Introduction
Stereotactic body radiation therapy (SBRT) has been
most frequently applied in the treatment of lung tumors (1–6),
including the use of SBRT based on the extracranial γ-knife
(1–2), which has gradually become the standard
treatment of early-stage non-small cell lung carcinoma (NSCLC).
However, Timmerman et al (2006) (7) reported that centrally-located tumor
cases have a higher proportion of serious adverse reactions
following SBRT. The central area is defined as the bronchial tree,
consisting of the carina, the left and right bronchus, the left and
right upper lobe bronchus, the right middle lobe bronchus, the
lingular lobe bronchus, the left and right lower lobe bronchus and
the area extending 2 cm from the bronchial tree. The administered
dose in this previous study was 60–66 Gy/3 fractions/1–2 weeks. In
total, 28 out of 70 patients succumbed; among them, five patients
succumbed to a tumor and six patients succumbed to causes related
to the treatment. Of the total patients, 14 exhibited grade 3–5
toxicity. Severe toxicity was observed in 46% of patients with a
centrally-located tumor. The study indicated that this radiotherapy
scheme should not be used for centrally-located tumors. Therefore,
the National Comprehensive Cancer Network (NCCN) guidelines for the
treatment of NSCLC, in the years 2010 and 2011, defined the central
area as a forbidden region for SBRT treatment. Along with the
increase in the clinical observations of SBRT, certain studies have
challenged this presumption. Chang et al (8) reported that the application of other
doses of SBRT was safe and effective for centrally-located tumors.
The NCCN guideline of 2012 rescinded the decision to make the
central location a forbidden area, and recommended that SBRT may be
tried on centrally-located tumors with other dose schedules
(8,9), but not the unsafe dose schedule of
54–60 Gy/3 fractions reported by Timmerman et al (7). More studies (10–14)
have explored the effects and the side-effects of SBRT for
centrally-located tumors. The adverse reactions are variable due to
the difference in ethnicity, equipment and SBRT dosage. The present
study reports the clinical observations when using the body γ-knife
and SBRT in the centrally-located tumors of Chinese patients.
Materials and methods
Subjects
Between May 2009 and May 2012, a total of 28
patients with 46 tumor lesions were treated by body γ-knife. A
total of 22 lesions were located in the hilar region, while 19
mediastinal lesions were 1 cm away from the esophagus and 5
mediastinal lesions were within 1 cm of the esophagus. Of the total
lesions, 23 were ≤2 cm, 18 were between 2–5 cm and 5 were ≥5 cm in
size. The characteristics of the patients are summarized in
Table I, and the pathology, staging
and medication are summarized in Table
II. In total, 15 patients received first-line chemotherapy,
eight patients received second and subsequent lines of chemotherapy
and five patients did not receive systemic chemotherapy; a
71-year-old patient refused chemotherapy, three patients were
extremely old so were presumed unable to tolerate chemotherapy (≥77
years old) and one patient exhibited idiopathic thrombocytopenia.
Written informed consent was obtained from the patients and the
study was approved by the Ethics Commitee of the Department of
Radiation Oncology, Affiliated Hospital of Academy of Military
Medical Sciences (Beijing, China).
 | Table ICharacteristics of 28 patients. |
Table I
Characteristics of 28 patients.
| Characteristic | Value |
|---|
| Gender, n |
| Male | 21 |
| Female | 7 |
| Age, n |
| <65 years | 18 |
| ≥65 years | 10 |
| Median age (range),
years | 58 (28–84) |
| ECOG score, n |
| 0–1 | 24 |
| 2 | 4 |
| Pathological type,
n |
| Lung cancer | 22 |
| Squamous | 6 |
| Adeno | 9 |
| SCLC | 7 |
| Other tumors | 6 |
 | Table IIPathology, stage and systemic
treatment of 28 patients. |
Table II
Pathology, stage and systemic
treatment of 28 patients.
| Pathology | Stage | Cases, n | No-medication cases,
n | First-line, n | Second-line, n | Third-line, n |
|---|
| NSCLC | IIIa | 2 | | 2 | | |
| IIIb | 5 | 1 (84-year-old) | 4 | | |
| IVa | 6 | 1 (71-year-old
refused) | 4 | 1 | |
| IVb | 2 | 1 (Idiopathic
thrombocytopenia) | | 1 | |
| SCLC | Limited | 3 | | 2 | 1 | |
| Extensive | 4 | | 3 | | 1 |
| Breast cancer | IV | 2 | | | | 2 |
| Colon cancer | IV | 1 | | | | 1 |
| Thymic carcinoma | IV | 1 | 1 (81-year-old) | | | |
| Laryngocarcinoma | IV | 1 | 1 (77-year-old) | | | |
| Thyroid
carcinoma | IV | 1 | | | 1 | |
Equipment
The Moon God γ-knife manufactured by Shenzhen ET
Medical Group (Futian, Shenzhen, China) was used in the study, with
42 cobalt-60 sources and a dose rate of >2 Gy/min.
Radiation
Computed tomography (CT) scans were performed for
three fractions, including the end of the expiratory phase, the end
of the inspiratory phase and the free-breathing phase. The images
were transferred to the γ-knife system. The tumor target area and
the associated normal tissues were delineated, and the displacement
of the tumor was measured in three directions (X, Y and Z)
(15). CT venography (CTV) was
consistent with the gross tumor volume (GTV). GTV plus the
displacement range was the internal target volume (ITV), and the
expansion by 2–3 mm in each direction was the planning target
volume (PTV). The prescribed dose and fraction are summarized in
Table III. The dose line (in the
range of 50–60%) covering 95% of the target area was the prescribed
dose. The dose schedule was 3–5 Gy for each fraction/10–12
fractions; total 36–50 Gy (1,2,16).
 | Table IIIDose and fraction received by 28
patients. |
Table III
Dose and fraction received by 28
patients.
| Total dose, Gy | Case, n | Dose,
Gy/fraction | Fractions, n | BED10,
Gy |
|---|
| 50 | 1 | 5 | 10 | 75 |
| 51.6 | 3 | 4.3 | 12 | 73.788 |
| 52.5 | 1 | 3.5 | 15 | 70.875 |
| 48 | 6 | 4 | 12 | 67.2 |
| 45 | 1 | 4.5 | 10 | 65.25 |
| 46.2 | 3 | 3.3 | 14 | 61.446 |
| 43.2 | 4 | 3.6 | 12 | 58.752 |
| 45 | 8 | 3 | 15 | 58.5 |
| 36.3 | 1 | 3.3 | 11 | 48.279 |
Follow-up
From the time of the γ-knife therapy, the follow-up
continued until the patient succumbed or until October 1, 2012.
Evaluation of efficacy and toxicity
The efficacy was evaluated according to the Response
Evaluation Criteria in Solid Tumors (World Health Organization,
2000) (17). The toxicity was
evaluated according to the National Cancer Institute Common
Toxicity Criteria, v3.0 (18).
Early toxicity was defined as toxicity ≤90 days after the start of
therapy. Late toxicity was defined as >90 days after the start
of therapy.
Statistics
Statistical analysis was carried out on multiple
factors that affected survival time, using SPSS software (SPSS,
Inc., Chicago, IL, USA). The Kaplan-Meier method was used to
evaluate the survival rates. The survival durations were evaluated
from the day of treatment. The log-rank test was used to compare
the different levels of a factor. Cox Regression model was used for
multivariate analysis of survival. P< 0.05 is considered to
indicate a statistically significant difference.
Results
Survival and local control rate
By October 1, 2012, 15 patients remained alive. The
median follow-up period was 14 months (range, 10–41 months). The
median survival time was calculated as 13 months (range, 4–41
months) from the start of SBRT. There were nine complete response
(CR) cases, 14 partial response (PR) cases and five stable disease
(SD) cases. CR plus PR accounted for 82.1% (23/28). No recurrent
cases occurred in the irradiated region during the follow-up
period. Of the 13 patients that succumbed, 11 succumbed to tumor
progression, one to heart failure and one to a brain
hemorrhage.
Among the 15 cases of NSCLC, 13 patients were male
and two patients were female. Nine patients exhibited
adenocarcinoma, six patients exhibited squamous cell carcinoma and
the median dose of the biologically effective dose
[BED10, where α/β=10; BED=nd(1+d/α/β)] was 58.8 Gy
(range, 48.3–73.8 Gy). In total, seven patients were classified as
phase IIIb and eight patients were classified as phase IV. The
median age was 64 years old (range, 48–84 years old). The Eastern
Cooperative Oncology Group (ECOG) scores were 0 for eight patients,
1 for four patients and 2 for three patients. The tumor diameter
was ≤2 cm in three patients, 2–5 cm in five patients and >5 cm
in seven patients. The median survival time was 19 months (range,
11–37 months). A total of 9 patients succumbed. The median
follow-up period of the 6 surviving patients was 19 months (range,
17–33 months). The tumor size (p=0.028) and ECOG score (p=0.025)
significantly affected survival time in the multivariate analysis
using the Cox proportional hazards model. Other factors had no
significant effect: Stage III vs. stage IV, p=0.812; pathological
type (squamous cell carcinoma vs. adenocarcinoma), p=0.717; age,
p=0.866; and dose (BED10), p=0.928. In the two groups of
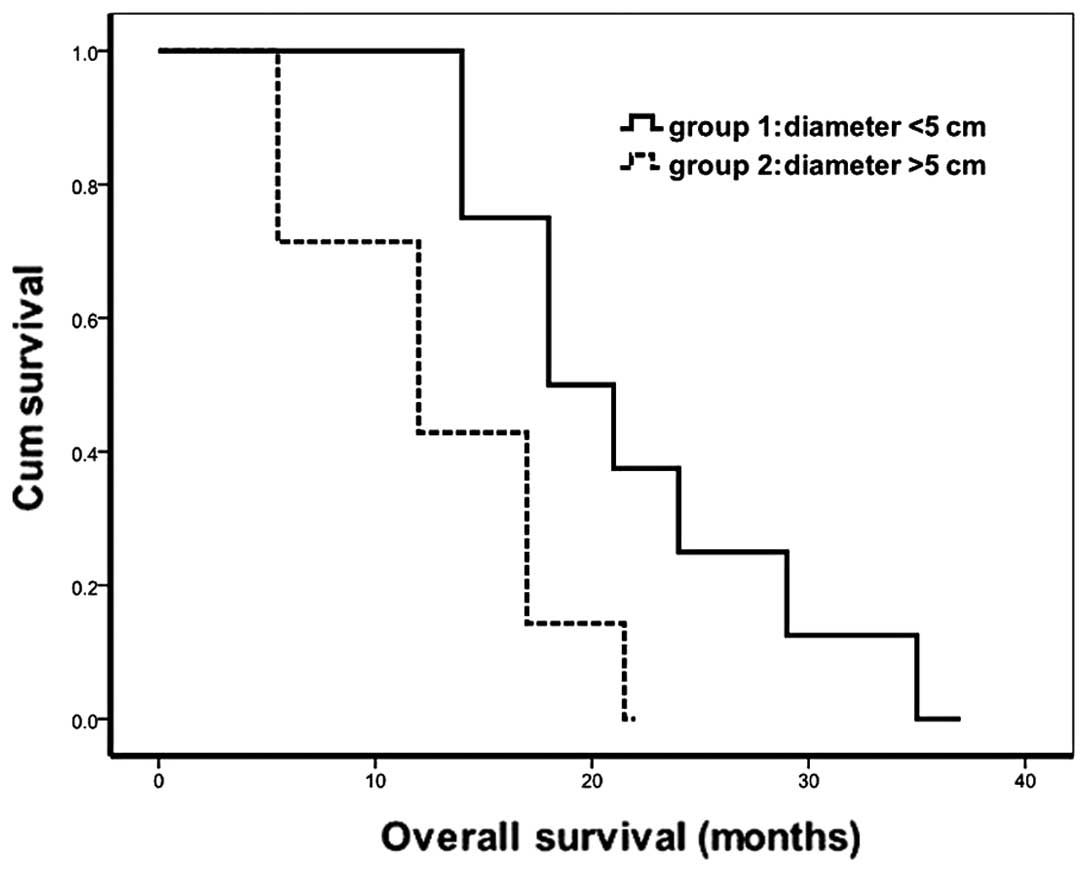
patients with tumor diameters of <5 cm or >5 cm, the median
survival time was 21 months (range, 17–37 months) and 13 months
(range, 11–22 months), respectively; log-rank test, p=0.042
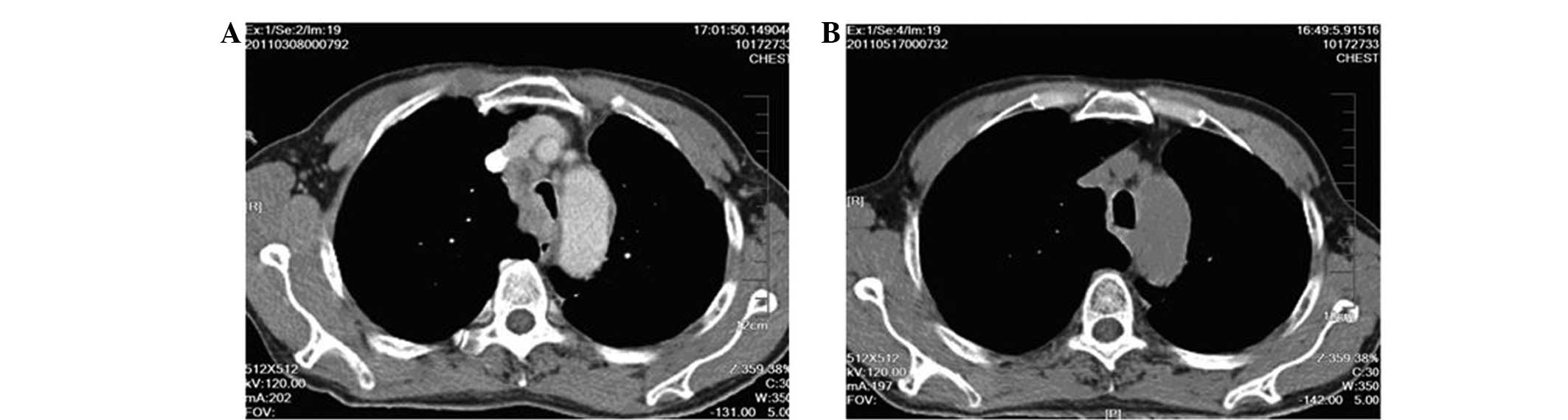
(Fig. 1). The tumor of a typical
patient is demonstrated in Fig
2.
Of the seven small cell lung carcinoma (SCLC)
patients, one patient succumbed. The patient was a 70-year-old male
in the extensive stage, with 7 lines of chemotherapy and
conventional radiotherapy of the primary lesion prior to SBRT. The
patient succumbed to disease progression 4 months after SBRT. The
medium follow-up period of the six survivors was 14 months (range,
13–41 months). When these patients received SBRT, three patients
were in the extensive stage and three patients were in the limited
stage.
Toxicity
In total, five patients had acute esophagitis; grade
1 in two patients and grade 2 in three patients. All patients
completely recovered from this condition following
anti-inflammatory and infusion therapy 2 months after radiotherapy.
Of the five patients, two survived and three succumbed (two
patients succumbed to disease progression and one patient succumbed
to heart failure). The tumors were all located ≤1 cm away from the
esophagus. The dose was 3 Gy in 15 fractions for three patients,
3.3 Gy in 14 fractions for one patient and 3.6 Gy in 12 fractions
for one patient.
Three patients were reported to have atelectasis.
The occurrence time was 3.5, 7 and 31 months after irradiation,
respectively, and the dose for all three was 3 Gy in 15 fractions.
The lobes of two patients were expanded through bronchoscopic
treatment of lavage and expansion. By the end of the statistics
period, 1 SCLC patient in the limited stage had been followed-up
for 40 months and had a good quality of life. One patient with
stage IV adenocarcinoma succumbed to lung disease progression; the
patient survived for 37 months after irradiation. One patient with
stage IIIB adenocarcinoma was reported to exhibit atelectasis 3.5
months after irradiation; the patient refused bronchoscopic lavage
and the condition improved following symptomatic treatment.
One of two patients with phase 2 radiation
pneumonitis had a history of chronic bronchitis for >10 years.
The right hilar, 4R and 7 area lymph nodes were treated by γ-knife
at the same time. Telephone follow-ups revealed that the patient’s
cough had intensified five months after irradiation, and thus was
treated with antibiotics. The other patient was a 58-year-old male
with multiple metastases revealed following a right upper lung
adenocarcinoma resection; a new lesion (diameter 2 cm) was observed
in the right lower lung 3 months after gefitinib treatment. The
patient had the first γ-knife radiation treatment and exhibited a
CR; the disease progressed 9 months after irradiation. The patient
then received GP regimen chemotherapy (1,000 mg/m2
gemcitabine on days 1 and 8, and 75 mg/m2 cisplatin for
the first 3 days) for 4 cycles and paclitaxel single-agent
chemotherapy for 3 cycles. A new lesion appeared in the hilum of
the right lung (near the tumor first treated by the γ-knife) 9
months after chemotherapy. The second γ-knife therapy was
conducted. Telephone follow-ups revealed that grade 2 radiation
pneumonitis occurred 3 months after irradiation, and this was
treated by antibiotics. The patient succumbed to a brain hemorrhage
5 months after the second SBRT.
Discussion
With regard to the SBRT dose, Timmerman et al
(7) reported that the toxicity in
centrally-located tumor cases caused by SBRT was 11 fractions of
that of the peripheral tumor. The dose was as high as 60–66 Gy/3
fractions, with a BED10 of 181–211 Gy and a
BED3 of 460–550 Gy. Joyner et al (2006) (14) studied nine patients with
centrally-located lung tumors that were treated by SBRT. The dose
was 36 Gy/3 fractions, with a BED10 of 79 Gy and a
BED3 of 180 Gy; grade III tracheal stenosis was observed
in one patient. The dose reported by Chang et al (2008)
(8) was 40–50 Gy/4 fractions, with
a BED10 of 80–103 Gy and a BED3 of 173–258
Gy. No serious adverse reactions were observed in the trachea and
the lung. The dose used by Haasbeek et al (10) was 60 Gy/8 fractions, with a
BED10 of 105 Gy and a BED3 of 210 Gy; grade
III chest pain was reported by two patients, and grade III dyspnoea
was observed in two patients. The SBRT dose based on extracranial
γ-knife was 3–5 Gy/10–15 fractions (1,2,16). The
dose in the present study was 45–51 Gy/10–15 fractions, with 48–75
Gy for BED10 and 76–133 Gy for BED3. These
doses resulted in higher local control rates and the side-effects
were acceptable.
Patients with an advanced stage of disease may not
necessarily be treated with an excessively high dose. Relatively
low dose schedules may be more appropriate for such patients, with
mild side-effects and good local control effects within the
survival time. For patients with long-term survival, the local
control rate and tolerance should be considered when determining
the radiation dose for centrally-located tumors. The NCCN
guidelines (2012) have recommended that the use of 54–60 Gy/3
fractions is unsafe for centrally-located tumors, but that other
dose regimens may be safe and effective. Under the premise of
complying with the limit dosage for normal tissues, tumors >5 cm
can be treated by SBRT.
Joyner et al (14) reported that 36 months after SBRT,
certain patients complained of airway stenosis. In the study by
Miller et al (19), the
incidence of airway stenosis was 7% one year after high-dose
radiotherapy and 38% four years after high-dose radiotherapy.
Oshiro et al (11) reported
one patient with atelectasis, combined with a severe cough and
expectoration, one year after radiotherapy. The symptoms were
alleviated after 2 months of repeated balloon dilation therapy.
However, Bral et al (20)
reported that one patient with a centrally-located tumor treated
for tracheal stenosis succumbed following the therapy. This
indicated that invasive procedures on the trachea are risky
following high doses of radiation. In the present study, three
patients that reported atelectasis had tumors near the opening of
the lobe and segmental bronchi, and there was re-expansion in two
patients following bronchoscopic treatment.
Acute esophagitis is associated with the location of
the tumor, and the concept of the central area should be classified
by the location. SBRT is a precise radiotherapy; the dose of
radiation on the adjacent tissues is higher, while the dose
received by remote tissues is greatly reduced. The adverse
reactions may demonstrate a greater difference with adjacent
tissues. Wulf et al (21)
reported that 3 months after irradiation, mediastinal lesions at 30
Gy/3 fractions and ulcerative esophagitis appeared. In the present
study, the patients irradiated due to hilar and front-middle
mediastinal lesions did not report acute esophagitis. Of the five
patients suffering from acute esophagitis, the distance of the
tumor to the esophagus for all was ≤1 cm, and these patients all
fully recovered in 2 months. Thus, it is feasible to treat these
tumors with a dose of 3 Gy in 15 fractions or 36 Gy in 12
fractions.
In total, two patients suffered from grade 2 late
radiation pneumonitis in the present study. One patient had a past
history of chronic bronchitis. For the other patient, the tissues
irradiated by the second irradiation were close to that of the
first irradiation. SBRT should be applied with more caution to
patients with poor lung function or a history of chronic lung
disease. Oshiro et al (11)
reported that following treatment of hilar tumors with SBRT in 21
patients, three were observed with dyspnea of grade 3 and above,
and two had a history of radiotherapy. Wulf et al (21) reported that one patient receiving
the second-line radiotherapy had hemoptysis and succumbed. The
second SBRT on the adjacent tumor or local tumor should be
performed with caution, and the radiation dose should be decreased
to reduce the occurrence of side-effects.
In the majority of cases, the doctors performing
radiotherapy are faced with similar anatomical site diseases from
different primary tumors. Overall survival time is associated with
the type of disease, staging and the biological behavior of the
primary tumor, and is further associated with systemic therapy and
the local treatment of other parts. The difference between the
survival time of patients with stage III and stage IV NSCLC was not
significant in the present study. Cheruvu et al (22) reported 146 cases of NSCLC. The
5-year survival rate of patients with stage III, stage IV and
recurrent stage IV, starting from diagnosis, was 7, 14 and 27%,
respectively. The 5-year survival rate of stage IV patients with ≤8
lesions was significantly improved in comparison to stage III
patients, who developed extensive metastasis and were not suitable
for SBRT. The survival rate was 14 and 0%, respectively
(P<0.00001).
Wu et al (2)
studied 43 cases of stage I/II NSCLC treated by SBRT. The median
follow-up period was 22 months (range, 3–102 months). The 1- and
2-year local control rate was 75 and 60% for tumors of ≤3 cm, 84
and 71% for tumors of 3–5 cm, 55 and 14.6% for tumors of 5–7 cm and
45 and 21% for tumors of >7 cm, respectively. Clinical staging
is an important factor affecting the local control rate (P=0.000)
and overall survival (P=0.015). The present study demonstrated that
tumor size significantly affected the survival time when SBRT was
applied to treat stage III and IV NSCLC.
Patient age had no effect on the survival time.
There were three 75-year-old patients, one with phase IIIb NSCLC,
one with mediastinal lymph node metastasis of a laryngocarcinoma
and one with thymic carcinoma; all survived well following SBRT by
the end of the statistical period.
In short, centrally-located lung tumors could be
treated by SBRT, with an appropriate dose schedule. In the present
study, the local control rate was high and the adverse reactions
were well tolerated. The centrally-located area should be
subdivided according to the different anatomical sites and the
distance from the esophagus. However, more thorough and detailed
studies are required.
References
|
1
|
Xia T, Li H, Sun Q, Wang Y, Fan N, Yu Y,
Li P and Chang JY: Promising clinical outcome of stereotactic body
radiation therapy for patients with inoperable Stage I/II
non-small-cell lung cancer. Int J Radiat Oncol Biol Phys.
66:117–125. 2006. View Article : Google Scholar
|
|
2
|
Wu D, Zhu H, Tang H, Li C and Xu F:
Clinical analysis of stereotactic body radiation therapy using
extracranial γ knife for patients with mainly bulky inoperable
early stage non-small cell lung carcinoma. Radiat Oncol.
6:842011.
|
|
3
|
Haasbeek CJ, Senan S, Smit EF, Paul MA,
Slotman BJ and Lagerwaard FJ: Critical review of nonsurgical
treatment options for stage I non-small cell lung cancer.
Oncologist. 13:309–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chi A, Liao Z, Nguyen NP, Xu J, Stea B and
Komaki R: Systemic review of the patterns of failure following
stereotactic body radiation therapy in early-stage non-small-cell
lung cancer: clinical implications. Radiother Oncol. 94:1–11. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palma D and Senan S: Stereotactic
radiation therapy: changing treatment paradigms for stage I
nonsmall cell lung cancer. Curr Opin Oncol. 23:133–139. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palma D, Visser O, Lagerwaard FJ,
Belderbos J, Slotman BJ and Senan S: Impact of introducing
stereotactic lung radiotherapy for elderly patients with stage I
non-small-cell lung cancer: a population-based time-trend analysis.
J Clin Oncol. 28:5153–5159. 2010. View Article : Google Scholar
|
|
7
|
Timmerman R, McGarry R, Yiannoutsos C,
Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C,
Williams M and Fletcher J: Excessive toxicity when treating central
tumors in a phase II study of stereotactic body radiation therapy
for medically inoperable early-stage lung cancer. J Clin Oncol.
24:4833–4839. 2006. View Article : Google Scholar
|
|
8
|
Chang JY, Balter PA, Dong L, Yang Q, Liao
Z, Jeter M, Bucci MK, McAleer MF, Mehran RJ, Roth JA and Komaki R:
Stereotactic body radiation therapy in centrally and superiorly
located stage I or isolated recurrent non-small-cell lung cancer.
Int J Radiat Oncol Biol Phys. 72:967–971. 2008. View Article : Google Scholar
|
|
9
|
Lagerwaard FJ, Haasbeek CJ, Smit EF,
Slotman BJ and Senan S: Outcomes of risk-adapted fractionated
stereotactic radiotherapy for stage I non-small-cell lung cancer.
Int J Radiat Oncol Biol Phys. 70:685–692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haasbeek CJ, Lagerwaard FJ, Slotman BJ and
Senan S: Outcomes of stereotactic ablative radiotherapy for
centrally-located early-stage lung cancer. J Thorac Oncol.
6:2036–2043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oshiro Y, Aruga T, Tsuboi K, Marino K,
Hara R, Sanayama Y and Itami J: Stereotactic body radiotherapy for
lung tumors at the pulmonary hilum. Strahlenther Onkol.
186:274–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Unger K, Ju A, Oermann E, Suy S, Yu X,
Vahdat S, Subramaniam D, Harter KW, Collins SP, Dritschilo A,
Anderson E and Collins BT: CyberKnife for hilar lung tumors: report
of clinical response and toxicity. J Hematol Oncol. 3:392010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nuyttens JJ, van der Voort van Zyp NC,
Praag J, Aluwini S, van Klaveren RJ, Verhoef C, Pattynama PM and
Hoogeman MS: Outcome of four-dimensional stereotactic radiotherapy
for centrally-located lung tumors. Radiother Oncol. 102:383–387.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joyner M, Salter BJ, Papanikolaou N and
Fuss M: Stereotactic body radiation therapy for centrally-located
lung lesions. Acta Oncol. 45:802–807. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen G, Wang YJ, Sheng HG, Duan XP, Wang
JL, Zhang WJ, Zhou ZS, Zhu GY and Xia TY: Double CT imaging can
measure the respiratory movement of small pulmonary tumors during
stereotactic ablative radiotherapy. J Thorac Dis. 4:131–140.
2012.PubMed/NCBI
|
|
16
|
Zhang LP, Nie Q, Kang JB, Wang B, Cai CL,
Li JG and Qi WJ: Efficacy of whole body γ-knife radiotherapy
combined with thermochemotherapy on locally advanced pancreatic
cancer. Ai Zheng. 27:1204–1207. 2008.(In Chinese).
|
|
17
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, et al: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colevas AD and Setser A: The NCI Common
Terminology Criteria for Adverse Events (CTCAE) v 3.0 is the new
standard for oncology clinical trials. J Clin Oncol. 22(July 15
Suppl): 60982004.
|
|
19
|
Miller KL, Shafman TD, Anscher MS, Zhou
SM, Clough RW, Garst JL, Crawford J, Rosenman J, Socinski MA,
Blackstock W, Sibley GS and Marks LB: Bronchial stenosis: an
underreported complication of high-dose external beam radiotherapy
for lung cancer? Int J Radiat Oncol Biol Phys. 61:64–69. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bral S, Gevaert T, Linthout N, Versmessen
H, Collen C, Engels B, Verdries D, Everaert H, Christian N, De
Ridder M and Storme G: Prospective, risk-adapted strategy of
stereotactic body radiotherapy for early-stage non-small-cell lung
cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys.
80:1343–1349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wulf J, Hädinger U, Oppitz U, Thiele W,
Ness-Dourdoumas R and Flentje M: Stereotactic radiotherapy of
targets in the lung and liver. Strahlenther Onkol. 177:645–655.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheruvu P, Metcalfe SK, Metcalfe J, Chen
Y, Okunieff P and Milano MT: Comparison of outcomes in patients
with stage III versus limited stage IV non-small cell lung cancer.
Radiat Oncol. 6:802011. View Article : Google Scholar : PubMed/NCBI
|