Introduction
The endoplasmic reticulum (ER) is an organelle that
regulates the synthesis, folding and aggregation of intracellular
proteins. ER stress (ERS) is a subcellular organelle pathology in
which the aggregation of unfolded or misfolded proteins in the ER
leads to its dysfunction. Previous studies have highlighted
evidence that ERS is closely associated with viral hepatitis,
non-alcoholic fatty liver disease, alcoholic liver damage, liver
cancer and other liver diseases (1–6).
The unfolded protein response (UPR) is a protective
response mediated by the ER chaperone, glucose-regulated protein 78
(GRP78), and three ERS receptor proteins, protein kinase-like ER
kinase (PERK), activating transcription factor 6 (ATF6) and
inositol-requiring enzyme 1 (IRE1). If ERS is absent, PERK, ATF6
and IRE1 combine with GRP78, making it inactive. When ERS is
present, GRP78 dissociates from these three transmembrane proteins
in favor of combining with the unfolded protein. Following
dissociation, the receptor proteins are activated and initiate the
UPR, which may raise the expression of GRP78 and folded protease by
inhibiting protein synthesis. In addition, the receptor proteins
promote ER-associated degradation to reduce the aggregation of
unfolded or misfolded proteins in the ER, protect the cell from
ERS-induced damage and restore normal cell function. In addition to
initiating the ERS-mediated adaptive response when ERS is marked or
long-term, PERK, ATF6 and IRE1 initiate ERS-mediated apoptosis,
inducing cell damage and apoptosis. Previous studies have indicated
that ERS induces apoptosis through the following pathways:
CCAAT/enhancer-binding protein homologous protein (CHOP); growth
arrest/DNA damage-inducible protein 153; C-Jun N-terminal kinase
(JNK); and caspase (7).
In the current study, intermittently administrated
diethylnitrosamine (DEN) was used to induce a rat liver cancer
model that simulated the occurrence and development of human liver
cancer. The critical regulatory factors in three ERS signaling
pathways were observed during the progression of hepatocellular
carcinoma (HCC) in order to clarify the mechanism of liver cancer
and to provide an experimental basis for its prevention and
targeted therapy.
Materials and methods
Rat liver cancer model
In total, 136 male, 5-week-old Wistar rats
[SCXK-(Ji) 2007-0003; Experimental Animal Center of Bethune Medical
College of Jilin University, Certificate of Conformity, Yanji,
China] with body weights of 140–160 g and which had been feeding
stably for 7 days, were divided into experimental and control
groups. The experimental group (n=120) was provided with sterile
drinking water containing 0.01% DEN (purity, 99.9%; Sigma-Aldrich,
St. Louis, MO, USA) ad libitum. The water containing DEN was
replaced every day. After 5 weeks, the rats were provided with
DEN-free water for three weeks and then 0.01% DEN solution for 12
weeks prior to withdrawal of the drug. The control group (n=16)
received sterilized drinking water without DEN for the duration of
the experiment. In total, 15 experimental rats were sacrificed at
5, 8, 10, 12, 14, 16, 18 and 20 weeks each, respectively, with two
control rats of the same age sacrificed at each of these
time-points. This study was approved by the ethics committee of
Yanbian University (Yanji, China).
Specimen collection and processing
Experimental rats were sacrificed, and the
appearance, color and texture of the livers were recorded. Specific
sections of liver or liver cancer tissues were fixed in 4%
paraformaldehyde, paraffin-embedded and sectioned for HE staining.
Other sections of the liver or liver cancer tissues (1×1×1 mm) were
fixed in 2.5% glutaraldehyde at 4°C, rinsed twice in 0.1 mol/l PBS
and then fixed in 1.0% osmium tetroxide and embedded in EPON812 for
ultra-thin sections, which were double-stained with uranyl acetate
and lead citrate and observed by a JEM1200EX transmission electron
microscope (JEOL, Tokyo, Japan).
Western blotting
Livers and tumors were lysed in lysis buffer (Pierce
Biotechnology, Inc., Rockford, IL, USA) and then centrifuged at
12,000 × g for 15 min. Protein concentration was determined using
the BCA kit (Pierce Biotechnology, Inc.) according to the
manufacturer’s instructions. A 70-μg protein sample was
fractionated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred to a polyvinylidene fluoride
membrane (Pall Corporation, Port Washington, NY, USA). Following
blocking for 1 h with 5% milk in Tris-buffered saline and Tween-20,
the following primary antibodies were added and the blots were
incubated at 4°C overnight: Rabbit polyclonal anti-GRP78, rabbit
polyclonal anti-PERK, rabbit polyclonal anti-ATF6, rabbit
monoclonal anti-IRE-1, goat monoclonal anti-CHOP, rabbit polyclonal
anti-eIF2α and rabbit monoclonal anti-TRAF2 or anti-caspase-12
(1:400; Boshide Biotechnology Co., Ltd., Wuhan, China). Following
incubation with secondary antibodies (1:5,000), the membranes were
visualized by chemiluminescence. The intensity of the protein bands
was quantitatively determined using an ultraviolet crosslinker
(Bio-Rad, Hercules, CA, USA) and normalized with the intensity of
the actin (rabbit polyclonal anti-calnexin; Nanjing KeyGen
Biotech., Co., Ltd., Nanjing, China) band in each gel.
Quantitative (q)PCR
Total RNA was extracted from the tumors using the
RNeasy Plus Mini kit (Nanjing KeyGen Biotech., Co., Ltd.) according
to the manufacturer’s instructions. cDNA was generated with the
iScript Select cDNA Synthesis kit and analyzed by qPCR using
SyberGreen qPCR primer assays and the iCycler iQ multicolor
real-time PCR detection system (all Nanjing KeyGen Biotech., Co.,
Ltd.). Relative expression levels were normalized against β-actin
expression run concurrently as a reference control. Primer
sequences are shown in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Target gene | Sequence 5′-3′ | Length, bp |
|---|
| β-actin | F:
GCAGAAGGAGATTACTGCCCT
R: GCTGATCCACATCTGCTGGAA | 136 |
| GRP78 | F:
TCGACTTGGGGACCACCTAT
R: GCCCTGATCGTTGGCTATGA | 77 |
| PERK | F:
GAAGTGGCAAGAGGAGATGG
R: GAGTGGCCAGTCTGTGCTTT | 61 |
| ATF6 | F:
GGACCAGGTGGTGTCAGAG
R: GACAGCTCTGCGCTTTGGG | 61 |
| IRE-1 | F:
TCATCTGGCCTCTTCTCTCGGA
R: TTGAGTGAGTGGTTGGAGGC | 77 |
| CHOP | F:
ACCACCACACCTGAAAGCAG
R: AGCTGGACACTGTCTCAAAG | 86 |
| eIF2α | F:
TTGAACTGTTGTGACCCCGAC
R: CGTAGTCTGCCCGATTTTGC | 71 |
| TRAF2 | F:
TGCTATCTTCTCCCCAGCCT
R: TCGCCATTCAAGTAGACCCG | 75 |
| Caspase-12 | F:
TGGATACTCAGTGGTGATAA
R: ACGGCCAGCAAACTTCATTA | 76 |
Statistical analysis
Data from all experiments are presented as the mean
± SD. Statistical differences were evaluated using one-way ANOVA
and independent t-tests of sample pairs with SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Rat liver pathological changes
Control rats
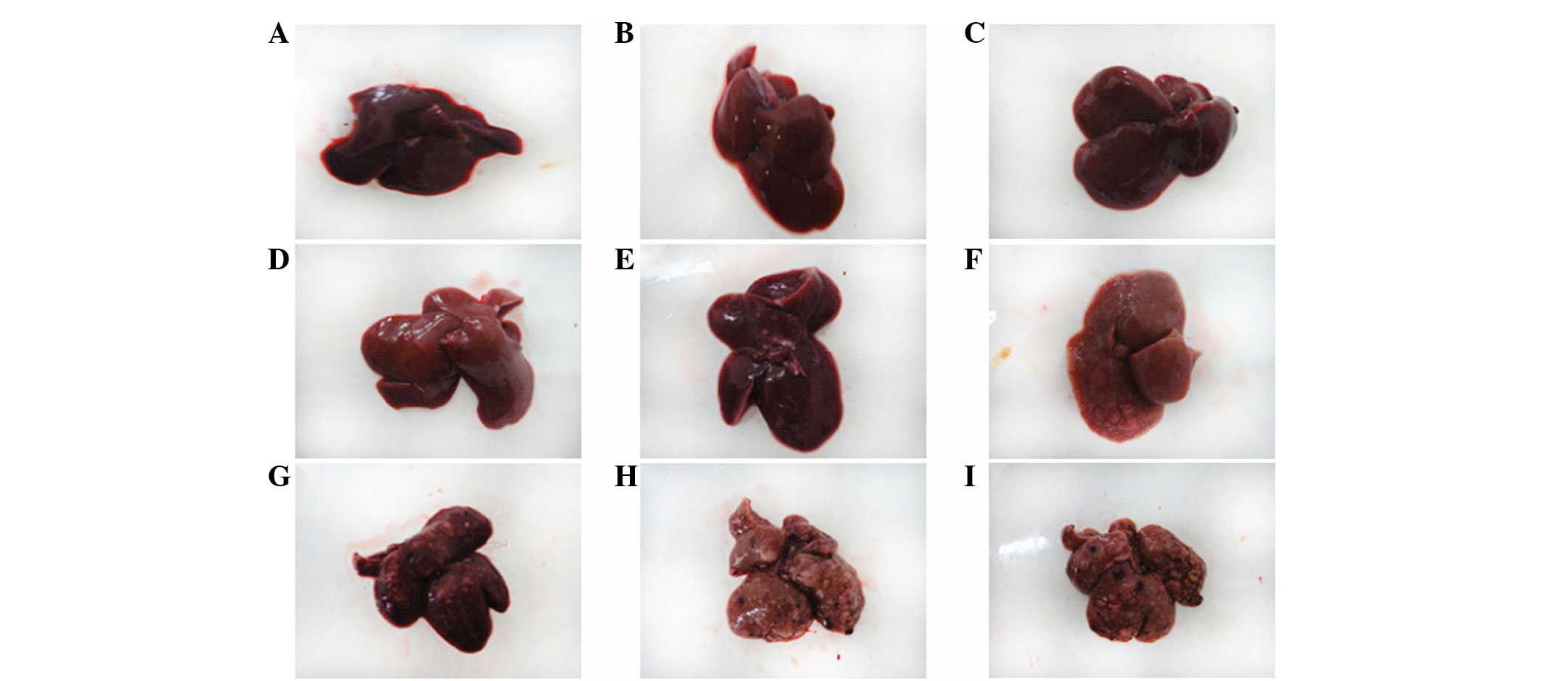
The surface of the liver was smooth and brown with
an evident gloss, and the texture was soft (Fig. 1A). Observed by light microscopy, the
structure of the hepatic lobule was complete, with hepatocytes
arranged in neat rows and clear nuclei (Fig. 2A). By electron microscopy, the cell
morphology was regular, rounded or oval and the ratio of nucleus to
cytoplasm was normal. Cytoplasmic organelles were abundant, with
well-developed mitochondria, rough and smooth ER and Golgi
complexes (Fig. 3A).
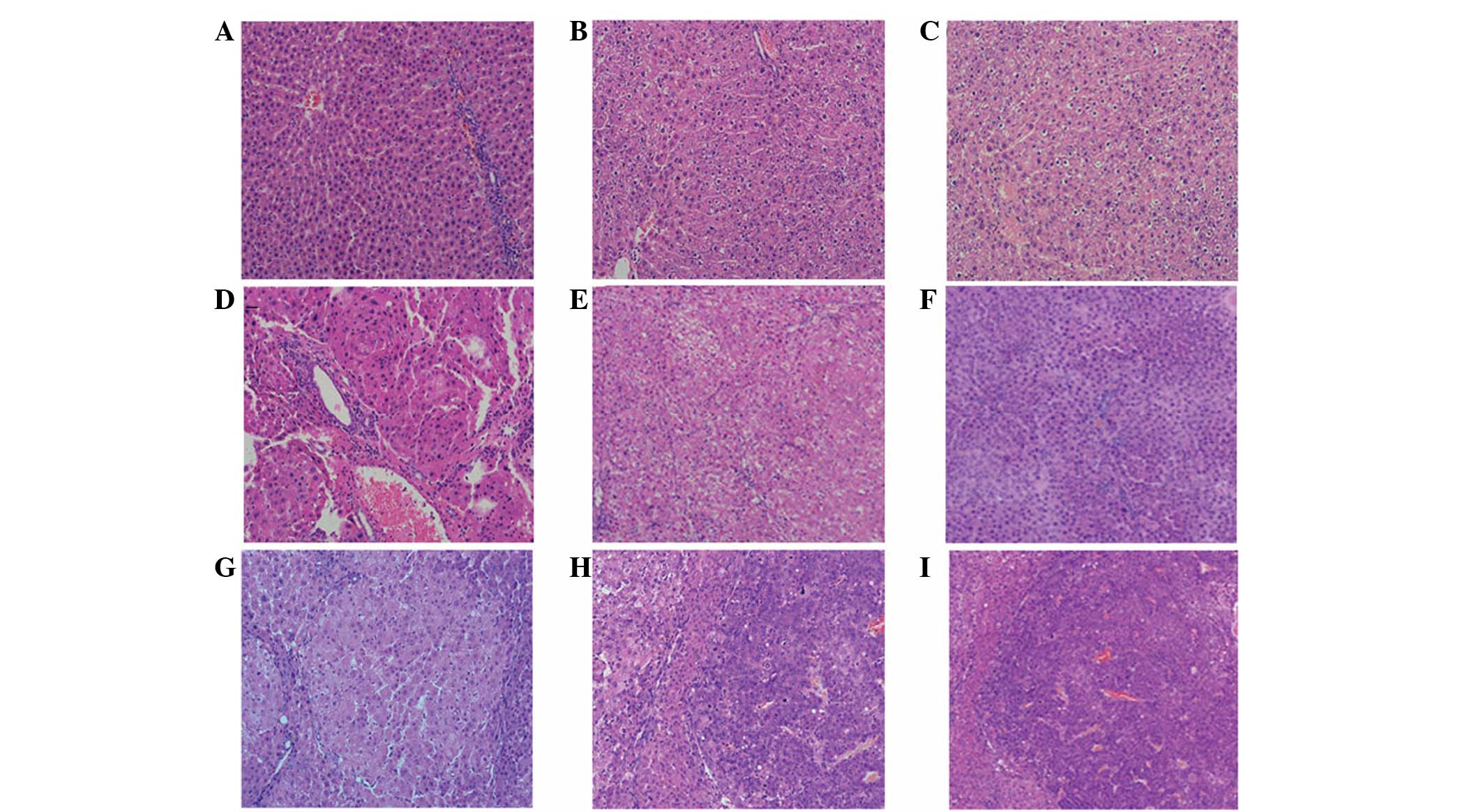 | Figure 2Changes in liver tissue during the
development of DEN-induced rat liver cancer, as observed by light
microscopy. (A) Normal group, the architecture of the hepatic lobes
was complete, with hepatocytes arranged in neat rows and clear cell
nuclei. Experimental group following the induction of rat liver
cancer at (B) 5 weeks; (C) 8 weeks, the architecture of the hepatic
lobes was basically complete and there was visible intralobular
focal necrosis with infiltrating inflammatory cells; (D) 10 weeks;
(E) 12 weeks; (F) 14 weeks, the architecture of the hepatic lobes
was damaged and the hepatocytes were proliferating, which was
accompanied by severe steatosis and the formation of visible
pseudolobules; (G) 16 weeks; (H) 18 weeks; and (I) 20 weeks, the
cancer cells exhibited evident atypia, with abnormally large nuclei
and diminished cytoplasm (HE staining; magnification, ×200). DEN,
diethylnitrosamine. |
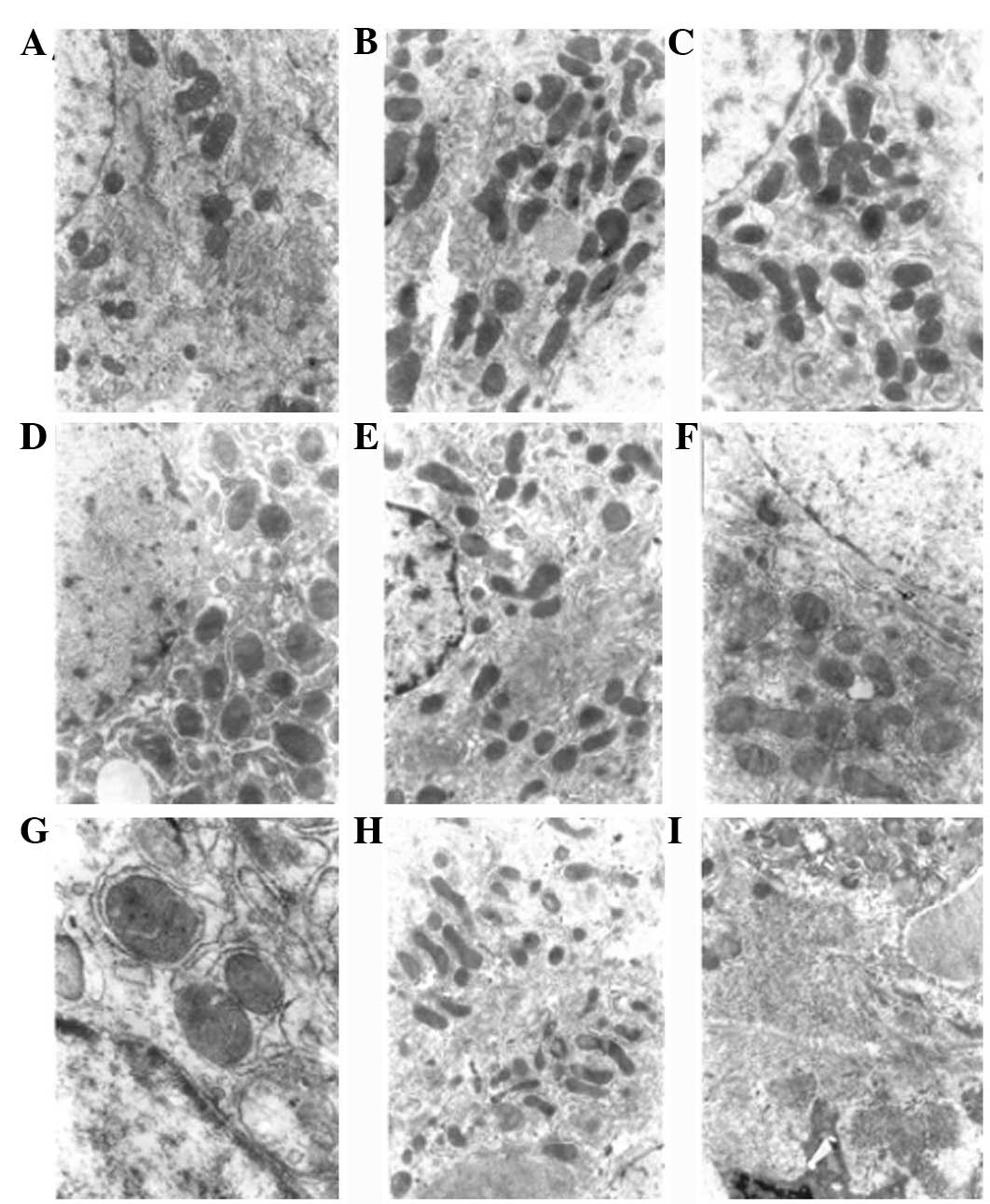 | Figure 3Electron microscopy showing changes in
liver tissue in DEN-induced rat liver cancer. (A) Normal group, the
hepatocytes were round or oval and in regular arrays, the ratio of
nucleus to cytoplasm was normal and cytoplasmic organelles were
abundant. Experimental group following the induction of rat liver
cancer at (B) 5 weeks; (C) 8 weeks, the hepatocytes were swollen,
with swollen mitochondria, the granular matrix had disappeared and
there was granulovacuolar degeneration; (D) 10 weeks; (E) 12 weeks;
(F) 14 weeks, aggregation of hepatocyte chromatin, increased number
of mitochondria, distrupted cristae of the mitochondria, dilation
of the rough ER, uneven nuclear membranes and migration of the
nucleoli to the side of the cell was observed; (G) 16 weeks; (H) 18
weeks; and (I) 20 weeks, larger hepatocyte nuclei, reduced number
of mitochondria, disrupted structure of the mitochondria, loss of
the layered structure of the rough ER, fragmented plasma membrane
and dissociation of the ribosomes from the rough ER was observed
(TEM; magnification, ×6,000). DEN, diethylnitrosamine; ER,
endoplasmic reticulum. |
Experimental rats
The experimental rat liver pathology was divided
into the following three temporal stages: Early
carcinogenesis-hepatocyte injury period (1–8 weeks); interim
carcinogenesis-sclerosis (9–14 weeks); and late
carcinogenesis-cancer period (15–20 weeks). In the early
carcinogenesis-hepatocyte injury period, the appearance of the
liver was not evidently abnormal (Fig.
1B and C). Observed by light microscopy, the architecture of
the hepatic lobes was basically complete, although a few of the
cells exhibited ballooning degeneration. There was visible
intralobular focal necrosis with inflammatory cell infiltration and
gradual emergence of fibrous tissue proliferation and regeneration
of hepatocytes (Fig. 2B and C).
Electron microscopy observed swelling hepatocytes, with swelling
mitochondria and the disappearance of the granular matrix, which
was accompanied by granulovacuolar degeneration (Fig. 3B and C). In the interim
carcinogenesis-sclerosis period (9–14 weeks), the surface of the
liver gradually roughened and varying numbers of large and small
gray lesions appeared (Fig. 1D–F).
As observed by light microscopy, the normal lobular structure was
destroyed, hepatocytes were replaced by fibrous tissue and the
typical pseudolobular structure had formed (Fig. 2D–F). Electron microscopy revealed
the aggregation of hepatocyte chromatin, increased numbers of
mitochondria, disrupted mitochondrial cristae, dilated rough ER,
uneven nuclear membranes and the migration of nucleoli to the edges
of the cells (Fig. 3D–F). In the
late carcinogenesis-cancer period (15–20 weeks), the surface of the
liver was covered with multiple large and small nodules (Fig. 1H–J). Observed by light microscopy,
the cancer cells exhibited evident atypia, with larger nuclei and
less cytoplasm than normal, and there were a number of monocytes
and mitotic figures (Fig. 2H–J).
Electron microscopy showed hepatocyte nuclei of an increased size,
fewer mitochondria, a disrupted mitochondrial structure, loss of
the layered structure of the rough ER, fragmentation of the plasma
membrane and dissociation of the ribosomes from the ER (Fig. 3H–J).
Test results of associated factors in the
ERS reaction pathway
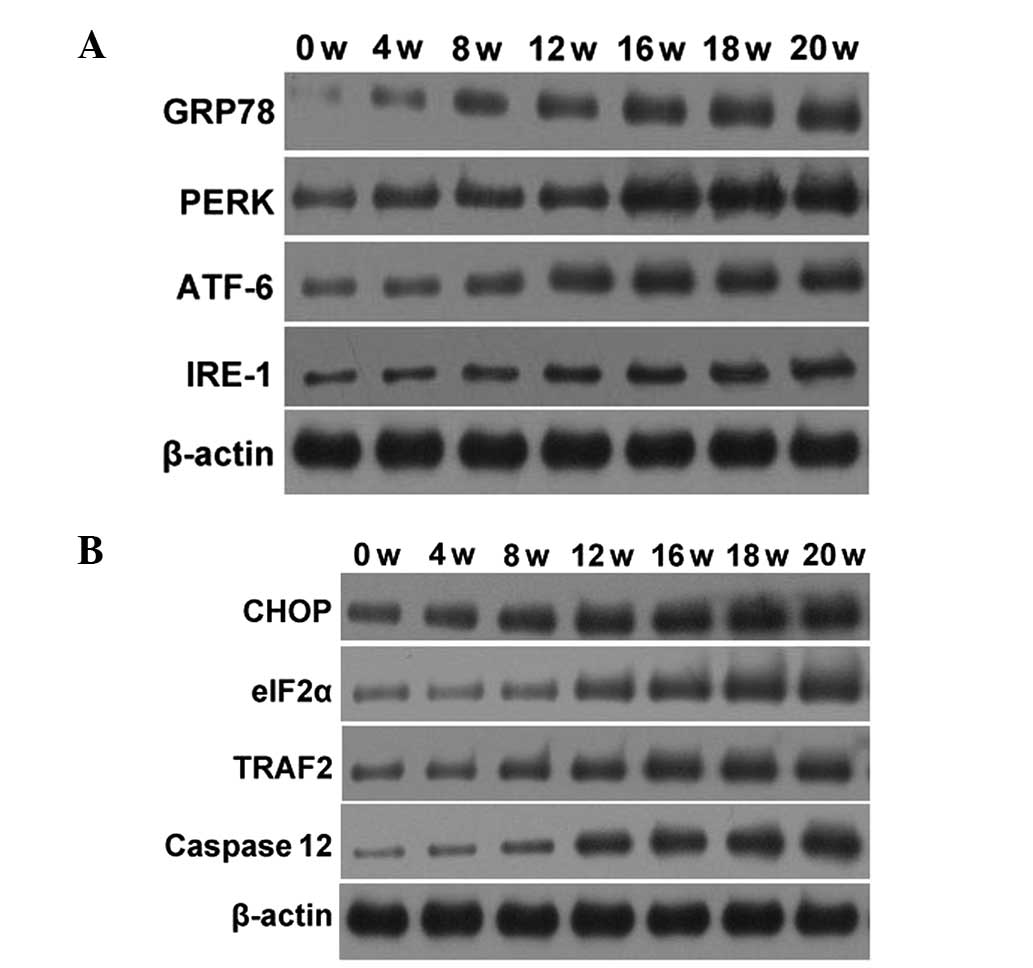
The expression of the GRP78, PERK, ATF6 and IRE-1
proteins was tested in the UPR associated with the ERS reaction
pathway. During early and mid-term carcinogenesis (1–14 weeks), the
expression of these proteins gradually increased. In late
carcinogenesis (15–20 weeks), the protein expression was
essentially constant subsequent to reaching a peak. The western
blotting results are shown in Fig.
4A. The expression of CHOP, eIF2α, TRAF2 or caspase-12
proteins, involved in the three pathways that induce apoptosis by
ERS, were further tested. During early and mid-term carcinogenesis
(1–14 weeks), the expression of these proteins gradually increased,
and in late carcinogenesis (15–20 weeks) the expression of the
proteins was essentially constant subsequent to reaching a peak.
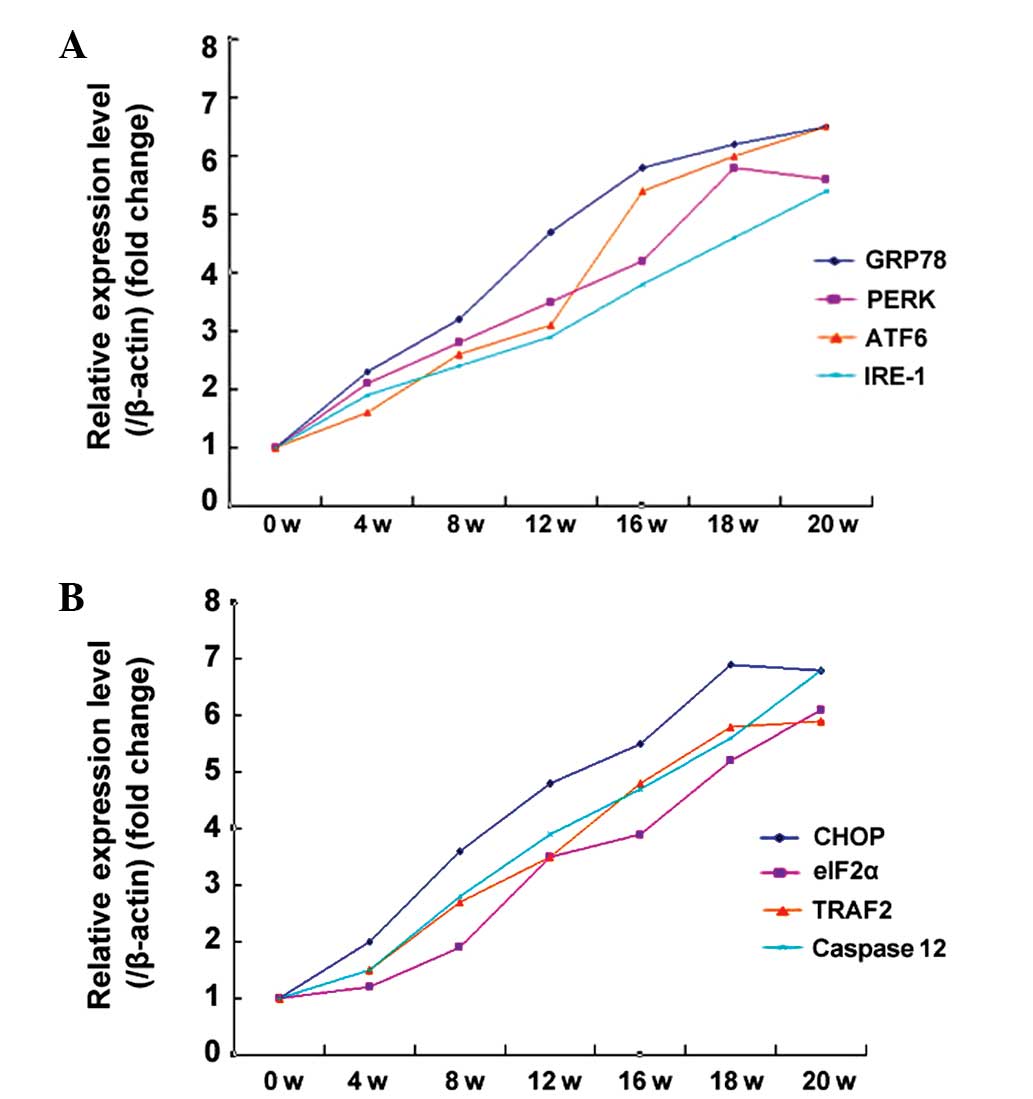
The western blotting results are shown in Fig. 4B. In order to explore any changes in
the transcriptional levels of ERS-associated factors, qPCR was used
to assess their RNA levels. The observed changes in the RNA levels
of GRP78, PERK, ATF6, IRE-1, CHOP, eIF2α and TRAF2 or caspase-12
were consistent with the protein expression results (Fig. 5).
Discussion
Human hepatoma evolves in the following multi-stage
process: i) Damage due to hepatitis B or C viral infection causing
chronic hepatitis or cirrhosis; ii) adenomatous hyperplasia
nodules; iii) early HCC; iv) advanced HCC; and v) HCC metastasis.
This multi-stage occurrence and development model has been
confirmed by pathological and clinical cases. In the present study,
the modified intermittent administration method, developed by Zhang
et al(7), was used to
successfully induce the hepatoma model in Wistar rats. The model is
simple, with a short tumorigenic cycle, and the pathological
process follows the general development of human liver cancer. The
results of the current study are similar to those described
previously (7), indicating that the
model is stable and has good reproducibility.
ER dysfunction is likely to lead to the accumulation
of misfolded and unfolded proteins in the ER lumen, thereby causing
ERS. Short-term ERS protects cells, but when ERS persists for a
prolonged duration or is highly intensive, unfolded proteins
accumulate in the lumen of the ER, leading to the dissociation of
the GRP78/BIP chaperone and the transmembrane proteins, PERK, ATF6
and IRE-1, thus inducing apoptosis (8,9). ERS
induces apoptosis through the activation of the following three
pathways (10,11): i) CHOP, when ERS persists for a
prolonged duration the translation initiation factor, eIF2α, is
phosphorylated, which directly stimulates the translation of
associated proteins, causing the activation of transmembrane
proteins, ATF6 and IRE-1, which induce the transcription and
expression of CHOP, leading to apoptosis; ii) ASK1/JNK, ERS
activates IRE-1, which binds with TRAF2 to activate ASK1 and
further activate JNK, which induces apoptosis; and iii) caspase,
when ERS occurs, caspase-12 is activated through a variety of
mechanisms to initiate the downstream caspase-3-mediated apoptosis
pathway.
In the present study, the ERS components and
downstream regulatory factors were assayed at the protein and RNA
levels. It was found that during early and medium-term
carcinogenesis (1–14 weeks), the expression of GRP78, PERK, ATF6
and IRE-1, involved in the UPR, as well as CHOP, eIF2α and TRAF2 or
caspase-12, associated with apoptosis, gradually increased. These
results indicated that during DEN-induced rat liver injury, liver
cell proliferation and cirrhosis, the liver undergoes the ERS
reaction, and ERS is involved in each of these processes. No
significant changes in the expression of ERS-associated proteins
were identified in the late stage of carcinogenesis (15–20 weeks)
once it reached a peak. This indicated that ERS may not be involved
in the growth of liver cancer cells following the initial formation
of DEN-induced rat liver cancer. This hypothesis remains to be
confirmed. The present study shows that ERS is involved in the
occurrence and development of primary liver cancer, highlighting a
new line of thinking for future studies and the treatment of liver
diseases.
Acknowledgements
The current study was supported by a grant from the
National Natural Science Foundation of China (no. 81260655).
References
|
1
|
Ciccaglione AR, Marcantonio C, Tritarelli
E, Equestre M, Vendittelli F, Costantino A, Geraci A and Rapicetta
M: Activation of the ER stress gene gadd153 by hepatitis C virus
sensitizes cells to oxidant injury. Virus Res. 126:128–138. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asselah T, Bièche I, Mansouri A,
Laurendeau I, Cazals-Hatem D, Feldmann G, Bedossa P, Paradis V,
Martinot-Peignoux M, Lebrec D, et al: In vivo hepatic endoplasmic
reticulum stress in patients with chronic hepatitis C. J Pathol.
221:264–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gregor MF, Yang L, Fabbrini E, Mohammed
BS, Eagon JC, Hotamisligil GS and Klein S: Endoplasmic reticulum
stress is reduced in tissues of obese subjects after weight loss.
Diabetes. 58:693–700. 2009. View Article : Google Scholar
|
|
4
|
Ji C and Kaplowitz N: Betaine decreases
hyperhomocysteinemia, endoplasmic reticulum stress, and liver
injury in alcohol-fed mice. Gastroenterology. 124:1488–1499. 2003.
View Article : Google Scholar
|
|
5
|
Nagy G, Szarka A, Lotz G, Dóczi J,
Wunderlich L, Kiss A, Jemnitz K, Veres Z, Bánhegyi G, Schaff Z, et
al: BGP-15 inhibits caspase-independent programmed cell death in
acetaminophen-induced liver injury. Toxicol Appl Pharmacol.
243:96–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu Y and Lee AS: Glucose regulated
proteins in cancer progression, drug resistance and immunotherapy.
Cancer Biol Ther. 5:741–744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang ZM, Wang G, Chen C, Xu W, Li Q, Hu
Q, Wang D and Li ZP: Pathologic and morphologic study on modified
DEN-induced hepatocarcinoma model in rats. Di San Jun Yi Da Xue.
12:1164–1167. 2007.
|
|
8
|
Kaufman RJ: Stress signaling from the
lumen of the endoplasmic reticulum coordination of gene
transcriptional and transcriptional controls. Genes Dev.
13:1211–1233. 1999. View Article : Google Scholar
|
|
9
|
Mori K: Tripartite management of unfolded
proteins in the endoplasmic reticulum. Cell. 101:451–454. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuzaki S, Hiratsuka T, Kuwahara R,
Katayama T and Tohyama M: Caspase-4 is partially cleaved by calpain
via the impairment of Ca2+ homeostasis under the ER stress.
Neurochem Int. 56:352–356. 2010.PubMed/NCBI
|
|
11
|
Lin JH, Li H, Yasumura D, Cohen HR, Zhang
C, Panning B, Shokat KM, Lavail MM and Walter P: IRE1 signaling
affects cell fate during the unfolded protein response. Science.
318:944–949. 2007. View Article : Google Scholar
|