Introduction
Kidney tumors are the third most common malignancy
of the urinary tract following prostate and bladder cancer
(1). Kidney tumors have no apparent
symptoms and are frequently fatal, and the majority are
incidentally found during routine abdominal imaging performed for
unrelated reasons. In addition, kidney biopsy is an invasive
technique that may result in complications and is unlikely to
provide accurate diagnosis in certain situations (2). Pre-existing screening tests are
available for organ-specific tumors, including prostate-specific
antigen and digital rectal examination for prostate cancer,
carcinoembryonic antigen, colonoscopy and testing for fecal occult
blood for colon cancer, and mammography and breast examinations for
breast cancer, however, there are no diagnostic modalities for the
early detection of renal cancer and no methods for the surveillance
of its recurrence. Thus, there is great interest in identifying
soluble biomarkers that may improve this situation.
Matrix metalloproteinases (MMPs) form a family of
>25 endopeptidases. MMPs may be described as multifunctional
enzymes capable of cleaving the basal membrane, extracellular
matrix components (such as fibrillar and non-fibrillar collagen,
proteoglycans, glycoproteins and denatured collagen), growth
factors, cytokines and cell surface-associated adhesion and
signaling receptors. Therefore, MMPs enhance tumor growth and
tumorigenicity. In particular, MMP-2 and -9, also known as
gelatinase A (72 kDa) and B (92 kDa), respectively, are most often
associated with the malignant phenotype of tumor cells. Growing
amounts of data indicate that circulating MMP-9 and/or MMP-2 levels
may be valuable in assessing prognosis or diagnosing a relapse
during follow-up (3–5). Due to their high degradation activity
and potentially disastrous effect on the cell microenvironment,
cellular MMPs are expressed in small amounts and their cellular
localization and activity are tightly controlled. Normally, MMPs
are inhibited by tissue inhibitors of metalloproteinases (TIMPs),
and the MMP/TIMP balance is considered to be a major factor in the
regulation of the net proteolytic activity of the individual MMPs.
In humans, four individual species of TIMPs are known (TIMP-1, -2,
-3 and -4). Of these, the C-terminal domain of TIMP-1 and -2 bind
the to the hemopexin domain of the proenzymes of MMP-9 and -2,
respectively (6,7). TIMP expression is regulated during
development and tissue remodeling and under pathological conditions
associated with unbalanced MMP activities. Changes in TIMP levels
are considered to be important since they directly affect the
levels of MMP activity. Additionally, TIMP-2 is unique in that, as
well as inhibiting the activity of MMP, it selectively interacts
with membrane type-1 MMP (MT1-MMP) to facilitate the cell surface
activation of the precursor of MMP-2 (pro-MMP-2). Thus, TIMP-2
functions to inhibit MMP activity and promote the cell surface
activation of pro-MMP-2 by MT1-MMP interaction. In addition to
metalloproteinase-inhibiting activities, TIMPs exhibit other
biological functions and have been implicated in the direct
regulation of the growth and apoptosis of cells (8–10).
Changes in MMPs and their inhibitors on the cellular
level may be reflected in body fluids. This is likely to allow
determination of MMPs and TIMPs in blood and/or urine as a simple
non-invasive tool for cancer diagnosis and monitoring.
In the present study, the serum and urinary levels
of MMP-2 and -9 and TIMP-1 and -2 were measured in patients with
oncocytoma or clear cell renal cell carcinoma (ccRCC) in order to
verify whether these molecules may offer a potential non-invasive
biomarker to provide useful clinical information for kidney
carcinoma.
Materials and methods
Patients
Peripheral venous blood and first morning urine
samples were collected from 20 selected patients prior to surgical
or other therapeutic intervention. Tumor specimens were obtained
from patients who had undergone surgical procedure. Standard
clinical laboratory criteria and histopathological observations
were used to diagnose and confirm the tumor type. The patient ages
ranged between 40 and 73 years (mean, 59.2±9.7 years), and in
total, there were 9 females and 11 males. The tumors were
classified by grade and stage according to the pTNM classification
(11). All patients provided
written informed consent and the study was approved by the local
ethics committee. A total of 53 normal healthy volunteers with no
concomitant illness were used as controls. The age of the healthy
volunteers ranged between 30 and 70 years (mean, 42±8 years) and
there were 30 females and 23 males. These volunteers provided
verbal permission. The subjects in the controls exhibited no signs
of infection, gastrointestinal hepatic or renal disease, tumors or
immunological disease. The basic laboratory parameter values of
these participants were within the reference limits.
Serum
Native serum was prepared using plastic tubes
without coagulation accelerators, to prevent the release of
gelatinase during platelet activation. The tubes were centrifuged
at 1,600 × g for 10 min, 30 min after blood collection. The sera
were aliquoted and stored at −20°C prior to use.
Urine sample preparation
The Multistix Combur test (Roche Diagnostic GmbH,
Mannheim, Germany) was used to examine the urine samples prior to
analysis. The urine samples that tested positive for leukocytes
were excluded due to confounding leukocytic gelatinases.
Microscopic hematuria, which was present in the majority of cancer
samples, was not quantified, however, macroscopic hematuric samples
were excluded. Immediately after collection, the samples were
frozen and stored at −20°C prior to being assayed. For this, the
samples were thawed and a 15-ml aliquot of each sample was
centrifuged at 1,000 × g for 10 min at 4°C. Supernatant was
collected and used to determine the MMP-2 and -9 and TIMP-1 and -2
levels by immunoassay.
Measurement of MMP-2 and -9 and TIMP-1
and -2 levels
MMP-2 and -9 and TIMP-1 and -2 levels were detected
by enzyme-linked immunosorbent assay (ELISA) using commercial kits
obtained from GE Healthcare (Amersham, UK). These assays are based
on a two-site sandwich format using two antibodies directed against
various epitopes of the molecule. The assay for MMP-2 recognizes
the pro-MMP-2, i.e., free pro-MMP-2 and that complexed with TIMP-2,
but not the active form of MMP-2. The assay for MMP-9 recognizes
the precursor of MMP-9 (pro-MMP-9), i.e., free pro-MMP-9 and that
complexed with TIMP-1. The assay for TIMP-1 recognizes free TIMP-1
and that complexed with MMPs. The assay for TIMP-2 recognizes free
TIMP-2 and that complexed with the active form of MMPs. All
analyses were performed according to the manufacturer’s
instructions.
Statistical analysis
All statistical analyses were performed using the
statistical computing environment R software (version 2.12.1; R
Foundation for Statistical Computing, Vienna, Austria). Data are
presented as the mean ± standard deviation (SD). Fisher’s exact
test was performed and P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients
A total of 20 patients with kidney disease were
evaluated over a 1-year period. Of these patients, 16 exhibited
ccRCC and 4 exhibited oncocytoma. A venous blood sample was
collected from each of the patients, and for all of the patients
with oncocytoma and for nine of the patients with ccRCC, first
morning urine samples were obtained. All four molecules, including
MMP-2 and -9 and their inhibitors TIMP-2 and -1, were measured in
the serum and urine samples. The levels of these molecules were
also measured in the sera and urine of 53 healthy subjects, who
were considered to be the control group, as normal values for these
molecules were unavailable.
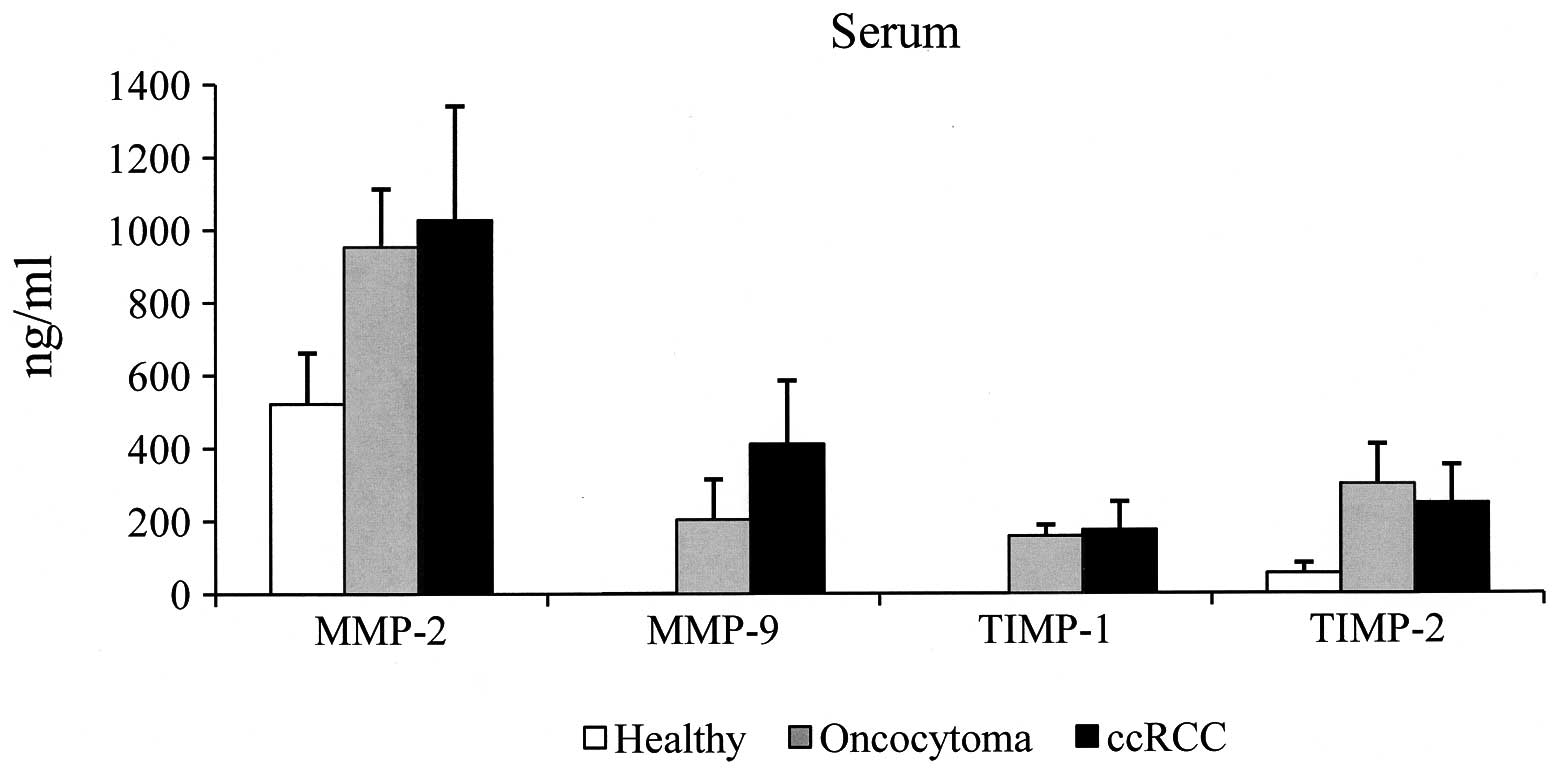
Serum samples
In the sera of the control group, MMP-2 was detected
in all the samples, with a value ranging between 475 and 798 ng/ml
(mean, 522±140 ng/ml). MMP-9 and TIMP-1 were undetectable, being at
or below the sensitivity of the assay. TIMP-2 was detected in all
the specimens and ranged between 33 and 118 ng/ml (mean, 55±28
ng/ml). The cut-off value was established by calculating the mean ±
2SD. The cut-off values of 802 ng/ml for MMP-2 and 111 ng/ml for
TIMP-2 were used. Samples with a value higher than that of the
cut-off were considered positive. The results obtained from the
sera of the patients with oncocytoma and ccRCC are shown in
Tables I and II, respectively. In the patients with
oncocytoma, the MMP-2 values were positive in 3/4 (75%) of the
specimens analyzed (range, 750–1,120 ng/ml; mean ± SD, 953±160
ng/ml), while in the patients with ccRCC, the values were positive
in 12/16 (75%) of specimens (range, 697–1,949; mean ± SD, 1,027±314
ng/ml). MMP-9 was detected in all the specimens analyzed, with mean
values of 203±111 ng/ml (range, 82–327 ng/ml) and 411±174 ng/ml
(range, 168–730 ng/ml) observed in the oncocytoma and ccRCC
patients, respectively. TIMP-1 was detected in all the specimens
analyzed; with a mean value of 157±31 ng/ml (range, 120–186 ng/ml)
in the oncocytoma patients and 174±78 (range, 63–429 ng/ml) in the
ccRCC patients. Since serum MMP-9 and TIMP-1 were undetectable in
all the healthy subjects, all pathological specimens were
considered positive, as all samples possessed serum MMP-9 and
TIMP-1 values higher than the sensitivity of the assay (assay
sensitivity was calculated as 2SDs above the zero dose binding of
80 determinations, and was 0.8 ng/ml for MMP-9 and 1.51 ng/ml for
TIMP-1). TIMP-2 was positive in all (100%) the individuals with
oncocytoma (range, 184–445 ng/ml; mean ± SD, 300±110 ng/ml) and in
15/16 (94%) of the ccRCC patients (range, 52–452 ng/ml; mean ± SD,
248±105 ng/ml). Considering the average value of each molecule, the
MMP-2 values were observed to be similar in the oncocytoma and
ccRCC patients, however, the mean value was ~2-fold higher in the
sera from the kidney disease patients compared with that of the
control group. In addition, the serum MMP-9 level was 2-fold higher
in the patients with ccRCC compared with those with oncocytoma
(Fig. 1). We observed that TIMP-1
and -2 values were similar in oncocytoma and ccRCC individuals. In
addition, serum TIMP-2 levels were ~5-fold higher in kidney disease
compared with the healthy specimens (Fig. 1).
 | Table ISerum MMP and TIMP content in
oncocytoma patients. |
Table I
Serum MMP and TIMP content in
oncocytoma patients.
| Case | Age, years | Gender | Stage | Grade | MMP-2, ng/ml | MMP-9, ng/ml | TIMP-1, ng/ml | TIMP-2, ng/ml |
|---|
| 1 | 42 | F | T1N0M0 | G1 | 1120 | 259 | 120 | 184 |
| 2 | 66 | M | T1N0M0 | G1 | 1030 | 142 | 177 | 310 |
| 3 | 59 | F | T2N0M0 | G1 | 750 | 82 | 186 | 261 |
| 4 | 59 | F | T1N0M0 | G1 | 910 | 327 | 143 | 445 |
 | Table IISerum MMP and TIMP content in ccRCC
patients. |
Table II
Serum MMP and TIMP content in ccRCC
patients.
| Case | Age, years | Gender | Stage | Grade | MMP-2, ng/ml | MMP-9, ng/ml | TIMP-1, ng/ml | TIMP-2, ng/ml |
|---|
| 5 | 69 | M | T1N0M0 | G1 | 1423 | 168 | 206 | 292 |
| 6 | 54 | M | T1N0M0 | G1 | 710 | 355 | 103 | 275 |
| 7 | 53 | M | T1N0M0 | G2 | 1175 | 173 | 226 | 123 |
| 8 | 60 | F | T1N0M0 | G2 | 697 | 609 | 125 | 52 |
| 9 | 51 | F | T1N0M0 | G2 | 1949 | 356 | 119 | 184 |
| 10 | 63 | F | T1N0M0 | G2 | 880 | 428 | 151 | 293 |
| 11 | 63 | M | T2N0M0 | G2 | 945 | 312 | 262 | 268 |
| 12 | 60 | F | T2N0M0 | G2 | 795 | 575 | 63 | 269 |
| 13 | 40 | M | T2N0M0 | G3 | 965 | 291 | 429 | 349 |
| 14 | 73 | M | T2N0M0 | G3 | 805 | 202 | 210 | 318 |
| 15 | 70 | M | T2N0M0 | G3 | 1060 | 497 | 150 | 326 |
| 16 | 61 | M | T2N0M0 | G3 | 1140 | 681 | 119 | 165 |
| 17 | 73 | F | T2N0M0 | G3 | 1030 | 730 | 82 | 124 |
| 18 | 43 | M | T2N0M0 | G3 | 1120 | 499 | 161 | 145 |
| 19 | 67 | M | T3N0M1 | G3 | 995 | 309 | 207 | 335 |
| 20 | 57 | F | T3bN0M1 | G3 | 745 | 393 | 172 | 452 |
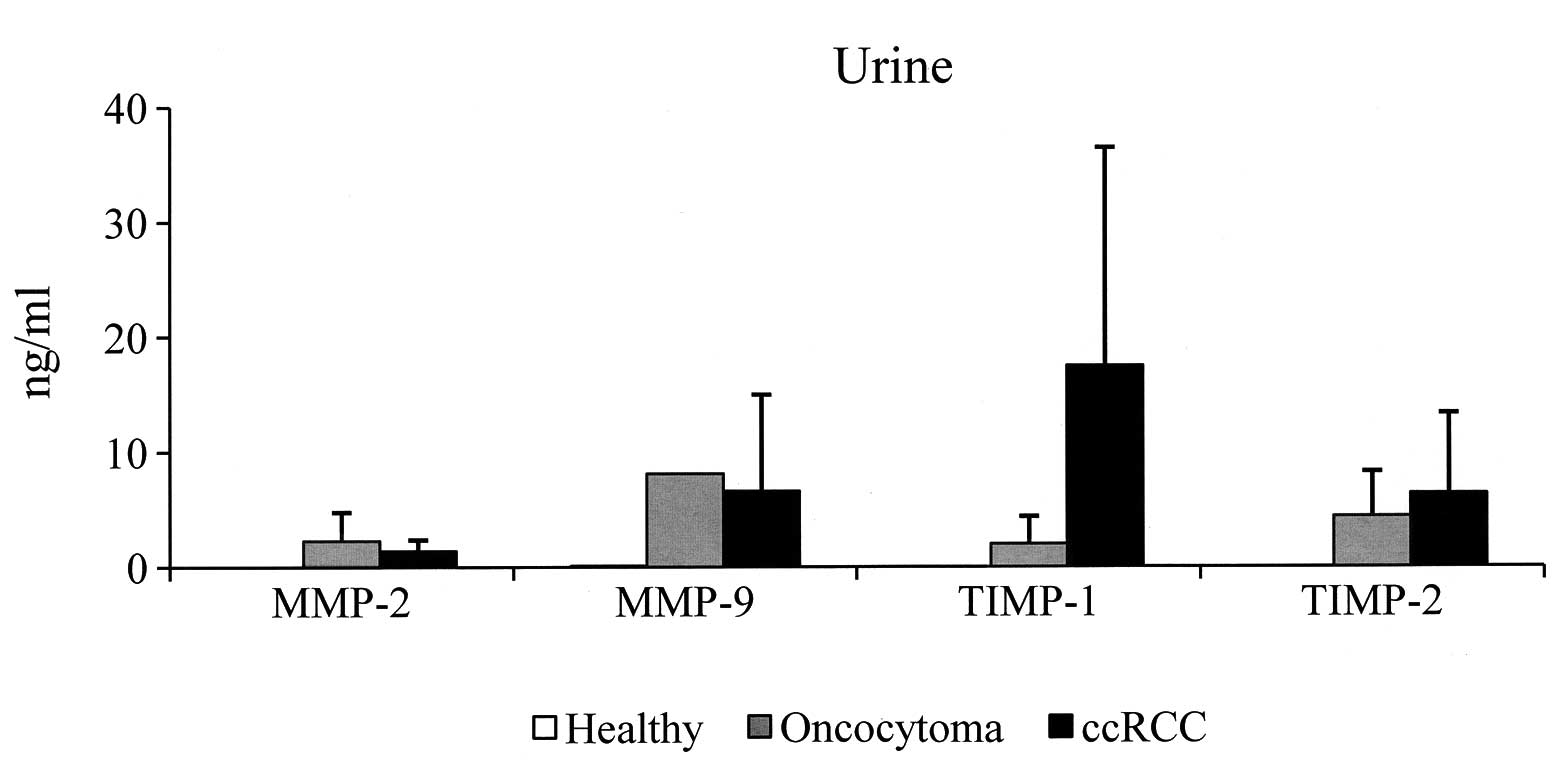
Urine samples
With regard to the urine specimens, since the four
molecules were undetectable in all the urine samples of the control
group, no cut-off values were established. In Tables III and IV, the results obtained in the urine from
patients with oncocytoma and ccRCC, respectively, are shown. In
particular, urinary MMP-2 was detected in two (50%) oncocytoma
specimens, with values of 0.58 and 4.11 ng/ml, respectively, and in
8/9 (89%) ccRCC samples (range, 0.72–3.41; mean ± SD, 1.4±1.0),
whereas urinary MMP-9 was detected in only one (25%) oncocytoma
specimen, with a value of 8.13 ng/ml, and in 6/9 (67%) of ccRCC
patients (range, 0.55–22.8 ng/ml). TIMP-1 was detected in two (50%)
oncocytoma individuals and in 8/9 (89%) of the ccRCC patients
(range, 0.17–55 ng/ml). Finally, TIMP-2 was detected in 3/4 (75%)
of the oncocytoma patients and in 7/9 (78%) of the ccRCC patients.
The mean values of MMP-2 and -9 and TIMP-1 and -2 of the positive
urine specimens are shown in Fig.
2. It is evident that the urinary levels were less than those
of the sera. In addition, the mean level of urinary TIMP-1 was
higher in the ccRCC patients compared with the oncocytoma
patients.
 | Table IIIUrine MMP and TIMP content in
oncocytoma patients. |
Table III
Urine MMP and TIMP content in
oncocytoma patients.
| Case | Age, years | Gender | Stage | Grade | MMP-2, ng/ml | MMP-9, ng/ml | TIMP-1, ng/ml | TIMP-2, ng/ml |
|---|
| 1 | 42 | F | T1N0M0 | G1 | 0.58 | 8.13 | 0.30 | N.D. |
| 2 | 66 | M | T1N0M0 | G1 | 4.11 | N.D. | N.D. | 4.14 |
| 3 | 59 | F | T2N0M0 | G1 | N.D. | N.D. | 3.77 | 8.51 |
| 4 | 59 | F | T1N0M0 | G1 | N.D. | N.D. | N.D. | 0.65 |
 | Table IVUrine MMP and TIMP content in ccRCC
patients. |
Table IV
Urine MMP and TIMP content in ccRCC
patients.
| Case | Age, years | Gender | Stage | Grade | MMP-2, ng/ml | MMP-9, ng/ml | TIMP-1, ng/ml | TIMP-2, ng/ml |
|---|
| 5 | 69 | M | T1N0M0 | G1 | 2.08 | 6.93 | 27.80 | 3.92 |
| 6 | 54 | M | T1N0M0 | G1 | 0.77 | N.D. | N.D. | 4.57 |
| 7 | 53 | M | T1N0M0 | G2 | 3.41 | 0.55 | 55.00 | 21.10 |
| 9 | 51 | F | T1N0M0 | G2 | 1.01 | N.D. | 1.70 | 1.84 |
| 13 | 40 | M | T2N0M0 | G3 | 0.72 | 1.68 | 0.51 | 6.70 |
| 14 | 73 | M | T2N0M0 | G3 | N.D. | N.D. | 7.10 | N.D. |
| 15 | 70 | M | T2N0M0 | G3 | 1.72 | 22.80 | 24.70 | N.D. |
| 19 | 67 | M | T3N0M1 | G3 | 0.87 | 6.13 | 23.10 | 2.03 |
| 20 | 57 | F | T3bN0M1 | G3 | 0.78 | 1.35 | 0.17 | 4.60 |
Discussion
To date, no reliable, non-invasive tumor markers for
renal cell carcinoma have been described. Among the current tumor
biomarkers, MMP members and their inhibitors (TIMPs) have the
potential to represent candidates to improve diagnosis and
follow-up surveillance. In humans, MMP expression has previously
been reported to be increased in the majority of malignancies
(3,4). TIMPs are multifunctional and act
indirectly through modulation of protease activity or directly
through cell surface receptors to direct cell fate (10). Tissue destruction in malignancies
correlates with an imbalance of MMPs over TIMPs. An aberrant
expression of TIMPs has been postulated to present an important
modulatory and prognostic factor in the invasive capacity of
specific tumors. Therefore, an imbalance between the expression,
activation and presentation of MMP-2 and -9 and their associated
TIMPs may have a role in the invasive phenotype. Previously, MMP-2
and -9 and TIMP-1 and -2 have been investigated, using diverse
techniques, in body fluids and tissues with variable results
(12–16). Tissue MMP-2 and -9 and TIMP-1 and -2
were found to be overexpressed in tumors and more frequently in
non-ccRCC (13,14). In particular, using
immunohistochemistry, Kallakury et al (14) reported that the increased expression
levels of MMP-2 and -9 and TIMP-1 and -2 individually correlate
with histological tumor types, with a vast majority of papillary
and sarcomatoid RCCs expressing these proteins as compared with
clear cell tumors. Furthermore, the increased expression of the
four molecules was found to correlate with poor prognostic
variables, including shortened patient survival (14). Using radioactive-labeled riboprobe
in situ hybridization and immunohistochemistry,
Bhuvarahamurthy et al analyzed the formalin-fixed,
paraffin-embedded tumor samples of 10 patients and found the
pronounced expression of MMP-2 and -9 in RCC at the mRNA and
protein levels. In addition, the expression of TIMP-1 and -2
appeared to be relevant in RCC (15). However, although these studies on
tissue markers are highly promising, there are certain limitations.
Immunochemistry is semi-quantitative and highly dependent on a
range variables, including choice of antibody, antibody
concentration, fixation techniques, variability in the
interpretation and stratification criteria and inconsistency in
specimen handling and technical procedures. Using an RT-PCR
technique, Kugler et al analyzed MMP-2 and -9 and TIMP-1 and
-2 in 17 RCC patients and demonstrated a marked correlation between
increased gene expression and tumor stage and aggressiveness
(16). Kamiya et al found
that the lytic activity is higher at the peripheries of tumors in
inflammatory sites, as observed by in situ zymography
(17). In addition, in 36 RCC
patients, Lein et al evaluated the content of the four
molecules using various techniques (RT-PCR, zymography,
immunohistochemistry and ELISA). The study found that in the tumor
tissues, MMP-9 and TIMP-1 were significantly higher than in the
normal counterparts. The level of MMP-2 did not differ between the
tumor and normal counterparts, and the measurement of the TIMP-2
values was not possible (18). With
regard to peripheral blood, Lein et al found that the plasma
MMP-9 levels were significantly higher in RCC patients than in
healthy controls, whereas MMP-2 and TIMP-2 concentrations were
higher in the healthy controls and the TIMP-1 concentrations were
not different. In particular, the study reported that plasma MMP-9
showed a sensitivity of only 36% in detecting RCC, and no
correlation was found with tumor type, grade or stage (18). Using a zymography technique, our
previous study showed that MMP-9 is enhanced in the sera from ccRCC
patients compared with that from oncocytoma patients, and that the
most abundant lytic activity was at 92 kDa (MMP-9), whereas MMP-2
was present in reduced quantities (12). The results of the ELISA in the
present study showed that MMP-2 and -9 and TIMP-1 and -2 were
present in the sera from all kidney disease patients analyzed. The
mean values of MMP-2 and TIMP-1 were similar in the ccRCC and
oncocytoma patients, whereas the mean values of MMP-9 were higher
in the ccRCC patients compared with those of oncocytoma patients.
Therefore, according to Lein et al, the results of the
current study support the hypothesis for the significance of MMP-9
in renal cancer, whereas MMP-2 does not appear to be important.
However, a broad overlap of the results was identified, and no
correlation was observed among the type of carcinoma, pathological
TNM stage or histological grading.
The ability to follow localized tumors or monitor
drug-based therapy results by a simple analysis of tumor-specific
markers in the easily available excretory product of the kidney is
desirable. However, to the best of our knowledge, insufficient
literature exists concerning urine markers for RCC. In the urine
samples of the present study, MMPs and TIMPs were detectable only
in certain pathological specimens, whereas they were undetectable
in the control group. No correlation was identified between the
urinary levels of the four molecules analyzed and the clinical
pathological observations. The results are consistent with the
results of the study by Cannon et al (19), but contradict the results of the
study by Sherief et al (20).
To date, despite tissue evidence, the analysis of
serum and urine MMP and TIMP levels appears to be an inadequate
test to identify kidney cancer. This conclusion may not be
transferable to the general population, however, due to the small
number of patients included in the studies. Therefore, further
evaluation is required. Future investigation involving a larger
cohort of patients may clarify whether MMP-2 and -9 and TIMP-1 and
-2 are useful biomarkers for ccRCC.
References
|
1
|
Jemal A, Siegel R, Xu and Wrad E: Cancer
statistics, 2010. Ca Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Cohen HT and McGoven FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar
|
|
4
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cell. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
6
|
Brew K, Dinakarpandian D and Nagase H:
Tissue inhibitors of metalloproteinases: evolution, structure and
function. Biochem Biophys Acta. 1477:267–283. 2000.PubMed/NCBI
|
|
7
|
Baker AH, Ahonen M and Kähäri VM:
Potential applications of tissue inhibitor of metalloproteinase
(TIMP) overexpression for cancer gene therapy. Adv Exp Med Biol.
465:469–483. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayakawa T, Yamashita K, Tanzawa K,
Uchijima E and Iwata K: Growth-promoting activity of tissue
inhibitor of metalloproteinase-1 (TIMP-1) for a wide range of
cells. A possible new growth factor in serum. FEBS Lett. 298:29–32.
1992. View Article : Google Scholar
|
|
9
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases.
Structure, functions and biochemistry. Cir Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stetler-Stevenson WG: Tissue inhibitors of
metalloproteinases in cell signaling: metalloproteinase independent
biological activities. Sci Signal. 1:re62008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin LH and Wittekind CH: International
Union Against Cancer (UICC) TNM classification of malignant tumors.
6th edition. Wiley-Liss; New York, NY: pp. 193–195. 2002
|
|
12
|
Di Carlo A: Matrix metalloproteinase -2
and -9 in the sera and in the urine of human oncocytoma and renal
cell carcinoma. Oncol Rep. 28:1051–1056. 2012.PubMed/NCBI
|
|
13
|
Struckmann K, Mertz K, Steu S, Storz M,
Staller P, Krek W, Schraml P and Moch H: pVHL co-ordinately
regulates CXCR4/CXCL12 and MMP2/MMP9 expression in human clear-cell
renal cell carcinoma. J Pathol. 214:464–471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kallakury BV, Karikehalli S, Haholu A,
Sheehan CE, Azumi N and Ross JS: Increased expression of matrix
metalloproteinases 2 and 9 and tissue inhibitors of
metalloproteinases 1 and 2 correlate with poor prognostic variables
in renal cell carcinoma. Clin Cancer Res. 7:3113–3119. 2001.
|
|
15
|
Bhuvarahamurthy V, Kristiansen GO,
Johannsen M, Loening SA, Schnorr D, Jung K and Staack A: In situ
gene expression and localization of metalloproteinases MMP1, MMP2,
MMP3, MMP9 and their inhibitors TIMP1 and TIMP2 in human renal cell
carcinoma. Oncol Rep. 15:1379–1384. 2006.PubMed/NCBI
|
|
16
|
Kugler A, Hemmerlein B, Thelen P,
Kallerhoff M, Radzun HJ and Ringert RH: Expression of
metalloproteinase 2 and 9 and their inhibitors in renal cell
carcinoma. J Urol. 160:1914–1918. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamiya N, Kishimoto T, Suzuki H, Sekita N,
Nagai Y, Oosumi N, Kito H, Tochigi N, Shinbo M, Nemori R, Ichikawa
T, Igarashi T, Ito H and Ishikura H: Increased in situ
gelatinolytic activity in renal cell tumor tissues correlates with
tumor size, grade and vessel invasion. Int J Cancer. 106:480–485.
2003. View Article : Google Scholar
|
|
18
|
Lein M, Jung K, Laube C, Hübner T,
Winkelmann B, Stephan C, Hauptmann S, Rudolph B, Schnorr D and
Loening SA: Matrix-metalloproteinases and their inhibitors in
plasma and tumor tissue of patients with renal cell carcinoma. Int
J Cancer. 85:801–804. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cannon GM Jr and Getzenberg RH: Urinary
matrix metalloproteinases activity is not significantly altered in
patients with renal cell carcinoma. Urology. 67:848–850. 2006.
View Article : Google Scholar
|
|
20
|
Sherief MH, Low SS, Miura M, Kudo N,
Novick A and Weimbs T: Matrix metalloproteinase activity in urine
of patients with renal cell carcinoma leads to degradation of
extracellular matrix proteins: possible use as a screening assay. J
Urol. 169:1530–1534. 2003. View Article : Google Scholar
|