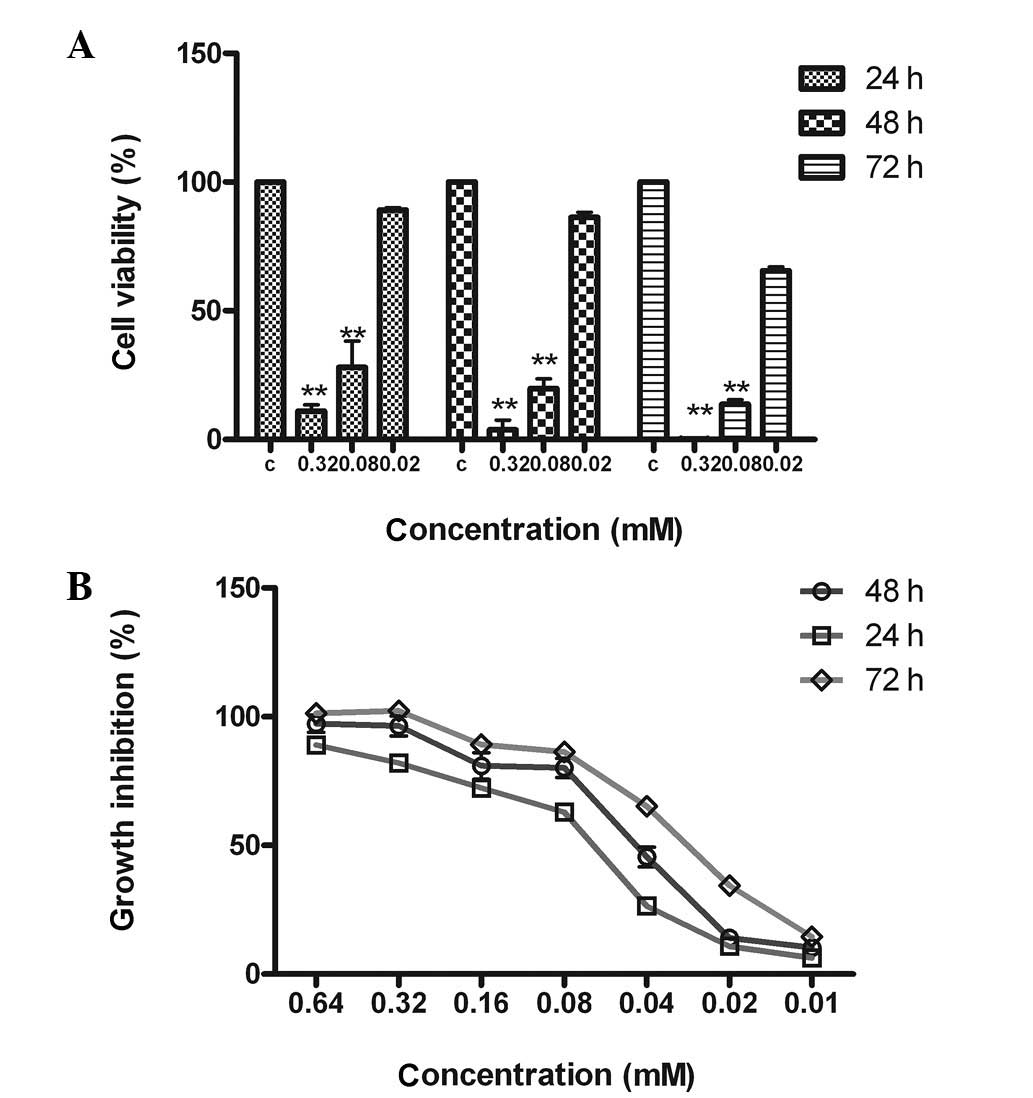
As shown in Fig. 2,
DA inhibited the proliferation of MCF-7 cells in a dose- and
time-dependent manner. Cell growth was suppressed by 98.2, 83.4 and
15.6% after treatment with 0.32, 0.08 and 0.02 mM DA, respectively.
The rate of tumor cell growth inhibition with 0.32 mM DA was
>90% and the IC50 values of the 24, 48 and 72 h time
courses were 0.080±0.022, 0.050±0.016 and 0.029±0.050 mM,
respectively.
DA induces the apoptosis of MCF-7
cells
DAPI nuclear staining was performed to detect
morphological changes in DA-treated cells. DA-treated cells
exhibited significant morphological changes, including nuclear
condensation, DNA fragmentation and pronuclear apoptotic bodies
(Fig. 3A). Annexin V/PI staining
also demonstrated apoptosis, which was induced by DA (Fig. 3B). Overall, it was shown that DA
visibly induced the apoptosis of MCF-7 cells in a dose-dependent
manner.
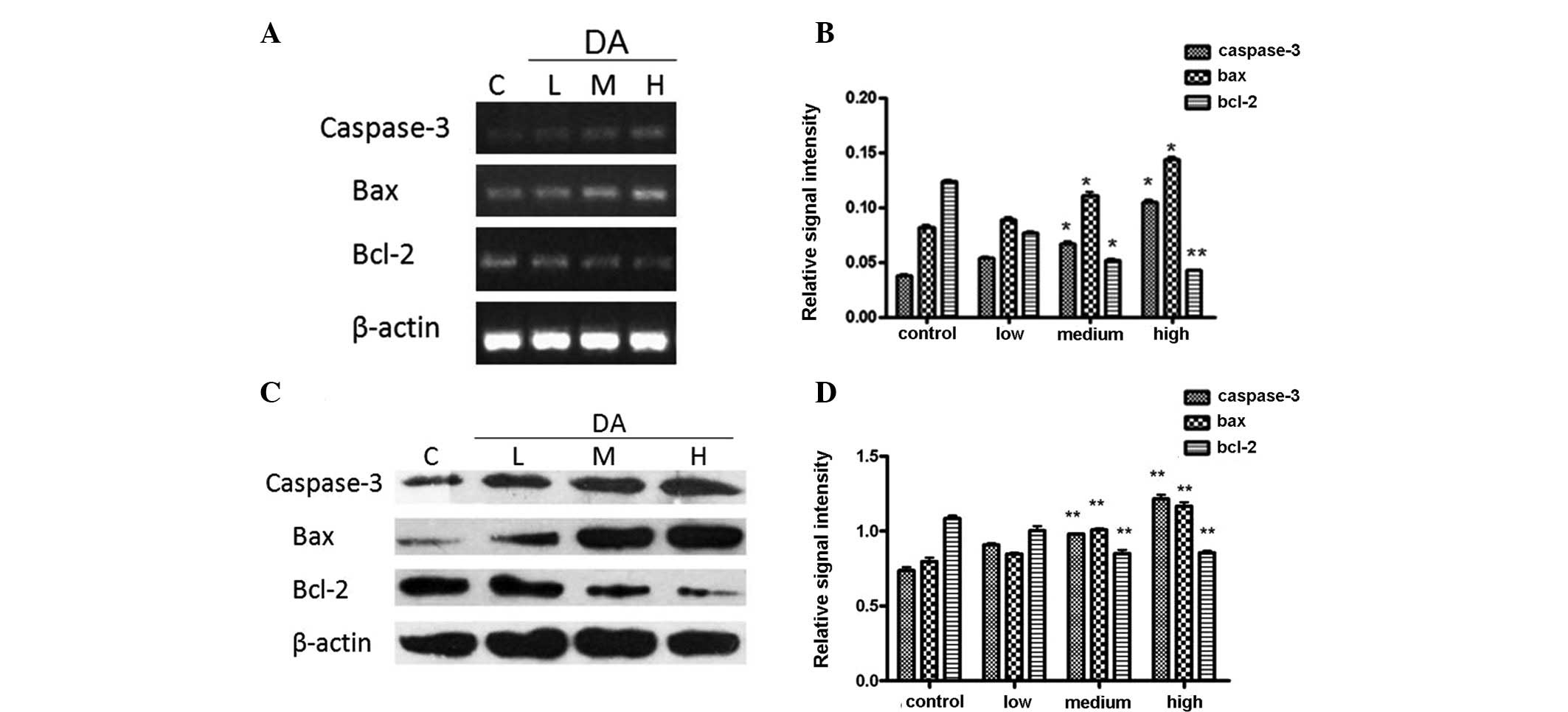
DA regulates the expression of
apoptosis-related genes and proteins
The expression levels of Bcl-2, Bax and caspase-3
were detected by RT-PCR. Following incubation with DA for 48 h, the
expression of Bax was noticeably enhanced in a dose-dependent
manner, while Bcl-2 expression decreased according to the
concentration of DA.
The expression levels of apoptosis-related proteins,
as detected by western blotting, were induced by DA in the same
manner. The ratio of Bax/Bcl-2 protein expression was elevated
markedly. Overall, it was observed that DA upregulated
apoptosis-related genes (Fig.
4).
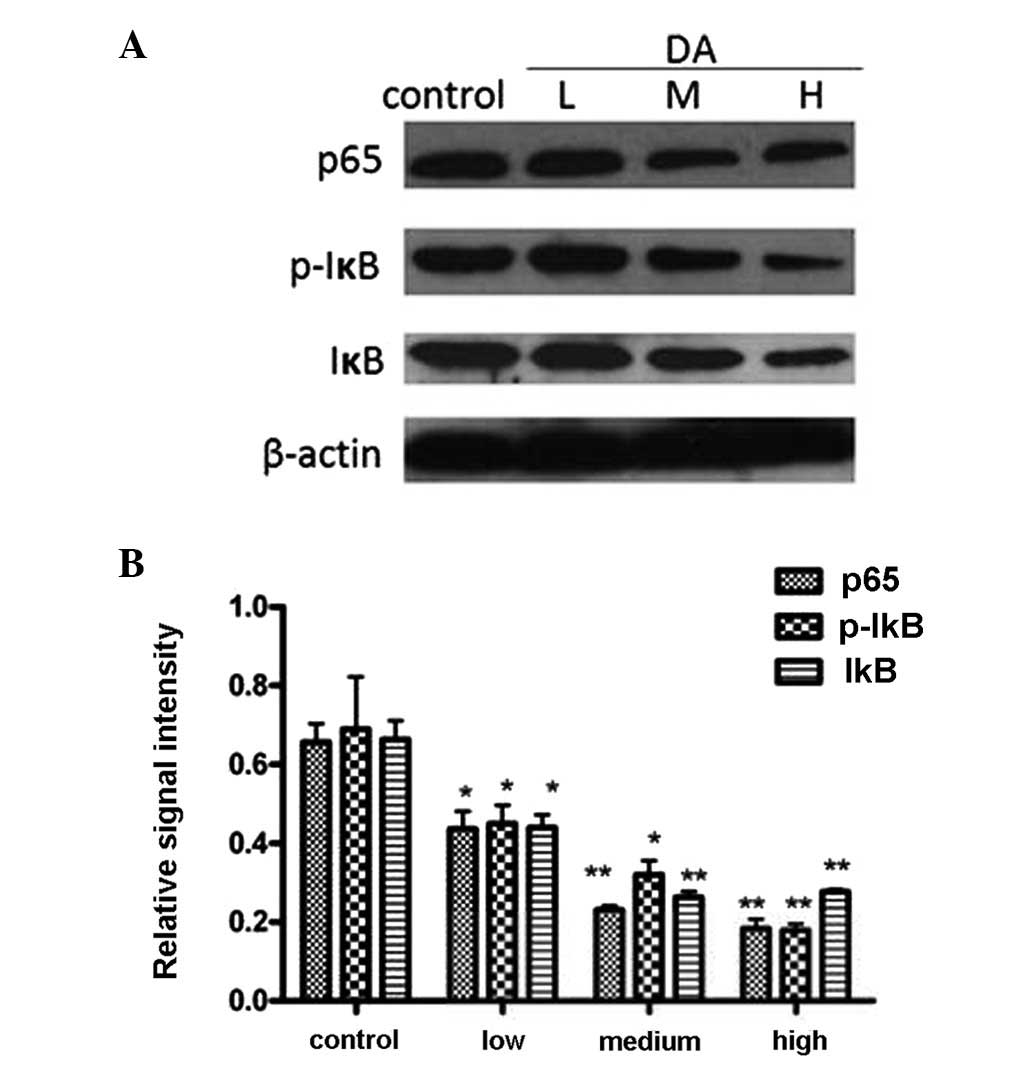
DA regulates the activity of the nuclear
factor (NF)-κB pathway
To investigate the potential mechanisms involved in
DA-induced apoptosis in MCF-7 cells, three important proteins
involved in the NF-κB pathway, p65, IκB and phosphorylated-IκB were
analyzed by western blotting. The results, as shown in Fig. 5, showed significant alterations in
the expression of phosphorylated-IκB and p65, while the expression
of IκB showed marginal change. This indicated that DA induced MCF-7
cell apoptosis by inactivating the NF-κB pathway.
Discussion
There has been growing interest in using naturally
occurring compounds to treat cancer. Shikonin, a compound isolated
from the TCM ‘Zicao’, has been used as an ointment for wound
healing. It has been demonstrated to inhibit the proliferation of
certain types of cancer cells and to induce apoptosis through
multiple signal transduction pathways (13,14).
Its antitumor effects were first shown by its activity against
S-180 tumor ascites at a dose of 5–10 mg/kg/day (15). It was also reported that the
administration of shikonin reduced the volume of intestinal
neoplasms induced by azoxymethane (16). After that, a number of other studies
also demonstrated shikonin’s potential anticancer activities in
several human tumors through inhibiting cancer cell growth,
inducing apoptosis (6), inhibiting
DNA topoisomerase I/II activity (17), anti-telomerase activity (18) and antiangiogenesis (5). However, its poor solubility and
toxicity have significantly hampered its clinical use (19). Over the past few years, studies have
been conducted with the aim of identifing novel shikonin
derivatives with little toxicity. DA, a shikonin derivative, has
been shown to have little toxicity, making it a promising
anticancer agent (12). However the
anticancer effects and mechanisms of DA in human breast cancer
cells have not been elucidated. In the present study, the
anticancer effects and mechanisms of DA in MCF-7 cells were
investigated in vitro and it was observed that DA was able
to suppress the proliferation of MCF-7 cells and induce cellular
apoptosis time and dose dependently.
Previous studies have demonstrated that
shikonin-like compounds cause cell apoptosis through the activation
of a caspase-dependent pathway in numerous types of cancer cells.
The treatment of chronic myelogenous leukemia K562 cells (5), human prostate cancer PC-3 cells
(20), melanoma cells (6) and osteosarcoma cells (21) with shikonin induced apoptosis
through increased caspase-3 activity. Our previous studies also
showed that DA induced apoptosis in hepatocellular carcionoma
SMMC-7721 cells (7) and gastric
cancer SGC-7901 cells (22). To
further investigate the underlying mechanisms of its
antiproliferative effects, the present study investigated the
expression of apoptosis-related proteins and the activity of the
NF-κB pathway in DA-treated MCF-7 cells.
Apoptosis, the stereotypic program of cellular
suicide regulated by a variety of factors, is critical in
tumorigenesis and tumor progression (23). Studies have shown that shikonin-like
compounds are able to induce apoptosis in multiple cancer cells
through various signal transduction pathways (9,10,12–15).
It was observed that following treatment with DA, MCF-7 cells
exhibited typical morphological apoptotic changes. Apoptosis is a
complex process involving a variety of molecules. The
mitochondrial-mediated signal transduction pathway is central in
the regulation of apoptosis. Members of the Bcl-2 family are the
key regulators of mitochondrial response to apoptotic signals, with
individual members promoting or suppressing apoptosis. Bcl-2, an
anti-apoptotic factor, negatively regulates this cellular suicide
machinery, whereas another Bcl-2-homologous protein, Bax, promotes
cell death by competing with Bcl-2 (24,25).
To determine whether apoptosis-related genes contribute to the
inhibitory effects of DA on MCF-7 cells, we measured the relative
Bcl-2 and Bax expression levels. It was noted that DA decreased the
expression of Bcl-2 but increased the expression of Bax in a
dose-dependent manner. Moreover, caspase-3 expression was also
upregulated by DA.
It was also observed that DA suppressed the activity
of NF-κB, an important transcription factor in the regulation of
the genes governing apoptosis. NF-κB serves as a prosurvival agent
similar to Bcl-2 in various circumstances (17). NF-κB complexes are mostly composed
of two heterodimeric subunits of p50 and p65. In unstimulated
cells, the NF-κB is in an inactive form within the cytosol,
complexed to an inhibitory IκB-α protein (26,27).
Following exposure to various carcinogens and growth stimuli, IκB-α
may be phosphorylated and degraded by releasing the free NF-κB
transcription factor. After the free NF-κB translocates into the
nucleus, the genes with κB reporter regions in their promoters may
be activated, the functions of which are closely associated with
abnormal proliferation and survival of cancer cells (28). In the present study, it was observed
that DA inactivated the NF-κB pathway through inhibiting the
phosphorylation of IκB-α and downregulating p65 subunit
expression.
In conclusion, DA was able to inhibit the
proliferation and growth of MCF-7 cells in vitro by
inducting apoptosis via the activation of caspase-3 and alteration
of the apoptosis-related genes Bcl-2 and Bax. These alterations may
be associated with inactivation of the NF-κB pathway through the
downregulation of p65 and inhibition of IκB-α phosphorylation.
These results suggest that DA has promise for potential clinical
use as an anticancer agent in treating breast cancer. However, its
mechanisms in other signaling pathways require discussion in
further studies.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
3
|
Hortobagyi GN: Treatment of breast cancer.
N Engl J Med. 339:974–984. 1998. View Article : Google Scholar
|
|
4
|
Chen X, Yang L, Zhang N, et al: Shikonin,
a component of Chinese herbal medicine, inhibits chemokine receptor
function and suppresses human immunodeficiency virus type 1.
Antimicrob Agents Chemother. 47:2810–2816. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao X, Yu CR, Li WH and Li WX: Induction
of apoptosis by shikonin through a ROS/JNK-mediated process in
Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell
Res. 18:879–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang H, Zhou P, Huang H, et al: Shikonin
exerts antitumor activity via proteasome inhibition and cell death
induction in vitro and in vivo. Int J Cancer. 124:2450–2459. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu YY, Wan LH, Zheng XW, et al: Inhibitory
effects of β,β-dimethylacrylshikonin on hepatocellular carcinoma in
vitro and in vivo. Phytother Res. 26:764–771. 2012.
|
|
8
|
Pietrosiuk A, Furmanowa M,
Skopińiska-Rózewska E, et al: The effect of acetylshikonin isolated
from Lithospermum canescens roots on tumor-induced cutaneous
angiogenesis. Acta Pol Pharm. 61:379–382. 2004.
|
|
9
|
Gong K and Li W: Shikonin, a Chinese
plant-derived naphthoquinone, induces apoptosis in hepatocellular
carcinoma cells through reactive oxygen species: A potential new
treatment for hepatocellular carcinoma. Free Radic Biol Med.
51:2259–2271. 2011. View Article : Google Scholar
|
|
10
|
Kim SH, Kang IC, Yoon TJ, et al: Antitumor
activities of a newly synthesized shikonin derivative,
2-hyim-DMNQ-S-33. Cancer Lett. 172:171–175. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Litchfield JT Jr and Wilcoxon F: A
simplified method of evaluating dose-effect experiments. J
Pharmacol Exp Ther. 96:99–113. 1949.PubMed/NCBI
|
|
12
|
Zeng Y, Liu G and Zhou LM: Inhibitory
effect of acetylshikonin on human gastric carcinoma cell line
SGC-7901 in vitro and in vivo. World J Gastroenterol. 15:1816–1820.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Yang L, Oppenheim JJ and Howard
MZ: Cellular pharmacology studies of shikonin derivatives.
Phytother Res. 16:199–209. 2002. View
Article : Google Scholar
|
|
14
|
Lu L, Qin A, Huang H, et al: Shikonin
extracted from medicinal Chinese herbs exerts anti-inflammatory
effect via proteasome inhibition. Eur J Pharmacol. 658:242–247.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sankawa U, Ebizuka Y, Miyazaki T, et al:
Antitumor activity of shikonin and its derivatives. Chem Pharm Bull
(Tokyo). 25:2392–2395. 1977. View Article : Google Scholar
|
|
16
|
Yoshimi N, Wang A, Morishita Y, et al:
Modifying effects of fungal and herb metabolites on
azoxymethane-induced intestinal carcinogenesis in rats. Jpn J
Cancer Res. 83:1273–1278. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahn BZ, Baik KU, Kweon GR, et al:
Acylshikonin analogues: synthesis and inhibition of DNA
topoisomerase-I. J Med Chem. 38:1044–1047. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plyta ZF, Li T, Papageorgiou VP, et al:
Inhibition of topoisomerase I by naphthoquinone derivatives. Bioorg
Med Chem Lett. 8:3385–3390. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang F, Chen Y, Duan W, et al: SH-7, a new
synthesized shikonin derivative, exerting its potent antitumor
activities as a topoisomerase inhibitor. Int J Cancer.
119:1184–1193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gaddipati JP, Mani H, Shefali, et al:
Inhibition of growth and regulation of IGFs and VEGF in human
prostate cancer cell lines by shikonin analogue 93/637 (SA).
Anticancer Res. 20:2547–2552. 2000.PubMed/NCBI
|
|
21
|
Chang IC, Huang YJ, Chiang TI, et al:
Shikonin induces apoptosis through reactive oxygen
species/extracellular signal-regulated kinase pathway in
osteosarcoma cells. Biol Pharm Bull. 33:816–824. 2010. View Article : Google Scholar
|
|
22
|
Zhen-Jun S, Yuan-Yuan Z, Ying-Ying F, et
al: β,β-Dimethylacrylshikonin exerts antitumor activity via Notch-1
signaling pathway in vitro and in vivo. Biochem Pharmacol.
84:507–512. 2012.
|
|
23
|
Green DR and Evan GI: A matter of life and
death. Cancer Cell. 1:19–30. 2002. View Article : Google Scholar
|
|
24
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen HM and Tergaonkar V: NFκB signaling
in carcinogenesis and as a potential molecular target for cancer
therapy. Apoptosis. 14:348–363. 2009.
|
|
27
|
Tergaonkar V, Correa RG, Ikawa M and Verma
IM: Distinct roles of IκB proteins in regulating constitutive NF-κB
activity. Nat Cell Biol. 7:921–923. 2005.
|
|
28
|
Basak S, Kim H, Kearns JD, et al: A fourth
IκB protein within the NF-κB signaling module. Cell. 128:369–381.
2007.
|