Introduction
The phosphatidylinositol-3-kinase
(PI3K)/Akt-mediated signal transduction pathway is closely
associated with tumorigenesis and has been used as a target of
caner therapy (1). PI3K is able to
phosphorylate the third OH group on the inositol ring of
phosphatidylinositol (PI), and the main product of the
phosphorylation is phosphatidylinositol-3,4,5-triphosphate (PIP3).
PIP3 acts as the second messenger to activate downstream components
of the signal transduction pathway. Akt, also known as protein
kinase B, is a serine/threonine-specific protein kinase that is
crucial in the regulation of cellular proliferation,
differentiation, apoptosis and migration. PIP3 is able to bind with
Akt and cause the translocation of Akt from the plasma to the
membrane and activate the enzyme (2,3).
PI3K/Akt-mediated signal transduction may be initiated by
interacting with receptor tyrosine kinases (RTKs) or by binding
with the small G protein, Ras (4).
The epidermal growth factor receptor (EGFR) is a key member of the
RTK family. When ligands bind with EGFR, autophosphorylation of
EGFR occurs and the phosphorylated tyrosine site is able to recruit
PI3K to the C-terminal domain of EGFR, causing the
phosphorylation/activation of PI3K, initiating the associated
signal transduction (5).
cGMP-dependent protein kinases (PKGs) are
serine/threonine kinases and, currently, two types of PKGs have
been identified in mammalian cells, Type I (PKG I) and Type II (PKG
II) (6,7). PKG I has been shown to suppress the
growth of tumor cells and has been identified as a tumor suppressor
(8). However, the antitumor effect
of PKG II has not been clearly elucidated. The expression and
activity of PKG II in human gastric cancer cell lines have been
observed to be significantly lower than those of normal gastric
mucosal cells, and the increase of PKG II activity may inhibit the
proliferation of gastric cancer cell lines (9,10).
Further study in our laboratory revealed that PKG II was able to
inhibit EGF-induced proliferation and migration, and inhibit
MAPK-mediated signal transduction in gastric cancer cells. Notably,
the inhibition was associated with the prevention of EGF-induced
phosphorylation/activation of EGFR (11,12).
As the activation of EGFR also initiates other signal transduction
pathways, including the PI3K/Akt- and JAK/STAT-mediated pathways
(5,13,14),
further investigation into whether PKG II has an inhibitory effect
on these signal pathways is required. The present study was
designed to elucidate the possible inhibition of PKG II on the
EGF-initiated PI3K/Akt-mediated signal transduction pathway.
Materials and methods
Cell line and reagents
The AGS human gastric cancer cell line was provided
by the Institute of Cell Biology (Shanghai, China). Adenoviral
vectors encoding β-galactosidase (pAd-LacZ) and PKG II (pAd-PKG II)
were provided by the University of California (San Diego, CA, USA).
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum
(FBS) were obtained from Gibco (Grand Island, NY, USA). The
antibody against PKG II (rabbit anti-human) was from Abgent
Biotechnology (San Diego, CA, USA). The horseradish peroxidase
(HRP)-conjugated antibody against β-actin (moust anti-human) and
the antibody against Bcl-2 (mouse anti-human) were obtained from
Santa Cruz (Santa Cruz, CA, USA). The antibody against p-EGFR
(Tyr1173; rabbit anti-human) was purchased from Cell Signaling
(Danvers, MA, USA). The antibodies against p-PI3K, p-Akt, p-mTOR,
NF-κB, Bax, DNA fragment factor (DFF), caspase-9 and LaminB1
(rabbit anti-human) were purchased from Bioworld Technology (St.
Louis Park, MN, USA). HRP-conjugated secondary antibodies (goat
anti-mouse and goat anti-rabbit) were obtained from Jackson Immuno
Research Laboratories (West Grove, PA, USA). The cellular permeable
cGMP analog 8-pCPT-cGMP was purchased from Calbiochem (San Diego,
CA, USA) and EGF was obtained from Sigma (St. Louis, MO, USA). The
electrochemiluminescence (ECL) reagents were purchased from
Millipore (Billerica, MA, USA). The terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL) In Situ Cell
Death Detection kit was purchased from Roche Diagnostics (Mannheim,
Germany), while the Nuclear and Cytoplasmic Extract kit was
purchased from Kangcheng Bio-tech (Hangzhou, China). All other
reagents used were of analytical grade.
Cell culture and treatment
The AGS cells were cultured in DMEM, supplied with
10% FBS and maintained at 37ºC in a humidified incubator with 95%
air and 5% CO2. One day prior to the infection, the
cells were planted into six-well plates. When the cells were 70–80%
confluent, they were infected with Ad-LacZ or Ad-PKG II (with a
multiplicity of infection of 100%) or mock infected. At 24 h
post-infection, the medium was replaced with serum-free medium and
the culture was continued for 12 h. The infected cells were
incubated with 100 or 250 μM 8-pCPT-cGMP for 1 h and incubated with
100 ng/ml EGF. The EGF incubation time was 5 min to observe EGFR
phosphorylation and 12 h to observe the change in protein
expression.
Preparation of cell extracts and nuclear
protein
The differentially treated cells were harvested at
various times by aspiration of the media and direct addition of
heated 2X SDS sample buffer. The cell lysate was scraped and
transferred to tubes, heated for 5 min at 100ºC and stored at
−20ºC. The nuclear extracts were prepared according to the
instructions of the manufacturer of the Nuclear and Cytoplasmic
Extract kit. Briefly, the cells were harvested into tubes
containing PBS and centrifuged at 500 × g for 5 min. Ice-cold
cytoplasmic extraction reagent (CER) I buffer was added to the cell
pellet and fully resuspended by vortexing. The tubes were then
incubated on ice for 10 min followed by an addition of ice-cold CER
II buffer, vortexed and incubated on ice for 1 min. The samples
were centrifuged for 5 min in a microcentrifuge (~16,000 × g). The
supernatants (cytoplasmic extracts) were immediately transferred to
a clean pre-chilled tube. The insoluble fractions (pellets) were
resuspended in ice-cold NER buffer. The samples were repeatedly
vortexed for 15 sec every 10 min (at 4ºC) for a total of at least
40 min and microcentrifuged (~16,000 × g) for 10 min. The
supernatant fractions (nuclear extracts) were immediately
transferred to pre-chilled tubes and stored at −20ºC until use.
Western blotting
The proteins were separated by SDS-PAGE (8–12%) gel
according to their molecular size and transferred onto a PVDF
membrane. The blots were blocked using 5% (w/v) non-fat milk in
TBS-T for 1 h at room temperature, and then incubated at 4ºC
overnight with the primary antibody. Subsequently, the blots were
incubated with the secondary antibody at room temperature for 1 h.
The signal was visualized using ECL detection reagents.
Detection of apoptosis using the TUNEL
method
TUNEL was performed using the In Situ Cell Death
Detection kit, according to the manufacturer’s instructions.
Briefly, the cells that were grown on 24-well plates were fixed and
the endogenous peroxidase activity was quenched using 2%
H2O2 for 5 min. The cells were incubated with
a reaction buffer containing terminal deoxynucleotidyl transferase,
1 mM Mn2+ and fluorescein-labeled dUTP in a humid
atmosphere at 37ºC for 60 min. The cells were washed with PBS and
incubated with antifluorescein antibody conjugated with HRP for 30
min. Subsequent to being rinsed with PBS, the cells were immersed
in DAB solution. Under a microscope, the cells that exhibited brown
nuclear staining were considered to be apoptotic. For each well,
five fields (magnification, ×200) were randomly selected, and the
number of positive cells and the total number of cells in each
field were counted. The average ratio (positive cell number/total
cell number) of the apoptotic cells was taken as the value for one
experiment. The assay was repeated three times.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using a two-tailed ANOVA with
SPSS statistical software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
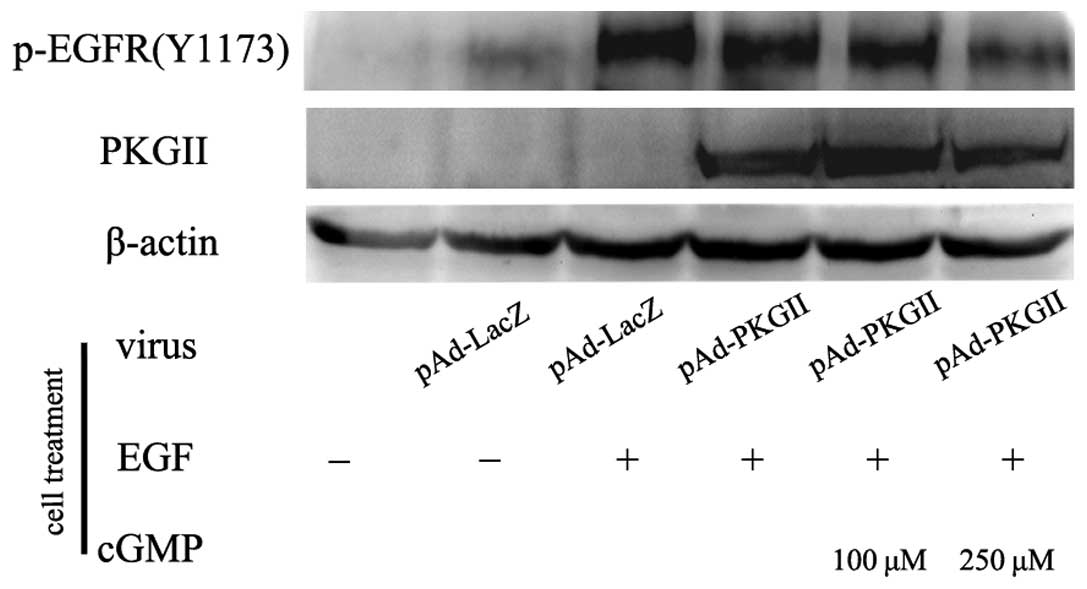
PKG II inhibits EGF-induced Tyrosine 1173
(Tyr1173) phosphorylation/activation of EGFR
When EGF binds with EGFR, Tyr1173 is one of the
autophosphorylation sites that is located on the receptor. The
phosphorylation of this site is associated with PI3K/Akt-mediated
signaling. In the present study, the inhibitory effect of PKG II on
the Tyr1173 phosphorylation of EGFR was investigated in the
differentially treated AGS cells, using western blotting. The
results revealed that in the Ad-LacZ-infected cells, there was a
marked increase in the phosphorylated Tyr1173 residues of EGFR when
the cells were incubated with 100 ng/ml EGF for 5 min. In the cells
that were infected with Ad-PKG II, stimulated with cGMP and
incubated with 100 ng/ml EGF for 5 min, the phosphorylation was
markedly decreased (Fig. 1). This
indicated that PKG II was able to inhibit Tyr1173
phosphorylation/activation of EGFR caused by EGF.
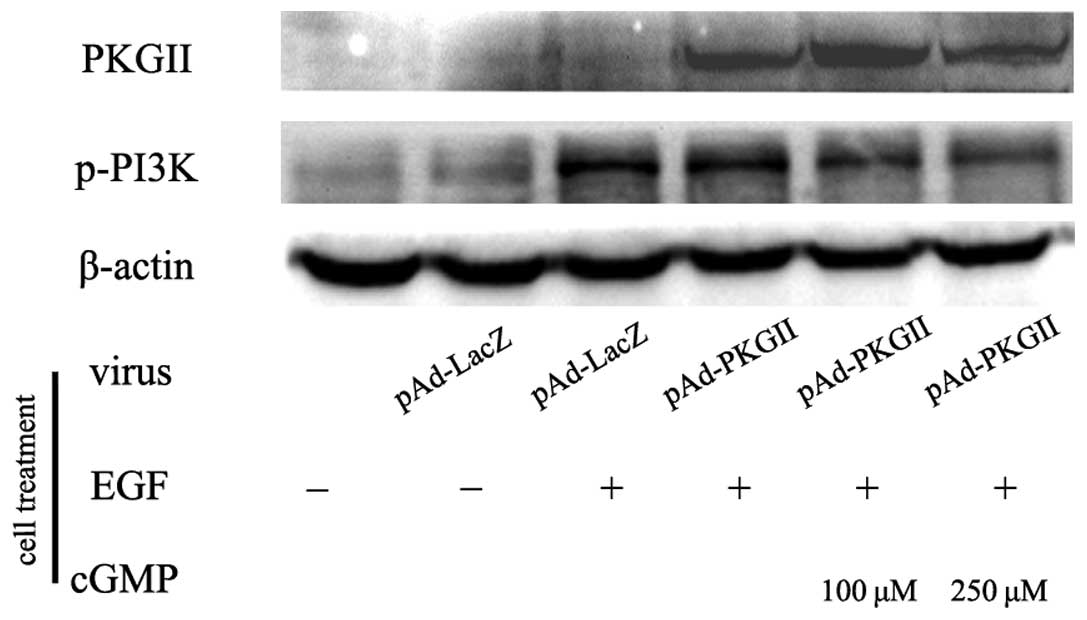
PKG II inhibits EGF-induced
phosphorylation/activation of PI3K
Phosphorylated EGFR is able to recruit the PI3K
regulatory subunit, p85α, to its Tyr1173 site and activate the
lipid kinase. During the activation processes, Tyr458 on p85α is
phosphorylated. Western blotting with an antibody against p-PI3K
p85 (Tyr458) was used to detect the phosphorylation of p85α. The
AGS cells were treated as previously described and the western
blotting results revealed that EGF treatment (100 ng/ml for 5 min)
increased the phosphorylation of PI3K p85α. In the cells that were
infected with Ad-PKG II, stimulated with cGMP and treated with EGF,
the phosphorylation level of PI3K p85α was markedly lower than that
of the cells that were infected with Ad-LacZ or treated with EGF
alone (Fig. 2). These results
revealed that PKG II inhibited the EGF-induced activation of
PI3K.
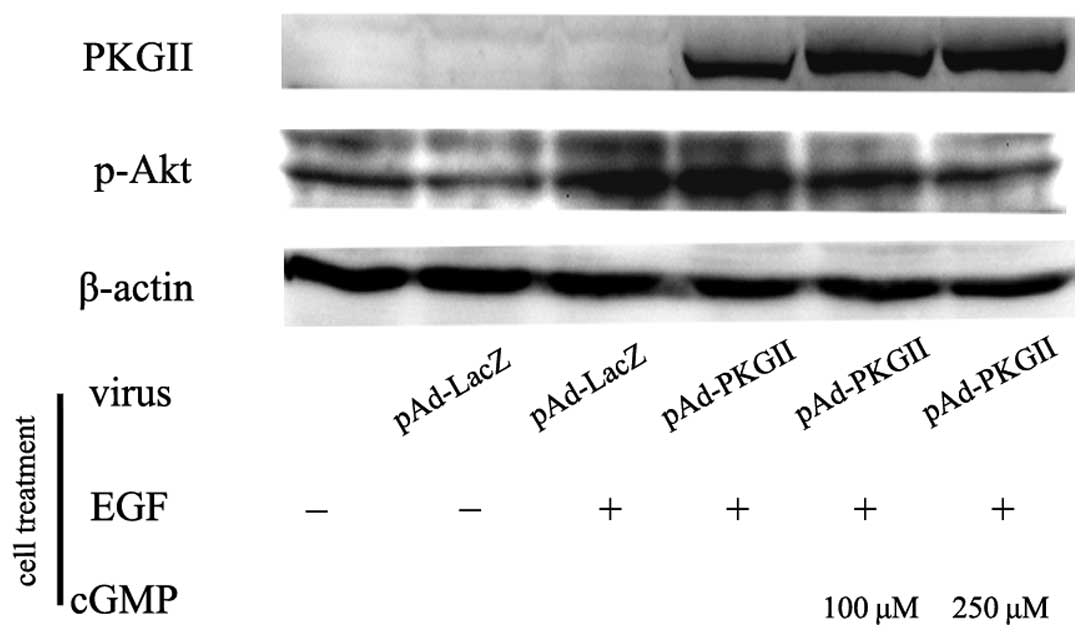
PKG II inhibits EGF-induced
phosphorylation/activation of Akt
Akt is a crucial signal transduction component in
the PI3K-mediated pathway. The activation of Akt is dependent on a
dual regulatory mechanism that requires its translocation to the
plasma membrane and dual phosphorylation on Thr308 and Ser473. In
the present study, the AGS cells were treated as previously
described and western blotting with an antibody against p-Akt
(Thr308) was used to detect the phosphorylation/activation of Akt
by EGF. The results revealed that EGF treatment induced a notable
increase in Thr308 phosphorylation on Akt, and the increase of PKG
II activity effectively inhibited the EGF-induced Akt
phosphorylation/activation of Akt (Fig.
3).
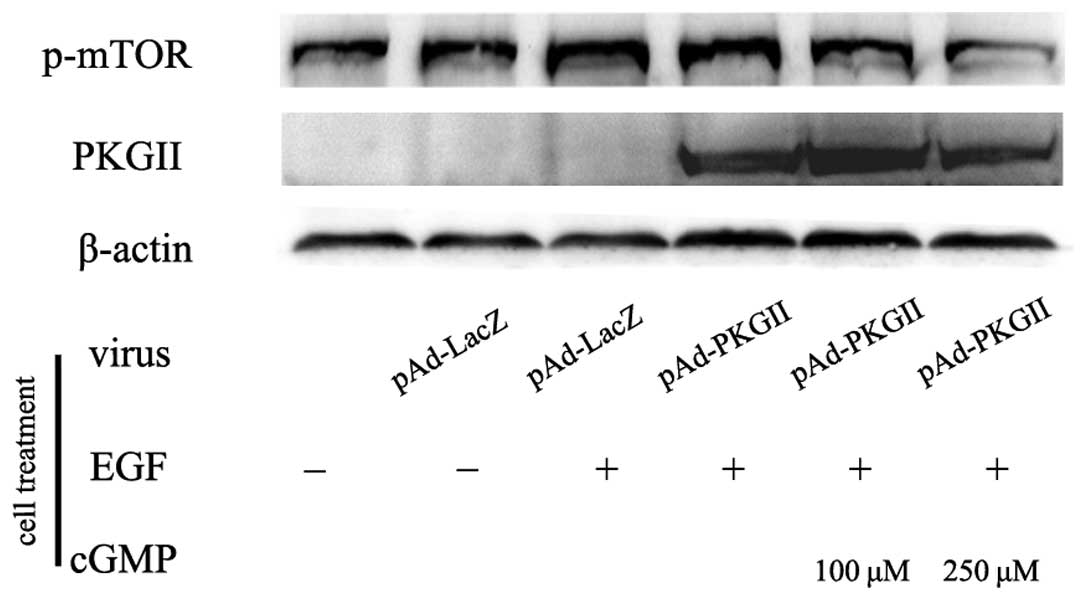
EGF-induced phosphorylation/activation of
mTOR is inhibited by activated PKG II
mTOR has been shown to be a direct substrate for
AKT. The proposed AKT phosphorylation site (Ser2448) in mTOR is
located within a C-terminal regulatory region. In the present
study, the AGS cells were treated as in Fig. 1 and western blotting with an anti
p-mTOR (Ser2448) antibody was used to detect the phosphorylation of
mTOR in the various cell groups. The results showed that EGF
treatment (100 ng/ml, 5 min) caused a marked increase of Ser2448
phosphorylation of mTOR, and the increase in PKG II activity
through infecting the cells with pAd-PKG II and stimulating the
cells with 8-pCPT-cGMP efficiently inhibited the EGF-induced
phosphorylation/activation of mTOR (Fig. 4).
PKG II inhibits EGF-induced activation of
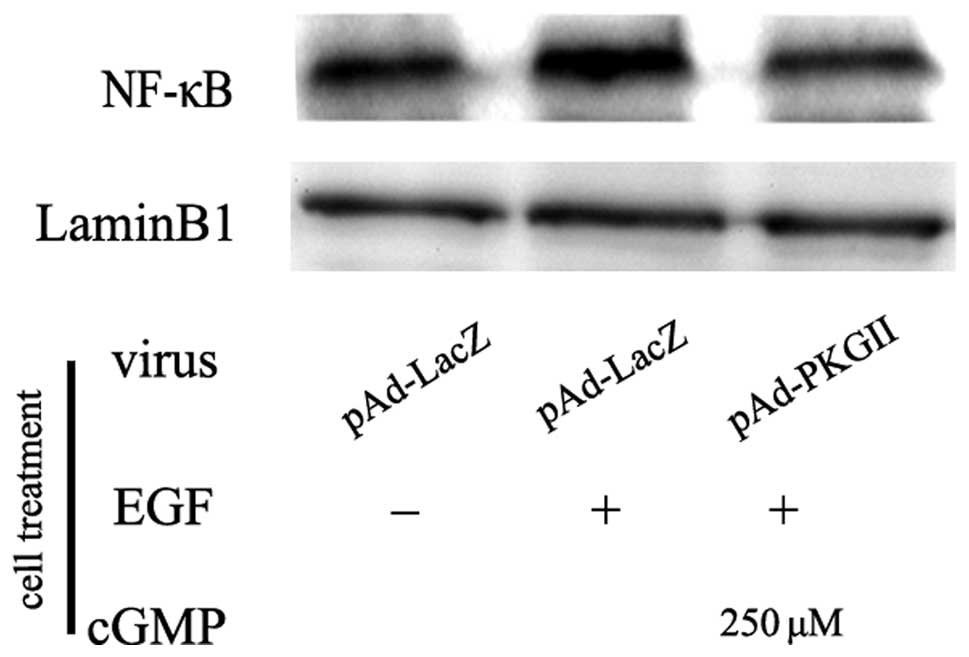
NF-κB
Transcription factor NF-κB is a dimer formed by a
group of subunits, including Rel (cRel), p65 (RelA, NF-κB3), RelB,
p50 (NF-κB1) and p52 (NF-κB2). The most common NF-κB is the
heterogeneous dimmer formed by p65 and p50. When NF-κB is
activated, the dimer translocates into the nucleus, binds with a
corresponding promoter and initiates gene transcription. In the
present study, western blotting with an anti-p65 (RelA) antibody
was used to detect the nuclear translocation of NF-κB. The results
revealed that the EGF treatment caused an increase in the nuclear
localization of p65 NF-κB. PKG II activity prevented the
translocation, indicating that PKG II is able to inhibit the
EGF-induced nuclear translocation/activation of NF-κB (Fig. 5).
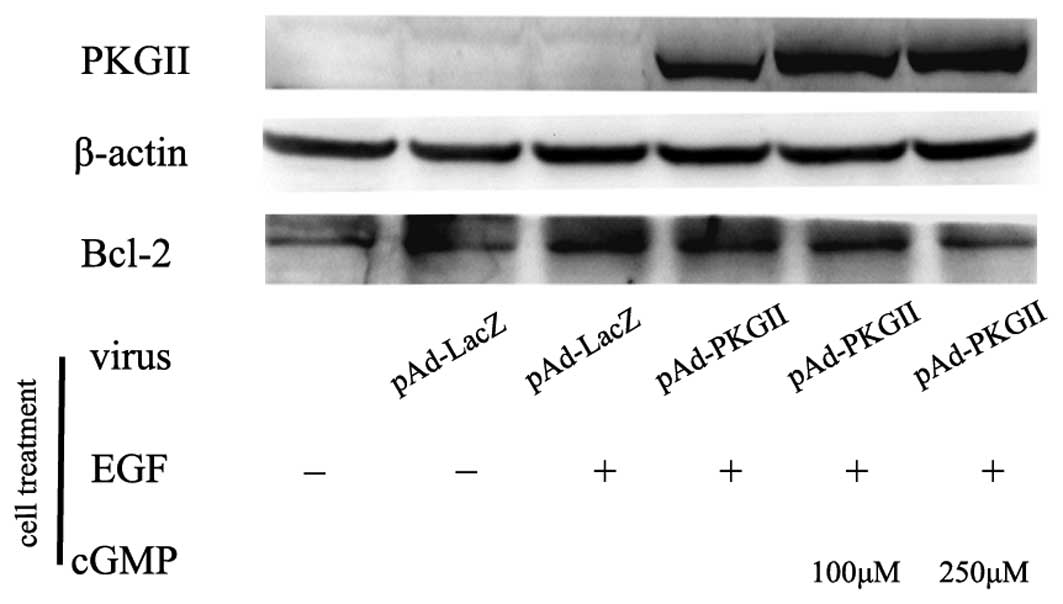
PKG II reverses EGF-induced expression of
apoptosis regulator proteins
Bcl-2 is an anti-apoptotic protein and a member of
the Bcl-2 family of the apoptosis regulator proteins. In the
present study, the expression of Bcl-2 in the EGF-treated AGS cells
was detected by western blotting. The results revealed that EGF
treatment (100 ng/ml, 12 h) caused an increase in the expression of
Bcl-2. Pre-infection with Ad-PKG II and treatment with 8-pCPT-cGMP
reversed the effect of EGF, causing a marked decrease in Bcl-2
expression (Fig. 6). In contrast,
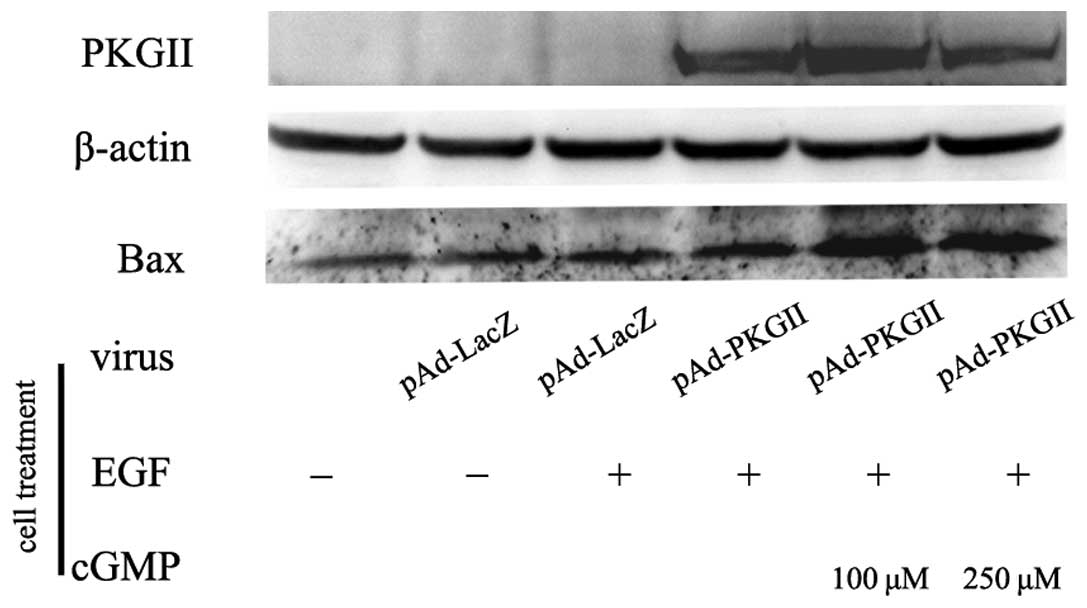
EGF treatment did not cause a marked change in the expression of
the pro-apoptotic protein, Bax, which, if activated, leads to the
activation of caspases and apoptosis. However, an increase of PKG
II activity caused a clear increase in Bax expression (Fig. 7).
PKG II stimulates the activation of
caspase-9
Caspases are essential in the regulation of cell
apoptosis and have been termed ‘executioner’ proteins. In the
present study, the changes in the protein levels of caspase-3 and
caspase-9 were detected by western blotting. The results revealed
that EGF treatment had no effect on the levels of caspase-3 (not
shown) and caspase-9. An increase in PKG II activity had no effect
on caspase-3 protein levels (not shown). However, PKG II did cause
a marked decrease in caspase-9. This suggests that PKG II had a
stimulating effect on the activation of caspase-9 (Fig. 8).
PKG II increases the activity of DFF
DFF is the component located at the end of apoptotic
pathway. Apoptotic signaling is able to stimulate DFF to break into
an active form, cause DNA fragmentation and initiate apoptosis.
Western blotting was used to detect the change in the protein
levels of DFF during EGF and PKG II/cGMP treatment. The results
revealed that EGF treatment had no effect on the protein levels of
DFF. However, an increase in PKG II activity caused a decrease in
DFF protein levels, indicating that PKG II activated DFF (Fig. 9). This suggests that PKG II is able
to increase the activity of DFF, cause DNA fragmentation and
increase the apoptosis of AGS cells.
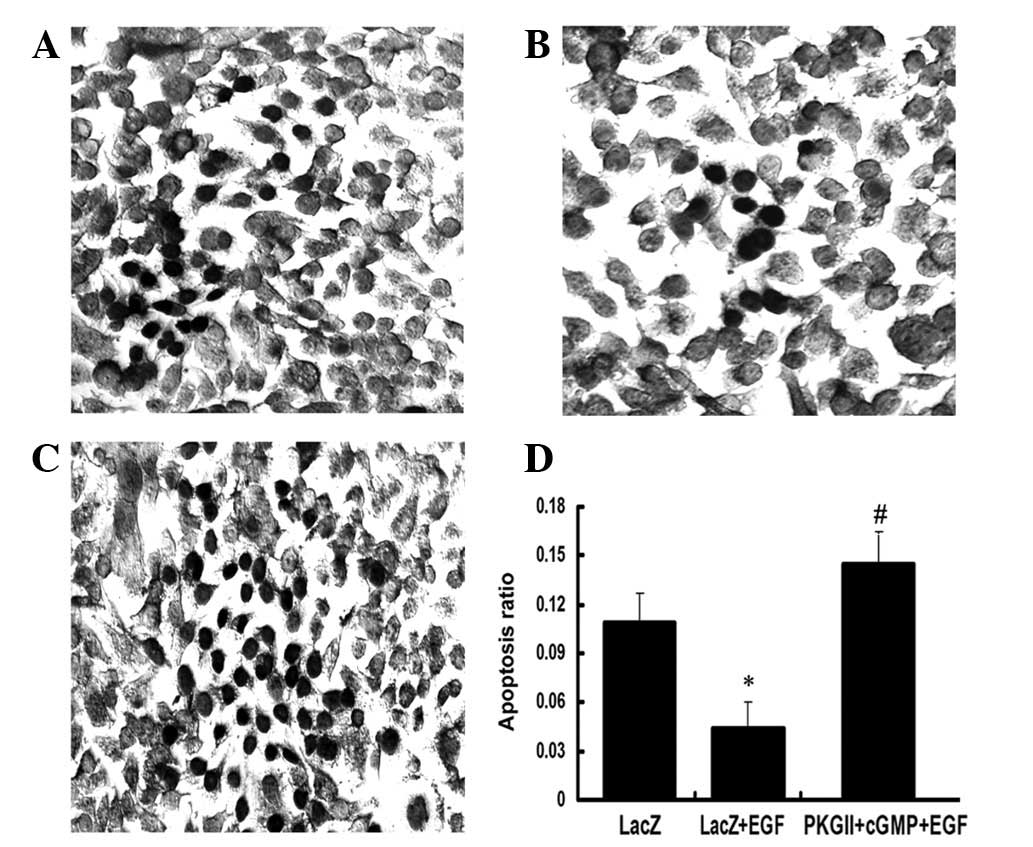
PKG II reverses the anti-apoptotic effect
of EGF
The TUNEL method was used to detect the apoptosis of
the differentially treated AGS cells. In the EGF treatment group
(100 ng/ml, 12 h) the ratio of apoptotic cells markedly decreased.
Ad-PKG II infection and incubating with 8-pCPT-cGMP efficiently
reversed the anti-apoptotic effect of EGF and caused a significant
increase in the apoptosis of the AGS cells (Fig. 10).
Discussion
PKG II has long been implicated in several
physiological functions, including intestinal secretion, bone
growth, learning and memory (15).
However, certain new functions of this kinase have been reported,
including the regulation of epithelial sodium channels and
mechanotransduction of osteoblasts (16–18). A
significant observation in studies with regard to PKG II is that
this kinase has a role in regulating the proliferation and
apoptosis of cells and is potentially associated with
tumorigenesis. Data have shown PKG II to inhibit the proliferation
of human prostate and neuroglioma cells, and induce the apoptosis
of human prostate and breast cancer cells (19–21).
The present study confirmed the inhibitory effect of PKG II on the
proliferation and migration of cancer cells, and the associated
mechanisms are currently under extensive investigation. A
significant finding is that PKG II is able to inhibit EGF-induced
activation of EGFR, which is the starting event of the signal
transduction of EGF/EGFR mediated pathways (11,12).
Activation of EGFR initiates the signal transduction
of several pathways, including the MAPK-, PI3K/Akt-, PLCγ1/IP3/DAG-
and JAK1/STAT-mediated pathways. The signal transductions of these
pathways are associated with the proliferation, apoptosis and
motility/migration of the cells (5,14,22).
Therefore, further study is required with regard to whether PKG II
also inhibits the signal transduction of other pathways besides
those that are mediated by MAPK, and whether it affects cellular
activities other than proliferation and migration. The present
study confirmed that in the gastric cancer AGS cell line, EGF
caused Tyr1173 phosphorylation of EGFR and the consequent
activation of the key components of the PI3K/Akt-mediated pathway,
including PI3K, Akt, mTOR and NF-κB. PKG II was also identified to
suppress the whole process of EGF-induced PI3K/Akt-mediated signal
transduction, including the activation of EGFR and each key
signaling component of the pathway.
The PI3K/Akt-mediated signal pathway is significant
in regulating apoptosis (23). In
numerous cancer tissues and cells, this pathway is overactive,
reducing apoptosis and allowing proliferation. The present study
identified that EGF treatment significantly reduced the apoptosis
of the AGS cells. This effect was prevented by a PI3K inhibitor
(data not shown), indicating that EGF was not able to reduce
apoptosis of the gastric cancer cells through the PI3K/Akt-mediated
pathway. PKG II was also able to efficiently reverse the
anti-apoptotic effect of EGF. This suggests that PKG II not only
inhibits the proliferation but also induces the apoptosis of
gastric cancer cells, and the two effects are associated with the
blockage of EGFR activation by this kinase.
EGFR is closely associated with tumorigenesis. The
overexpression and mutations of EGFR are identified in the majority
of cancer types (24). In
vitro experiments have confirmed that blocking EGFR activation
inhibited the proliferation of certain types of tumor cells
(25). Furthermore, a clinical
study has shown that cancer patients with an overexpression of EGFR
usually have a poor prognosis (26). Therefore, EGFR is a potential cancer
therapy target and methods of inhibiting EGFR activity and
associated signal transduction have been intensively studied,
including specific antibodies against EGFR and inhibitors of EGFR
(27). The present data have shown
that PKG II inhibits EGF/EGFR-induced proliferation and migration
and associated signal transduction of MAPK-mediated pathways of
cancer cells. The present study revealed that in gastric cancer
cells, PKG II is able to inhibit the EGF/EGFR-induced signal
transduction of the PI3K/Akt-mediated pathway and reverse the
anti-apoptotic effects of EGF/EGFR. This strongly suggests that PKG
II is a cancer suppression factor and may influence the strategy of
cancer therapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81272755, 31040002 and
81201959); the Graduate Student Research and Innovation Project in
Colleges and Universities of Jiangsu Province (grant no.
CXZZ13_0701); and the Specialized Research Fund for Senior
Personnel Program of Jiangsu University (grant no. 11JDG032). The
authors would like to thank Dr Gerry Boss and Dr Renate Pilz
(University of California) for the adenoviral constructs.
References
|
1
|
Marone R, Cmiljanovic V, Giese B and
Wymann MP: Targeting phosphoinositide 3-kinase: moving towards
therapy. Biochem Biophys Acta. 1784:159–185. 2008.PubMed/NCBI
|
|
2
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edward LA, Thiesen B, Dragowska WH, et al:
Inhibiton of ILK in PTEN-mutant human glioblastomas inhibits
PKB/Akt activation, induces apoptosis and delays tumor growth.
Oncogene. 24:3596–3605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berg M and Soreide K: EGFR and downstream
genetic alterations in KRAS/BRAF and PI3K/AKT pathways in
colorectal cancer: implications for targeted therapy. Discov Med.
14:207–214. 2012.PubMed/NCBI
|
|
5
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: its functions and alteration in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orstavik S, Natarajan V, Taskén K, et al:
Characterization of the human gene encoding the type I alpha and
type I beta cGMP-dependent protein kinase (PRKG1). Genomics.
42:311–318. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Orstavik S, Solberg R, Taskén K, et al:
Molecular cloning, cDNA structure and chromosomal localization of
the human type II cGMP-dependent protein kinase. Biochem Biophys
Res Commun. 220:759–765. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Browing DD: Protein kinase G as a
therapeutic target for the treatment of metastatic colorectal
cancer. Expert Opin Ther Targets. 12:367–376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang SQ, Chen YC, Wang Y, et al:
Expression of cGMP dependent protein kinase II in cancer cell lines
was obviously decreased. J Jiangsu Univ (Medicine Edition). 18:1–5.
2008.
|
|
10
|
Chen YC, Ren F, Sang JR, et al: Type II
cGMP-dependent protein kinase inhibits proliferation of the gastric
cancer cell line BGC-823. Mol Med Rep. 3:361–366. 2010.PubMed/NCBI
|
|
11
|
Wu Y, Chen Y, Qu R, et al: Type II
cGMP-dependent protein kinase inhibits EGF-triggered signal
transduction of the MAPK/ERK-mediated pathway in gastric cancer
cells. Oncol Rep. 27:553–558. 2012.PubMed/NCBI
|
|
12
|
Lan T, Chen Y, Sang J, et al: Type II
cGMP-dependent protein kinase inhibits EGF-induced MAPK/JNK signal
transduction in breast cancer cells. Oncol Rep. 27:2039–2044.
2012.PubMed/NCBI
|
|
13
|
Dong P, Xu Z, Jia N, et al: Elevated
expression of p53 gain-of-function mutation R175H in endometrial
cancer cells can increase the invasive phenotypes by activation of
the EGFR/PI3K/AKT pathway. Mol Cancer. 8:1032009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quesnelle KM, Boehm AL and Grandis JR:
STAT-mediated EGFR signaling in cancer. J Cell Biochem.
102:311–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hofmann F: The biology of cyclic
GMP-dependent protein kinases. J Biol Chem. 280:1–4. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nie HG, Chen L, Han DY, et al: Regulation
of epithelial sodium channels by cGMP/PKGII. J Physiol.
587:2663–2676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rangaswami H, Marathe N, Zhuang S, et al:
Type II cGMP-dependent protein kinase mediates osteoblast
mechanotransduction. J Biol Chem. 284:14796–14808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rangaswami H, Schwappacher R, Marathe N,
et al: Cyclic GMP and protein kinase G control a Src-containing
mechanosome in osteoblasts. Sci Signal. 3:ra912010.PubMed/NCBI
|
|
19
|
Cook AL and Haynes JM: Protein kinase G
II-mediated proliferative effects in human cultured prostatic
stromal cells. Cell Signal. 16:253–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swartling FJ, Ferletta M, Kastemar M, et
al: Cyclic GMP-dependent protein kinase II inhibits cell
proliferation, Sox9 expression and Akt phosphorylation in human
glioma cell lines. Oncogene. 28:3121–3131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fallahian F, Karami-Tehrani F, Salami S
and Aghaei M: Cyclic GMP induced apoptosis via protein kinase G in
oestrogen receptor-positive and -negative breast cancer cell lines.
FEBS J. 278:3360–3369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie Z, Peng J, Pennypacker SD and Chen Y:
Critical role for the catalytic activity of phospholipase C-γ1 in
epidermal growth factor-induced cell migration. Biochem Biophys Res
Commun. 399:425–428. 2010.
|
|
23
|
Yap TA, Garrett MD, Walton MI, et al:
Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and
promises. Curr Opin Pharmacol. 8:393–412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Normanno N, Bianco C, De Luca A, et al:
Target-based agents against ErbB receptors and their ligands: a
novel approach to cancer treatment. Endocr Relat Cancer. 10:1–21.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hynes NE and MacDonald G: ErbB receptors
and signaling pathways in cancer. Curr Opin Cell Biol. 21:177–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Quatrale AE, Porcelli L, Silvestris N, et
al: EGFR tyrosine kinases inhibitors in cancer treatment: in vitro
and in vivo evidence. Front Biosci. 16:1962–1972. 2011. View Article : Google Scholar : PubMed/NCBI
|