Introduction
Cervical cancer is the second most common type of
cancer and the sixth leading cause of cancer-related mortality in
females worldwide (1). Cervical
cancer affects ~16 females per 100,000 per year and ~8 per 100,000
per year are projected to succumb to the disease (2). Globally, in 2008, it was estimated
that there were 473,000 cases of cervical cancer and 253,500
mortalities per year (3,4). Early cervical cancer may be cured by
removing or destroying the precancerous or cancerous tissue.
However, patient outcome greatly suffers once the cancer has
metastasized to distant organs. The standard treatments for
cervical cancer include surgery, chemotherapy and radiation
therapy. These treatments indiscriminately lead to harm or damage
to adjacent or distant normal tissues, and may facilitate cancer
cell invasion and metastasis. Cervical cancer metastasis may cause
the treatment failure of conventional therapy, including surgery,
radiotherapy and chemotherapy (5).
Therefore, these studies indicate that new and more effective
treatments for cervical cancer require development.
Gallic acid, a naturally occurring plant phenolic
compound, is present in a wide variety of plant-based foods. Gallic
acid is one of the major active component of Chinese gall, and is
widely distributed in natural herbal plants and in large amounts of
prescribed Chinese herbs (6,7). In
the present study, gallic acid was isolated as a natural
antioxidant from Chinese gall. In the past, gallic acid has been
reported as a free radical scavenger, able to function to induce
differentiation and apoptosis in leukemia and lung cancer and to
suppress tumor angiogenesis and cell metastasis. We conjectured
that gallic acid plays a significant role in anticancer activities
(8–10). Additionally, there are few papers
with regard to the effect of gallic acid in cervical cancer cells
(11). Therefore, the current study
investigated the effect of gallic acid in human cervical cancer
HeLa and HTB-35 cells.
Materials and methods
Materials
Gallic acid was obtained from Tianjin Yi Fang Ke Ji,
Inc. (Tianjin, China). Gallic acid (100 mg) was dissolved in 1 ml
dimethyl sulfoxide (DMSO; Shanghai Yanhui Bio-tech Co., Ltd.,
Shanghai, China) as a stock solution (100 mg/ml). This stock
solution was further diluted to varying concentrations (0, 10, 15,
20, 25, 30 and 40 μg/ml) using cell culture medium immediately
prior to use. The control group was always treated with the same
concentration of DMSO.
Cell culture
Human cervical cancer HeLa and HTB-35 cells were
obtained from the American Type Culture Collection (Rockville, MD,
USA) and maintained in DMEM containing 10% fetal bovine serum (FBS;
Hangzhou Sijiqing Biology Engineering Materials Co., Ltd.,
Hangzhou, China), 100 U/ml penicillin, 50 μg/ml streptomycin and
100 μg/ml amphotericin. Cell cultures were were maintained in an
incubator with 5% CO2 at 37ºC. HUVECs were isolated from
fresh human umbilical cord veins and maintained in MEMα
(Invitrogen, Carlsbad, CA, USA) supplemented with 20% FBS, 0.04%
hydrocortisone, 0.1% VEGF, 0.1% IGF-1, 0.4% hFGF-B, 0.1% hEGF, 0.1%
ascorbic acid and 1% heparin.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5
diphenyltetrazolium bromide) assay
The MTT assay functions as an indicator of cell
survival and/or growth. The assay determines the presence of live
cells with functional mitochondria and may be used to detect
cytotoxicity and cell growth. Briefly, cervical cancer cells or
normal cells were placed into 96-well plates. Subsequent to
incubation overnight, the cultured cells were treated with varying
concentrations (0, 10, 15, 20, 25, 30 and 40 μg/ml) of gallic acid
for 24 h. MTT (Sigma, Shanghai, China) was added to each well and
incubated for an additional 4 h at 37ºC. Following centrifugation,
the medium containing non-metabilized MTT was then aspirated, and
150 μl DMSO was added to solubilize the formazan. The absorption
was then measured at a wavelength of 495 nm by an ELISA plate
reader (Varioskan Flash multimode reader, Thermo Fisher Scientific,
Shanghai, China).
Sulforhodamine B (SRB) assay
Skehan et al developed the SRB assay in order
to measure drug cytotoxicity and cell proliferation for large-scale
drug screening. The basis of this assay is the ability of SRB to
bind with cellular protein at differing pH values. The optical
density reading of the SRB assay is linear with cell number and
cellular protein (12). Briefly,
the cells were cultured and treated with various concentrations (0,
10, 15, 20, 25, 30 and 40 μg/ml) of gallic acid. Following 24 h of
incubation, the cells were fixed with 10% trichloroacetic acid and
stained with 0.4% SRB (Sigma) for 30 min. The excess dye was
removed by washing repeatedly with 1% acetic acid, then the
protein-bound dye was dissolved in 10 mM Tris base solution for
examination of the optical density at 510 nm using an ELISA plate
reader.
Bromodeoxyuridine (BrdU) proliferation
assay
The BrdU cell proliferation assay is an immunoassay
for the quantification of BrdU, which is incorporated into newly
synthesized DNA during the proliferative period of the cells. A
total of 10,000 cells in 250 μl culture medium were placed into
8-well chambers. The cells were treated with various concentrations
(0, 10, 12.5 and 15 μg/ml) of gallic acid, then incubated with BrdU
(25 μg/ml; Sigma) and fixed in 4% paraformaldehyde for 30 min. The
cells were then incubated with 2N HCl at 37ºC for 10 min.
Subsequent to incubation with 0.1 M boric acid for 3 min and being
blocked with 1% bovine serum albumin for 1 h, the cells were
incubated with anti-BrdU antibody (Millipore, Beijing, China)
overnight. Next, the cells were incubated with a FITC-conjugated
secondary antibody (Guangzhou Biological Technology Co., Ltd.,
Guangzhou, China) and then with DAPI (Sigma) at 10 μg/ml for 10
min, prior to being mounted using coverslips. Four random fields
from each well were counted under a fluorescent microscope (Nikon,
Japan).
Wound scratch assay
The wound scratch assay is an easy, economical and
well-developed method to quantify the migration rate of cells in
vitro. Brief, an artificial gap was created on the cell
monolayers with a plastic pipette tip following treatment with
various concentrations (0, 10, 15 and 20 μg/ml) of gallic acid.
Images of the wound area were captured following 24 h of incubation
under a phase-contrast microscope. Images of three random fields
were captured, and the cell migration ability was quantified as the
closure of the gap distance.
Invasion assay
Based on chemoattraction, the Matrigel invasion
assay is an extremely fast, low-cost and flexible method to
quantify the invasive ability of the majority of cell types. This
assay may be used to examine the migration activity associated with
matrix degradation. In the present study, Matrigel invasion
chambers (BD, Shanghai, China) were used to examine the ability of
cervical cancer cells to penetrate the extracellular matrix (ECM).
Subsequent to being treated with gallic acid for 24 h, the cells
were resuspended in serum-free medium and added to the upper
chamber, while medium containing 10% FBS was placed into the lower
chamber, thus serving as a chemoattractant. Following incubation
for 24 h, the cells on the bottom surface of the base membrane were
stained with Cell Tracker Green (Molecular Probes, Eugene, OR, USA)
and fixed in 4% formaldehyde for 10 min. Images of four fields were
captured randomly and the cells were counted in each field under a
fluorescence microscope at ×200 magnification. Data were expressed
as the invasive cell number compared with the control. All the
experiments were performed in triplicate and the results expressed
as the mean ± SEM of three independent experiments.
Tube formation assay
One of the most popular in vitro assays to
model the reorganization stage of angiogenesis is the tube
formation assay. Usually, this assay is employed to determine the
capacity of various compounds to increase or inhibit the formation
of capillary-like structures (tube formation). In the present
study, 70% ECM gel (100 μl; BD) was added to each well of a 96-well
plate, then placed in an incubator at 37ºC to allow the formation
of a gel. Subsequent to being treated with various concentrations
(0, 5, 10 and 15 μg/ml) of gallic acid, the HUVECs were
re-suspended in 150 μl serum-free medium, then placed onto the
solidified ECM gel and incubated for 2 h. The endothelial tubes of
5 random fields were examined under a phase-contrast microscope
(Nikon), and the extent of tube formation was estimated by counting
the overall tube length per area.
Western blot analysis
Subsequent to being treated with the various
concentrations (0, 10, 15 and 20 μg/ml) of gallic acid for 24 h,
the HeLa and HTB-35 cells were harvested and rinsed with PBS,
followed by extraction in 200 μl RIPA lysis buffer. Equal amounts
of each sample were separated by 10% Tris-Glycine gels then
transferred to PVDF membranes (Whatman, Hangzhou, China). The
membranes were blocked using skimmed milk, followed by incubation
with primary antibodies against ADAM17, EGFR, Akt, p-Akt, Erk,
p-Erk and actin (Santa Cruz Biotechnology, Dallas, TX, USA). The
membranes were analyzed after being incubated with horseradish
peroxidase-conjugated secondary antibodies, followed by the use of
a SuperSignal West Pico chemiluminescent protein detection kit
(Pierce, Rockford, Il, USA).
Statistical analysis
Data are presented as the mean ± SEM. Statistical
significance was analyzed by one-way ANOVA using the GraphPad Prism
software (version 4.0; La Jolla, CA, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Gallic acid reduces the viability of
cervical cancer cells
Gallic acid is an effective chemopreventive agent
in vivo and in vitro(6,7,13). In
the first experiment, two major active components, tannic acid and
gallic acid, from Chinese gall were compared with its aqueous crude
extract. Cell viability was determined by MTT assay using
logarithmically growing HeLa and HTB-35 cervical cancer cells
treated with various concentrations for 24 h. Tannic acid had no
greater cytotoxic effect on the HeLa and HTB-35 cells than the
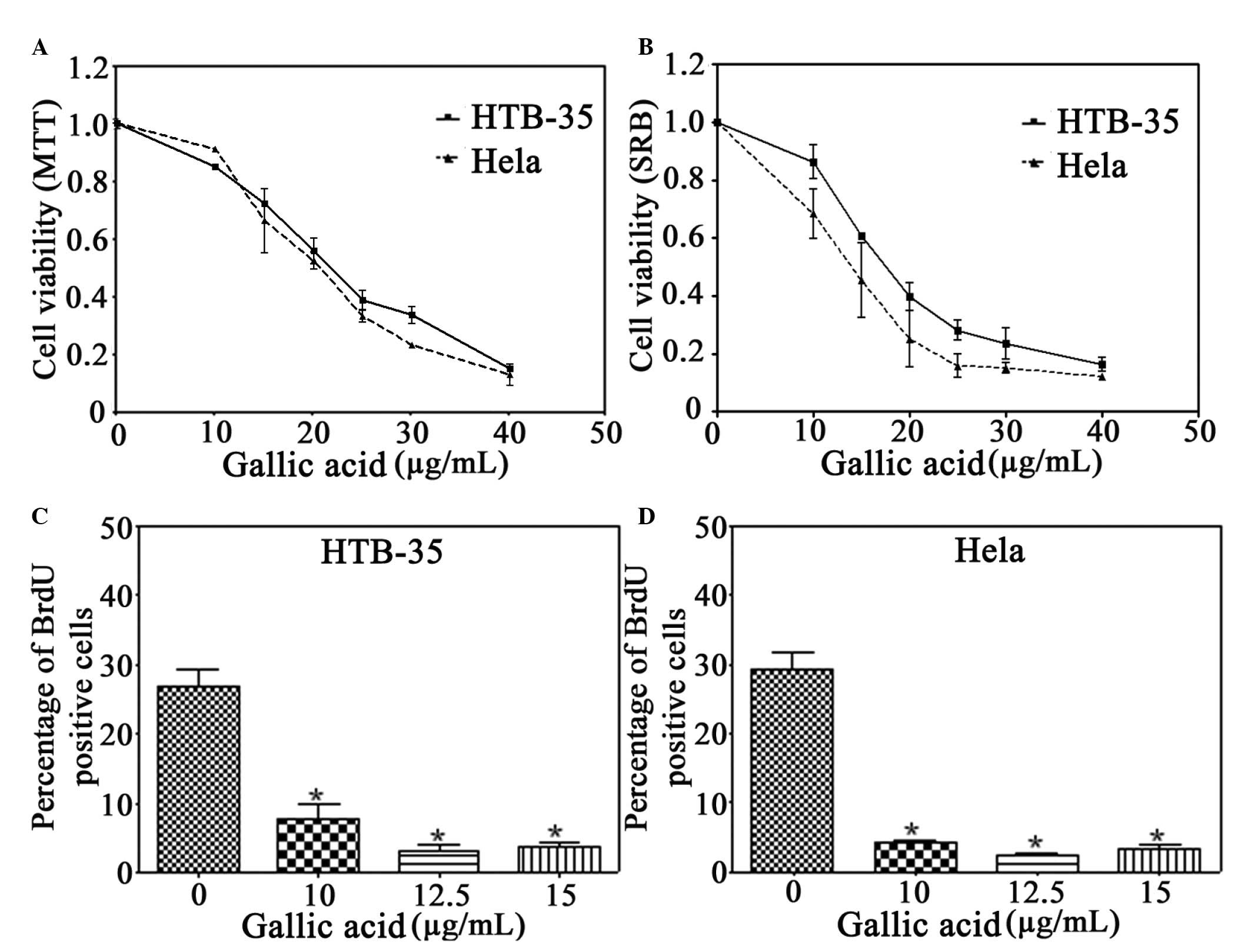
crude extract (data not shown). However, gallic acid significantly
reduced the cell viability in a dose-dependent manner (Fig. 1). Therefore, gallic acid was
selected for the subsequent experiments. Following treatment with
gallic acid for 24 h, the cell viability was significantly
decreased in the HeLa and HTB-35 cervical cancer cells, as examined
by MTT and SRB assays (Fig. 1A and
B). In comparison with the cytotoxic effect on the HeLa and
HTB-35 cervical cancer cells, gallic acid exhibited less
cytotoxicity in normal HUVECs. Gallic acid reduced cell viability
to ~92, 84 and 66% of the control in the HeLa cells and to ~94, 88
and 64% of the control in the HTB-35 cells at concentrations of 5,
10 and 15 μg/ml, respectively. However, at the same concentrations,
gallic acid was only able to decrease the cell viability to ~120,
111 and 75% of the control, respectively, in the HUVECs (data not
shown). These results provide direct evidence that gallic acid has
selective dose-dependent cytotoxicity for cervical cancer
cells.
To determine whether gallic acid had a
time-dependent effect on the cervical cancer cells, the cells were
treated with concentrations of 0, 10, 12.5 or 15 μg/ml, and an MTT
assay was performed at 24, 48, 72 and 96 h (Fig. 1C). Gallic acid was able to induce
significant inhibition of cell proliferation in the HeLa and HTB-35
cells, but only in a dose-dependent manner. The HeLa cells were
more sensitive to gallic acid than the HTB-35 cells at the same
concentration of 10 μg (Fig.
1C).
Gallic acid inhibits proliferation of
cervical cancer cells
To elucidate whether gallic acid contributes to the
inhibition of cell proliferation, a BrdU incorporation assay was
performed on the HeLa and HTB-35 cells treated with 10, 12.5 and 15
μg/ml of gallic acid for 24 h (Fig.
1D). Gallic acid significantly decreased the percentage of
BrdU-positive HeLa cells from 27% of the control group to 3.7%. By
contrast, the percentage of BrdU-positive HTB-35 cells was reduced
from 29% of the control group to 3.3%. These results indicate that
gallic acid elicits an antiproliferative effect in cervical cancer
cells.
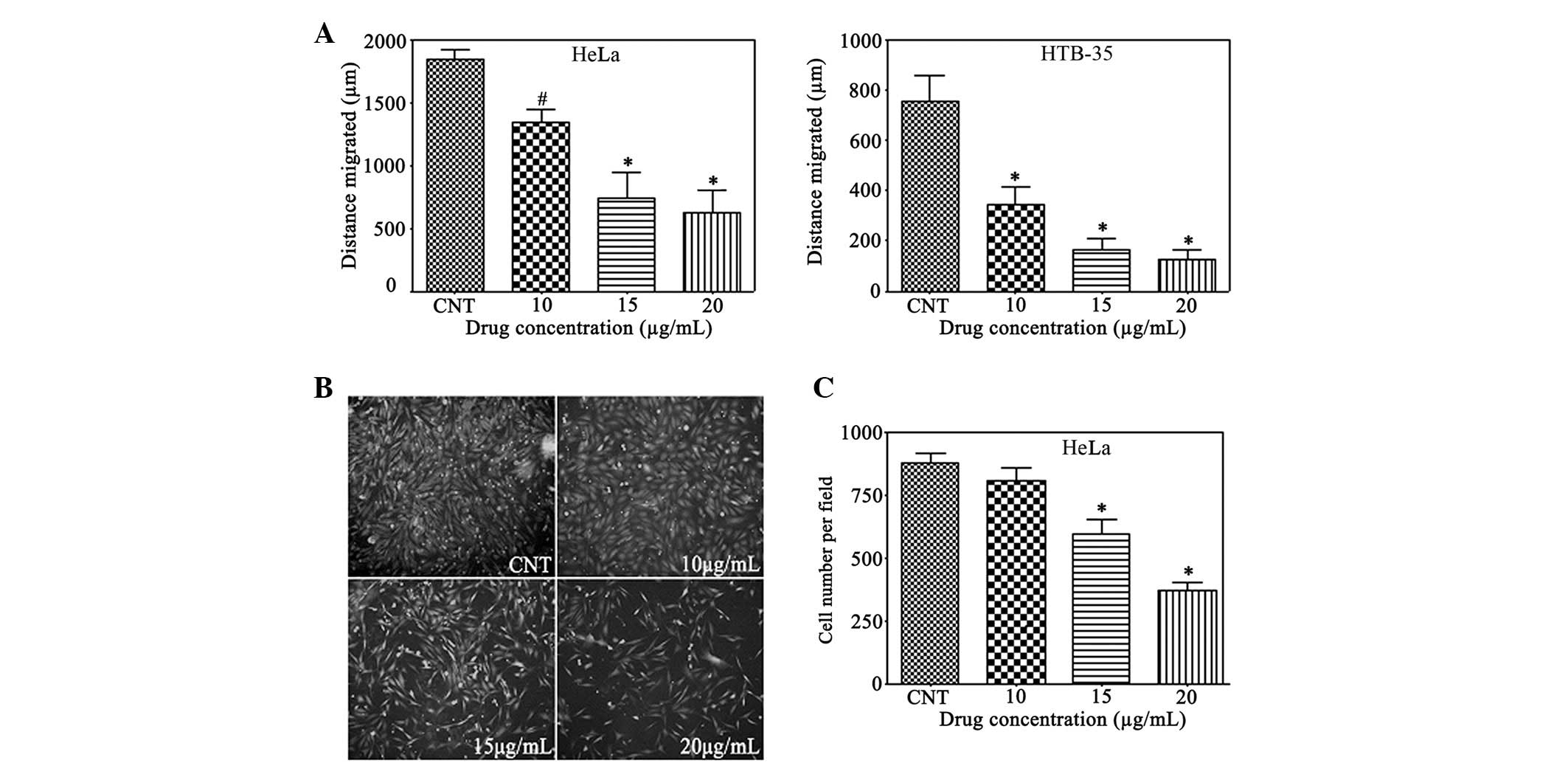
Gallic acid reduces cervical cancer cell
migration and invasion
If untreated, certain cervical high-grade
pre-invasive squamous intraepithelial lesions progress to become
invasive squamous cell carcinoma and spread to other areas of the
body through the blood and lymphocyte system (14). To study the contribution of gallic
acid to the cancer cell migration and invasion ability, a
wound-scratch assay and Matrigel invasion assay were performed on
the cervical cancer cells. In comparison with the untreated group,
in the HeLa and HTB-35 cervical cancer cells (Fig. 2A), the closure of the gap distance
was inhibited significantly and dose-dependently by gallic acid at
10, 15, and 20 μg/ml. Gallic acid displayed differing inhibitory
effects on the different cell lines. In comparison with the HeLa
cells, gallic acid decreased the gap to a greater extent at the
same concentrations in the HTB-35 cells. At concentrations of 10,
15, and 20 μg/ml, gallic acid was able to dramatically inhibit cell
migration to 73, 40 and 34% in the HeLa cells and to 45, 22 and 17%
in the HTB-35 cells, respectively, compared with the control.
Invasiveness is an important characteristic of cervical cells and a
target for the development of anticancer agents (14). As shown in Fig. 2B and C, gallic acid significantly
reduced the invasiveness of the HeLa cells (P<0.05) to ~92, 68
and 29% of the control at the concentrations of 10, 15, and 20
μg/ml.
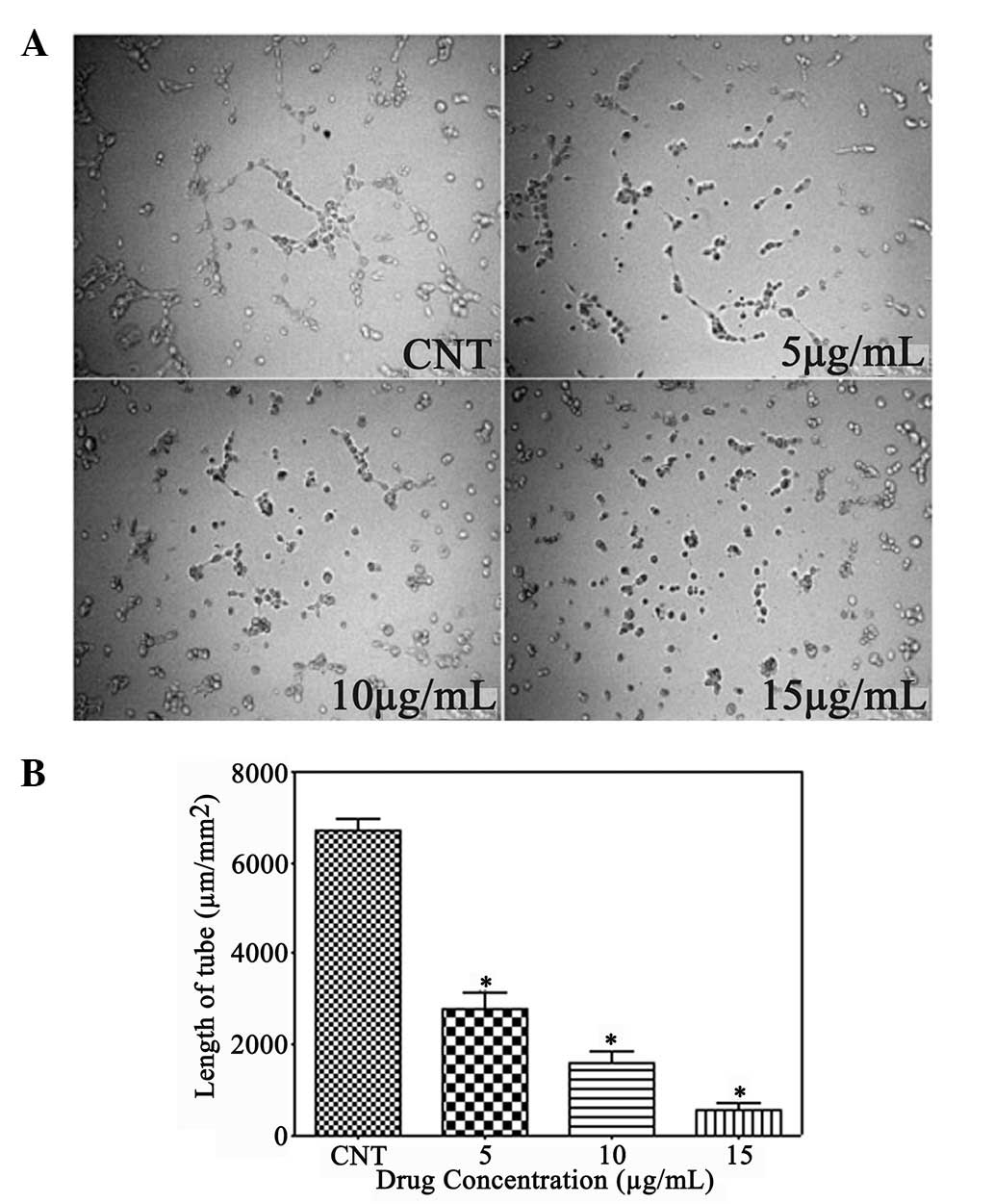
Gallic acid inhibits angiogenesis
Angiogenesis is the formation of new blood vessels,
which is considered a critical step for the growth of solid tumors.
Due to the neovascular nature of cervical cancer (15,16),
the present study investigated whether gallic acid had the ability
to inhibit tube formation in HUVECs. The untreated control group
was composed of multiple cells that gathered together and adhered
to each other. However, gallic acid showed significant inhibition
of the elongation of the tubes at all concentrations, and the tube
length per area was decreased to ~16.5, 15.3 and 30.3% of the
control group, respectively (Fig. 3A
and B).
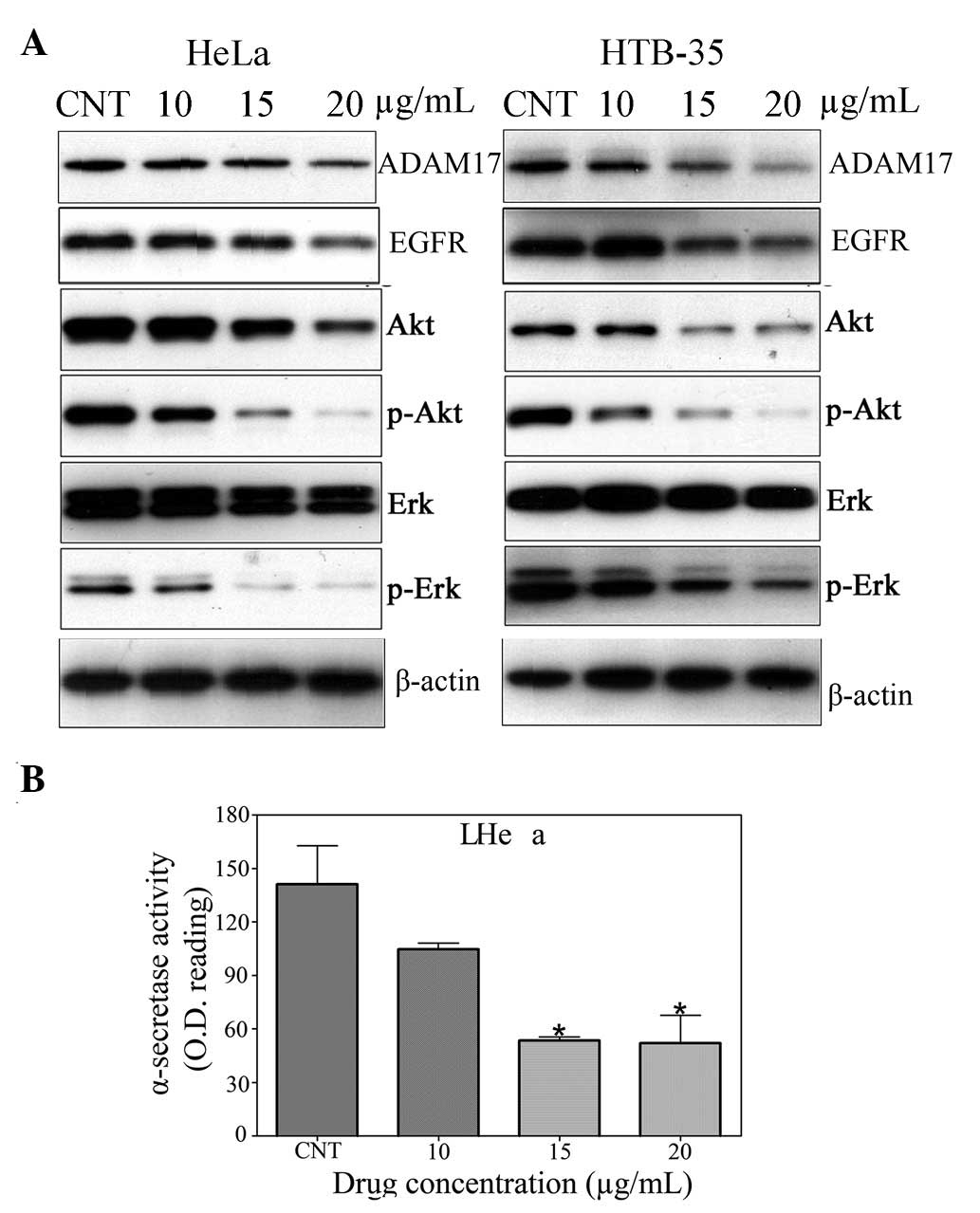
Gallic acid suppresses ADAM17 and EGFR
expression in cervical cancer cells
It has been reported that ADAM17 contributes to
cancer cell progression through activation of the EGFR/PI3K/Akt and
EGFR/Ras/MAPK/Erk signaling pathways (17,18).
It is evident that EGFR is expressed in normal and cervical cancers
with varying degrees of EGFR expression (19–21).
Therefore, these make cervical cancer amenable for targeted
therapy. EGFR expression acts as a biomarker for forming a
prognosis or for administering treatment (22). To elucidate whether gallic acid was
able to inhibit cervical cancer cell invasion by decreasing the
levels of ADAM17 and EGFR, the expression of ADAM17, Erk/p-Erk and
Akt/p-Akt was examined by western blotting in the HeLa and HTB-35
cervical cancer cells following treatment with gallic acid (10, 15,
20 μg/ml) for 24 h. In each cell line, the expression of ADAM17,
EGFR, p-Erk and p-Akt was significantly inhibited by gallic acid
(Fig. 4A). To further verify the
effect of gallic acid on ADAM17, an α-secretase activity assay kit
was employed to measure ADAM17 activity. Subsequent to being
treated with gallic acid at 20, 30, and 40 μg/ml for 24 h, ADAM17
activity was significantly decreased to approximately 74.7
(P<0.05), 38.8 (P<0.01) and 37.6% (P<0.01) of the control
group, respectively (Fig. 4B).
These results indicate that gallic acid elicits a reduction in
tumor invasiveness through the downregulation of ADAM17 and EGFR
expression and the dephosphorylation of Erk and Akt.
Discussion
The present study examined the effect of gallic
acid, a major active component of Chinese gall, which is a
traditional Chinese medical herb that has been used to treat
various cancers. The results of the MTT and SRB assays showed that
gallic acid significantly decreased the cell viability of the HeLa
and HTB-35 cancer cells in a dose-dependent manner. In comparison
with the effect on the cervical cancer cells, gallic acid showed a
substantial reduction of the cytotoxic effects on normal HUVECs at
the same concentrations (data not shown). These results indicated
that gallic acid exhibits significantly selective cytotoxicity in
cervical cancer. This data therefore demonstrates that gallic acid
reduces cervical cancer cell proliferation. The HeLa and HTB-35
cells were severely affected by gallic acid in the BrdU
incorporation assay. Previous studies have reported that gallic
acid has the ability to induce apoptosis in esophageal cancer
(6), human prostate cancer
(13) and HL-60 promyelocytic
leukemia (23) cells. However,
there was no significant apoptosis observed in either cell line
following the employment of Hoechst 33342 staining and a TUNEL
apoptosis assay in the pilot study.
The vascular network provides all the cells in the
body with oxygen and nutrients. It is evident that oxygen possesses
the most significant role in the regulation of solid tumor growth.
Due to aberrant growth and the subsequent imbalance of the supply
and demand of oxygen, the solid tumors, including those of cervical
cancer cells, cause hypoxia, which promotes the growth of new
capillaries to acquire more oxygen (16,22,24).
Previous studies have reported that gallic acid may be responsible
for the decrease of angiogenesis in vitro and in
vivo(25). With the employment
of a human placental vein angiogenesis model, gallic acid has been
shown to significantly inhibit the initiation of angiogenesis and
neovessel growth (25).
Additionally, following the intraperitoneal administration of 250
mg Rubus extract for 2 days, serum from the rats also
exhibited significant inhibition of angiogenic initiation and
subsequent neovessel growth. However, subsequent to being gavaged
with the extract, serum from the rats did not significantly reduce
angiogenic initiation and neovessel growth. Therefore, in the
present study, an in vitro tube formation assay was
performed to examine whether gallic acid has anti-angiogenesis
abilities. The HUVECs were less sensitive to the cytotoxicity
induced by gallic acid compared with HeLa and HTB-35 cells at the
concentrations of 5, 10 and 15 μg/ml. However, at all three
concentrations, gallic acid significantly decreased the capillary
tube formation in the HUVECs. Therefore, based on this data, gallic
acid may be considered useful for targeting angiogenesis. However,
further clarification of the underlying mechanisms is
necessary.
In the present study, a wound healing assay was
employed to investigate the migration of the HeLa and HTB-35 cells,
and the Matrigel invasion assay was performed to evaluate the
capacity of the HeLa cells to degrade the ECM and migrate through
the pores in the membrane. These data showed that the migration of
the HeLa and HTB-35 cells was significantly reduced with the
treatment of gallic acid. Also, the reduction of the invasion
potential by gallic acid was observed in the HeLa cells. To
elucidate the underlying mechanisms of decreased invasiveness, the
present study analyzed the protein expression of ADAM17 and EGFR
and the dephosphorylation state of Erk and Akt with the use of
western blotting in the HeLa and HTB-35 cells. Gallic acid
significantly reduced the level of ADAM17 and EGFR expression and
the level of Akt and Erk phosphorylation in the two cell lines. In
addition to the inhibition of protein expression, gallic acid
significantly reduced ADAM17 activity at all indicated
concentrations.
EGFR is a 170-kDa transmembrane glycoprotein
receptor encoded by the Her-1 proto-oncogene located on chromosome
7p12. EGFR functions through dimerization, which activates a
tyrosine kinase domain to regulate multiple functions, including
cell growth, differentiation, gene expression and development
(26). EGFR is present in numerous
normal tissues and is expressed in a wide variety of solid tumors,
including cervical cancer (27).
ADAMs are best known as ectodomain sheddases, and their domains
function as metalloproteases (28,29).
The disintegrin metalloproteinases of the ADAM family are
associated with the process of proteolytic ‘shedding’ of
membrane-associated proteins and hence the rapid modulation of key
cell signaling pathways in the tumor microenvironment. ADAM17 is an
important member of the ADAM family involved in the proteolysis of
collagen IV of the ECM and also the release of several integrins
from the cell surface, indicating that ADAM17 affects the migration
activity of a variety of cells, including cervical cancer cells.
ADAM17 is a primary upstream component for multiple EGFR
pro-ligands (30). EGFR binds with
its ligands and subsequently activates downstream MAPK/ERK and
PI3K/Akt pathways, which contribute to invasiveness and other
malignant phenotypes (17,18,30).
Gallic acid significantly decreases the phosphorylation of members
of the PI3K/AKT and MAPK/ERK signaling pathways, which play key
roles in cell proliferation and invasion. The present results
indicated that the inhibition of ADAM17 by gallic acid may be
responsible for decreased invasiveness through the suppression of
the EGFR/PI3K/AKT and EGFR/MAPK/ERK pathways.
In summary, the present study demonstrated that
gallic acid significantly reduces cell viability, proliferation,
invasion and tube formation. Suppression of ADAM17 and EGFR may
contribute to the inhibition of invasiveness through the
inactivation of PI3K/AKT and MAPK/ERK signaling pathways. These
results demonstrate the importance of validating the use of
traditional Chinese medicinal herbs in tumor prevention and
therapy, and show that gallic acid may potentially serve as a
candidate for cervical cancer treatment.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China, 2011–2013.
References
|
1
|
Armstrong EP: Prophylaxis of cervical
cancer and related cervical disease: a review of the
cost-effectiveness of vaccination against oncogenic HPV types. J
Manag Care Pharm. 16:217–230. 2010.PubMed/NCBI
|
|
2
|
Globocan. 2008, IARC. http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=900#WOMEN.
Accessed September 20, 2013
|
|
3
|
Alvarez-Salas LM and DiPaolo JA: Molecular
approaches to cervical cancer therapy. Curr Drug Discov Technol.
4:208–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haroon S and Cui M: Role of Pap smear in
early diagnosis of cervical cancer - A case study of women in Saudi
Arabia. Life Science Journal. 9:1027–1036. 2012.
|
|
5
|
Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Suppression of human cervical cancer cell
lines Hela and DoTc2 4510 by a mixture of lysine, proline, ascorbic
acid, and green tea extract. Int J Gynecol Cancer. 16:1241–1247.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Faried A, Kurnia D, Faried LS, et al:
Anticancer effects of gallic acid isolated from Indonesian herbal
medicine, Phaleria macrocarpa (Scheff.) Boerl, on human
cancer cell lines. Int J Oncol. 30:605–613. 2007.PubMed/NCBI
|
|
7
|
Raina K, Rajamanickam S, Deep G, Singh M,
Agarwal R and Agarwal C: Chemopreventive effects of oral gallic
acid feeding on tumor growth and progression in TRAMP mice. Mol
Cancer Ther. 7:1258–1267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
You BR and Park WH: Gallic acid-induced
lung cancer cell death is related to glutathione depletion as well
as reactive oxygen species increase. Toxicol In Vitro.
24:1356–1362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
You BR, Kim SZ, Kim SH and Park WH: Gallic
acid-induced lung cancer cell death is accompanied by ROS increase
and glutathione depletion. Mol Cell Biochem. 357:295–303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verma S, Singh A and Mishra A: Gallic
acid: Molecular rival of cancer. Environ Toxicol Pharmacol.
35:473–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
You BR, Moon HJ, Han YH and Park WH:
Gallic acid inhibits the growth of HeLa cervical cancer cells via
apoptosis and/or necrosis. Food Chem Toxicol. 48:1334–1340. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Skehan P, Storeng R, Scudiero D, et al:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agarwal C, Tyagi A and Agarwal R: Gallic
acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via
ATM-Chk2 activation, leading to cell cycle arrest, and induces
apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther.
5:3294–3302. 2006. View Article : Google Scholar
|
|
14
|
Zhai Y, Bommer GT, Feng Y, Wiese AB,
Fearon ER and Cho KR: Loss of estrogen receptor 1 enhances cervical
cancer invasion. Am J Pathol. 177:884–895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Leo S, Caschetto S, Garozzo G, et al:
Angiogenesis as a prognostic factor in cervical carcinoma. Eur J
Gynaecol Oncol. 19:158–162. 1998.
|
|
16
|
Triratanachat S, Niruthisard S,
Trivijitsilp P, Tresukosol D and Jarurak N: Angiogenesis in
cervical intraepithelial neoplasia and early-staged uterine
cervical squamous cell carcinoma: clinical significance. Int J
Gynecol Cancer. 16:575–580. 2006. View Article : Google Scholar
|
|
17
|
Lin P, Sun X, Feng T, et al: ADAM17
regulates prostate cancer cell proliferation through mediating cell
cycle progression by EGFR/PI3K/AKT pathway. Mol Cell Biochem.
359:235–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao LJ, Lin P, Lin F, et al: ADAM17
targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to
promote prostate cancer cell invasion. Int J Oncol. 40:1714–1724.
2012.PubMed/NCBI
|
|
19
|
Jeong JH, Jeong YJ, Cho HJ, et al:
Ascochlorin inhibits growth factor-induced HIF-1α activation and
tumor-angiogenesis through the suppression of EGFR/ERK/p70S6K
signaling pathway in human cervical carcinoma cells. J Cell
Biochem. 113:1302–1313. 2012.PubMed/NCBI
|
|
20
|
Mathur SP, Mathur RS and Young RC:
Cervical epidermal growth factor-receptor (EGF-R) and serum
insulin-like growth factor II (IGF-II) levels are potential markers
for cervical cancer. Am J Reprod Immunol. 44:222–230. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lakshmi S, Nair M, Jayaprakash P and
Pillai M: Epidermal growth factor and its receptor in cervical
cancer. Oncol Rep. 4:1103–1106. 1997.PubMed/NCBI
|
|
22
|
Gaffney DK, Haslam D, Tsodikov A, et al:
Epidermal growth factor receptor (EGFR) and vascular endothelial
growth factor (VEGF) negatively affect overall survival in
carcinoma of the cervix treated with radiotherapy. Int J Radiat
Oncol Biol Phys. 56:922–928. 2003. View Article : Google Scholar
|
|
23
|
Madlener S, Illmer C, Horvath Z, et al:
Gallic acid inhibits ribonucleotide reductase and cyclooxygenases
in human HL-60 promyelocytic leukemia cells. Cancer Lett.
245:156–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hawighorst H, Knapstein PG, Weikel W, et
al: Angiogenesis of uterine cervical carcinoma: characterization by
pharmacokinetic magnetic resonance parameters and histological
microvessel density with correlation to lymphatic involvement.
Cancer Res. 57:4777–4786. 1997.
|
|
25
|
Liu Z, Schwimer J, Liu D, et al: Gallic
acid is partially responsible for the antiangiogenic activities of
Rubus leaf extract. Phytother Res. 20:806–813. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soonthornthum T, Arias-Pulido H, Joste N,
et al: Epidermal growth factor receptor as a biomarker for cervical
cancer. Ann Oncol. 22:2166–2178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baselga J: Why the epidermal growth factor
receptor? The rationale for cancer therapy. Oncologist. 7(Suppl 4):
2–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu X, Lu D, Scully M and Kakkar V: ADAM
proteins - therapeutic potential in cancer. Curr Cancer Drug
Targets. 8:720–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kheradmand F and Werb Z: Shedding light on
sheddases: role in growth and development. Bioessays. 24:8–12.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng X, Jiang F, Katakowski M, Zhang ZG,
Lu QE and Chopp M: ADAM17 promotes breast cancer cell malignant
phenotype through EGFR-PI3K-AKT activation. Cancer Biol Ther.
8:1045–1054. 2009. View Article : Google Scholar : PubMed/NCBI
|