Introduction
Glial tumors are the most common brain malignancies,
accounting for major morbidity and mortality of adults and
children. These tumors are subclassified by the World Health
Organization (WHO) into four grades according to pathological
appearance, mitotic activity, vascular proliferation and regional
necrosis (1). Glioblastoma (GBM;
WHO grade IV) is the most aggressive type of glioma, with a median
survival time of 8–14 months (2).
The majority of GBM tumors are considered primary tumors, whereas
only 5% are secondary- to low-grade glioma, characterized by
various clinical and molecular features. Despite improved
understanding of the biology of glioblastoma and newly available
treatment modalities, survival rates have not changed in the past
decades.
DNA methylation, one of the most common epigenetic
modifications, has been widely described in cancer, which exhibits
global DNA hypomethylation in addition to significant
hypermethylation (and thus downregulation) of tumor suppressor
genes (3). Aberrant DNA methylation
is well described in GBM (4,5).
Related to testes-specific, vespid and pathogenesis
protein-1 (RTVP-1), or glioma pathogenesis-related protein 1, is
highly expressed in GBM and glioma cell lines, but not in normal
adult brain, nor in low grade astrocytomas, oligodendrogliomas or
other nervous system tumors (6–9).
Previous studies have reported that RTVP-1 can be epigenetically
regulated (10–13), but this has not been demonstrated in
central nervous system tissue or tumors.
In the current study, RTVP-1 promoter methylation
status in GBM and the association of promoter methylation with
disease progression and patient outcome were examined.
Materials and methods
Tissue and tumor samples
Tissue and tumor samples and patient data were
obtained from Sheba Brain Tumor Bank (Tel Aviv, Israel) and Henry
Ford Hospital (Detroit, MA, USA). Patient data was subsequently
anonymised. In total, 69 tissue samples from different patients
were received: 43 GBM, 16 oligodendroglioma and 10 samples of
non-tumor brain (excised from epilepsy patients). All samples were
from unrelated patients and none were from secondary tumor or tumor
relapse. The median age of GBM patients was 57 years (range, 35–74
years) and 47 years (range, 24–81 years) for oligodendroglioma
patients. Males accounted for 56% of patients. Each specimen was
assigned a 3-character code for identification between the sample
and the anonymous data. The specimen collection was approved by the
institutional ethical committee of each center.
DNA extraction and bisulfite
treatment
DNA was extracted according to the manufacturer’s
instructions of the DNeasy kit (spin-column) (Qiagen, Germantown,
MD, USA). Bisulfite treatment was performed on 1 μg DNA
using an EZ DNA Methylation-Gold kit (Zymo Research, Orange, CA,
USA) according to the Sequenom protocol (San Diego, CA, USA)
(14). The final elution volume of
C–T converted DNA was 50 μl.
Methylation analysis
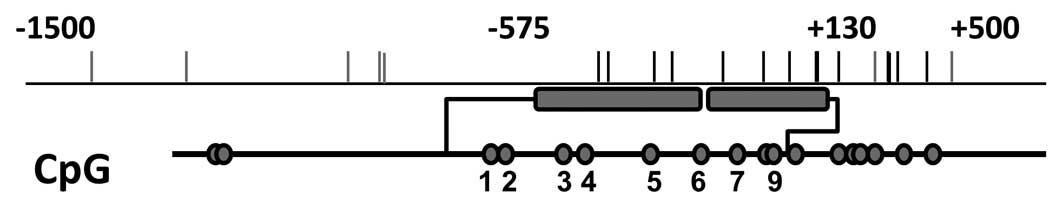
Promoter sequences were searched using the
University of California, Santa Cruz genome browser (http://genome.ucsc.edu). CpG sites were searched
within the promoter area between −1,500 bp upstream and +500 bp
downstream of the transcription start site (TSS). Methylation
analysis was performed by mass spectrometry (Sequenom Bruker mass
spectrometer and Sequenom Samsung MassARRAY Nano dispenser,
Sequenom Inc., San Diego, CA, USA) of base-specific cleavage
products, according to the Sequenom protocol previously described
(14). Amplicons and primers were
designed using Sequenom EpiDesigner (http://www.epidesigner.com), as presented in Fig. 1 and Table I. Polymerase chain reaction (PCR)
was carried out with 50 ng bisulfite-treated DNA made up to a total
volume of 25 μl with GoTaq buffer (Promega, Madison, WI,
USA), 200 μM deoxyribonucleotide triphosphates (dNTPs), 5 mM
MgCl2, 10 pmol forward and reverse primers and 2 units
GoTaq polymerase (Promega). PCR conditions were as follows: 95°C
for 10 min, followed by 35 cycles at 94°C for 20 sec, 60°C for 30
sec and 72°C for 1 min, and later 3 min at 72°C. Aliquots of the
PCR product were tested on 2% agarose gel. Shrimp alkaline
phosphatase treatment of the PCR product, in vitro
transcription to RNA and T-specific cleavage by RNAse A were
performed according to the Sequenom protocol using a MassCLEAVE kit
(Sequenom). The reaction product was robotically dispensed onto
silicon chips for Sequenom mass spectrometry detection.
 | Table IPCR and qPCR primers. |
Table I
PCR and qPCR primers.
| Gene | Sense | Primer sequence,
5′-3′ | Amplicon length,
bp |
|---|
| PCR (C–T converted
designa) |
| RTVP-1 |
| Amplicon 3 | Forward |
TTTTATTTAATAGGTGGTTGAGGTT | 424 |
| Reverse |
CCAAAAAAAATTCTAAAATCTCCAAA | |
| Amplicon 4 | Forward |
TTGGAGATTATTATTTTTGGAGATTT | 323 |
| Reverse |
CCTTAAAAAACTACAATCCAAAACC | |
| qPCR |
| RTVP-1 | Forward |
CAAGTGTTTGGACAATCTCTGTGTTA | 80 |
| Reverse |
GCCAGCCTGGATATACAACAGAGT | |
| TaqMan probe |
FAM-CCGACAGCGAGACCAAGTCAAACGT-BHQ-1 | |
| GAPDH | Forward |
CCTCCCGCTTCGCTCTCT | 64 |
| Reverse |
GGCGACGCAAAAGAAGATG | |
| TaqMan probe |
FAM-TCCTCCTGTTCGACAGTCAGCC-BHQ-1 | |
RNA extraction and quantitative
(q)PCR
RNA was extracted using TRIzol (Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
For qPCR, 2 μg RNA was reverse transcribed to cDNA according
to the instructions of the High Capacity cDNA Reverse Transcription
kit (Applied Biosystems, Foster City, CA, USA). TaqMan Real Time
PCR (Life Technologies) was carried out with 0.2 μg cDNA
made up to a total reaction volume of 20 μl with buffer,
including ROX (Life Technologies), 0.2 mM dNTP, 5 mM
MgCl2, 30 μg bovine serum albumin, 5 pmol both
forward and reverse primers, 5 pmol TaqMan probe (Life
Technologies) and 0.5 units IMMOLASE (Bioline, Taunton, MA, USA).
Primer sequences are shown in Table
I. GAPDH was selected as a housekeeping gene for relative
expression analysis. Samples were run on Real Time PCR 7500
(Applied Biosystems) at 95°C for 10 min, followed by 40 cycles at
95°C for 15 sec and 60°C for 1 min.
Statistical analysis
CpG methylation was considered a continuous
variable. Asymmetric distribution required non-parametric testing
with Kruskal-Wallis one-way analysis and Mann-Whitney U tests. For
qPCR, Student’s t-test was performed. qPCR results were analysed
using the ΔΔCT method and compared with GAPDH. Correlations were
calculated using Spearman’s rank correlation. Overall survival was
calculated with Kaplan-Meier analysis and validated by log-rank
tests. Statistical analysis was performed with GraphPad Prism 5 for
Windows (GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Tumor and control samples
A total of 69 samples were used: 43 GBM, 16
oligodendroglioma and 10 samples of non-tumor brain (excised from
epilepsy patients). All samples were obtained at diagnosis and no
relapse or secondary tumors were evaluated.
RTVP-1 promoter methylation analysis using
EpiDesigner software (Sequenom) revealed 9 CpG sites with 2
selected amplicons, covering 600 bp upstream and downstream of the
RTVP-1 TSS (Fig. 1). Methylation
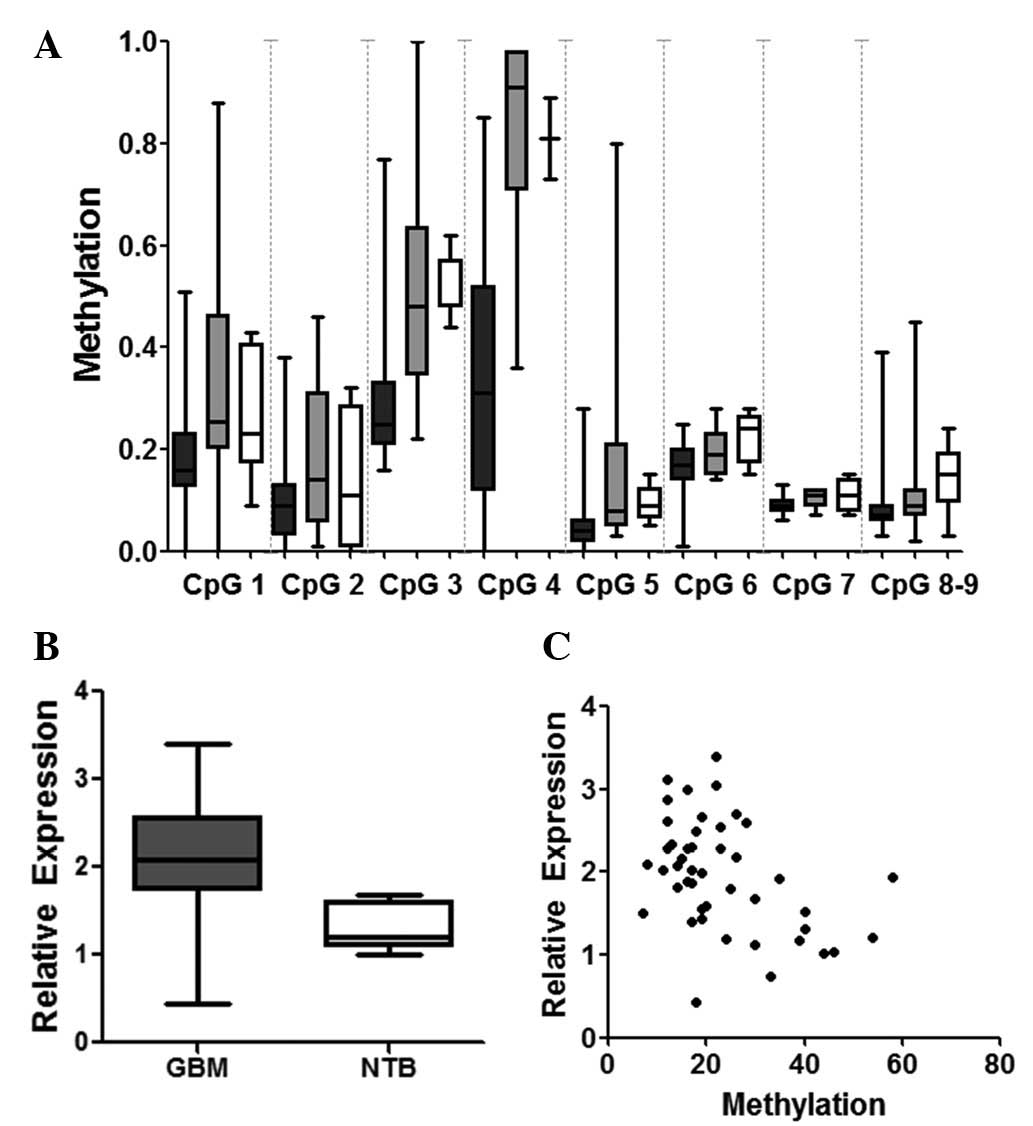
analysis was performed on all 69 samples. Average RTVP-1 promoter
methylation was significantly lower in GBM (15%) compared with
non-tumor brain samples (25%; P=0.001). Specific CpG analysis
revealed that all 9 CpG sites examined in the promoter area had
lower methylation in GBM samples compared with controls (Fig. 2A). CpG 3 and CpG 4 (located −251 and
−210 bp upstream of the RTVP-1 TSS, respectively) demonstrated the
most significant methylation difference between sample groups
(P<0.001). RTVP-1 promoter methylation in GBM was also
significantly lower when compared with oligodendroglioma (P=0.001).
RTVP-1 mRNA expression was significantly higher in GBM compared
with the control group (median log expression with interquartile
range, 2.06±0.69 in GBM vs. 1.33±0.26 in non-tumor brain;
P<0.001); (Fig. 2B). RTVP-1
expression was inversely correlated with average promoter
methylation (r=−0.4584; P=0.001; Fig.
2C).
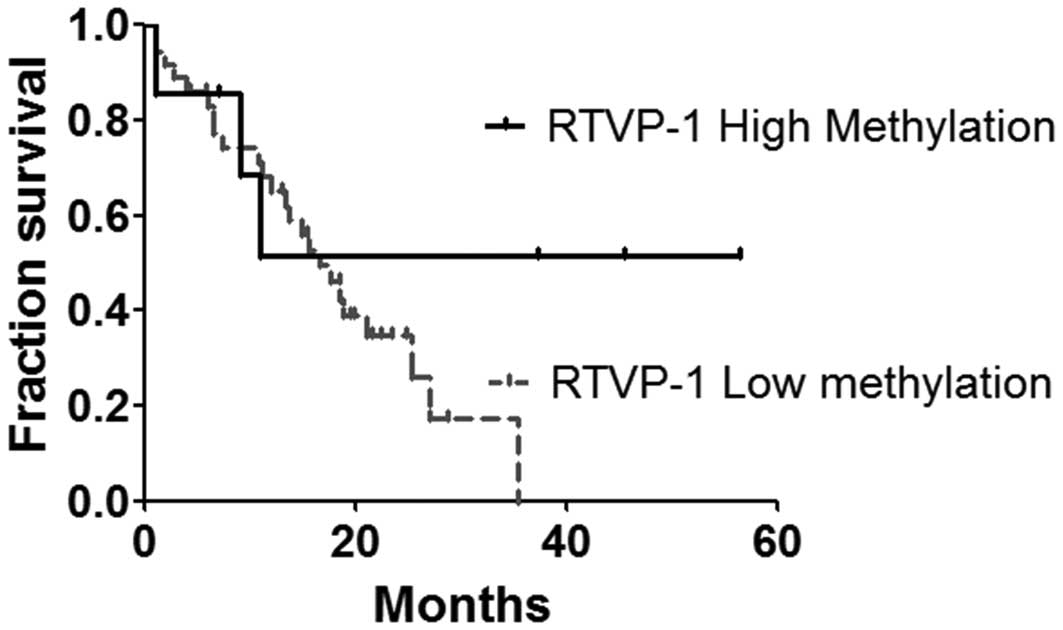
Promoter methylation status as a
prognostic marker in GBM
The methylation pattern of RTVP-1 had great
inter-sample variability in GBM compared with the narrow
methylation ranges observed in oligodendroglioma samples and
non-cancerous controls. Thus, several GBM samples were found to
differ from the general malignant methylation pattern. The clinical
significance of this pattern was assessed.
The RTVP-1 promoter was hypermethylated in 7 GBM
samples, similar to non-tumor samples. These samples, defined as
RTVP-1high-meth, were compared with the 36 samples of
hypomethylated RTVP-1 (RTVP-1low-meth). No significant
differences were identified between RTVP-1high-meth and
RTVP-1low-meth in basic characteristics, including
median age (54 and 57 years, respectively), gender (57 and 55% in
male patients, respectively) and tumor location. There was a trend
towards increased overall survival in RTVP-1high-meth
patients verses RTVP-1low-meth patients which did not
reach statistical significance (Fig.
3).
Discussion
In this study, the promoter methylation profile of
RTVP-1 in GBM was evaluated. Compared with non-tumor brain, GBM
samples were found to have hypomethylated RTVP-1 promoters.
RTVP-1 is normally expressed in the heart, spleen,
muscle, bone marrow, placenta, adrenal and prostate (8). RTVP-1 behaves differently in various
malignancies. In GBM, RTVP-1 is an overexpressed oncogene
associated with increased proliferation, enhanced invasion and
inhibition of apoptosis (6–9,15). By
contrast, RTVP-1 is underexpressed in prostatic carcinoma, in which
it is described as a tumor suppressor gene, acting via
p53-dependent and -independent regulation (16). Furthermore, overexpression of RTVP-1
has been found to induce apoptosis in prostate cancer cell lines
and in vivo models of prostate cancer (10,16–18).
RTVP-1 expression in prostate cancer is downregulated
epigenetically via methylation of the promoter (10). Promoter hypermethylation has also
been found to reduce RTVP-1 expression in acute myeloid leukemia
patients compared with lymphoblastic leukemia, chronic myeloid
leukemia and remission bone marrow (11). Promoter hypomethylation with high
RTVP-1 expression was identified in Wilms’ tumor (12). A similar correlation was recently
described in melanoma (13).
To the best of our knowledge, this report is the
first to identify similar promoter regulation in brain
malignancies. Additionally, the expression of RTVP-1 was
significantly higher in GBM compared with control non-tumor brain,
in agreement with previous reports (6,7). An
inverse correlation between RTVP-1 expression and promoter
methylation was successfully demonstrated.
Recently, microRNA-137 (mir-137) was described as a
regulator of RTVP-1 (15).
Downregulation of mir-137 contributes to the high expression of
RTVP-1 in glioblastoma. The current study describes further loss of
regulatory control of RTVP-1 in GBM by promoter methylation. Unlike
GBM, RTVP-1 was hypermethylated in oligodendroglioma, another
astrocytic tumor. While this may indicate specificity of RTVP-1
hypomethylation in GBM, it may also reflect general
hypermethylation observed in oligodendrogliomas (19,20),
similar to the methylation pattern in secondary but not primary GBM
(21).
The majority of known methylation-controlled
promoters contain CpG-rich sites (CpG islands). The RTVP-1 promoter
region contains a low number of CpG sites. Only 16 sites were
identified within 1,000 bp upstream and downstream of the TSS. The
method used for methylation analysis in the present study (Sequenom
mass spectrometry detection of T-cleaved products of
bisulfite-treated DNA) provided quantitative data through the
ability to assess single-CpG methylation. This method has been used
previously in similar studies and appears to have the benefit of
being a sensitive quantitative method (14,15).
This offers an advantage in CpG-poor regions, including the RTVP-1
promoter, and in identification of specific CpG sites, the
methylation of which may significantly impact mRNA expression
(22).
Finally, among glioblastoma samples, few were
significantly hypermethylated, correlating with lower expression of
RTVP-1, similar to non-tumor brain samples. In subjects with highly
methylated RTVP-1, a trend towards prolonged survival was detected
which did not reach statistical significance. Since RTVP-1 has
oncogenic features in GBM, silencing via hypermethylation may have
prognostic benefits. Further testing using additional samples are
required to confirm this.
In conclusion, this study has shown for the first
time that RTVP-1 is regulated by methylation in tissues of brain
origin. In GBM, high expression of RTVP-1 is associated with
promoter hypomethylation of this gene. GBM samples with
hypermethylated RTVP-1 were associated with a tendency toward
improved overall survival.
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jeswani S, Nuño M, Folkerts V and Bigg J:
Comparison of survival between cerebellar and supratentorial
glioblastoma patients: surveillance, epidemiology, and end results
(SEER) analysis. Neurosurgery. 73:240–246. 2013. View Article : Google Scholar
|
|
3
|
Esteller M: Epigenetics in cancer. N Engl
J Med. 358:1148–1159. 2008. View Article : Google Scholar
|
|
4
|
Noushmehr H, Weisenberger DJ, Diefes K, et
al: Identification of a CpG island methylator phenotype that
defines a distinct subgroup of glioma. Cancer Cell. 17:510–522.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martinez R, Martin-Subero JI, Rohde V, et
al: A microarray-based DNA methylation study of glioblastoma
multiforme. Epigenetics. 4:255–264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murphy EV, Zhang Y, Zhu W, et al: The
human glioma pathogenesis-related protein is structurally related
to plant pathogenesis-related proteins and its gene is expressed
specifically in brain tumors. Gene. 159:131–135. 1995. View Article : Google Scholar
|
|
7
|
Rich T, Chen P, Furman F, et al: RTVP-1, a
novel human gene with sequence similarity to genes of diverse
species, is expressed in tumor cell lines of glial but not neuronal
origin. Gene. 180:125–130. 1996. View Article : Google Scholar
|
|
8
|
Rosenzweig T, Ziv-Av A, Xiang C, et al:
Related to testes-specific, vespid, and pathogenesis protein-1
(RTVP-1) is overexpressed in gliomas and regulates the growth,
survival, and invasion of glioma cells. Cancer Res. 66:4139–4148.
2006. View Article : Google Scholar
|
|
9
|
Xiang C, Sarid R, Cazacu S, et al: Cloning
and characterization of human RTVP-1b, a novel splice variant of
RTVP-1 in glioma cells. Biochem Biophys Res Commun. 362:612–618.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren C, Li L, Yang G, et al: RTVP-1, a
tumor suppressor inactivated by methylation in prostate cancer.
Cancer Res. 64:969–976. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang T, Tan T, Xiao Y, et al: Methylation
and expression of glioma pathogenesis-related protein 1 gene in
acute myeloid leukemia. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
34:388–394. 2009.(In Chinese).
|
|
12
|
Chilukamarri L, Hancock AL, Malik S, et
al: Hypomethylation and aberrant expression of the glioma
pathogenesis-related 1 gene in Wilms tumors. Neoplasia. 9:970–978.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Awasthi A, Woolley AG, Lecomte FJ, et al:
Variable expression of GLIPR1 correlates with invasive potential in
melanoma cells. Front Oncol. 3:2252013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ehrich M, Nelson MR, Stanssens P, et al:
Quantitative high-throughput analysis of DNA methylation patterns
by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci
USA. 102:15785–15790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bier A, Giladi N, Kronfeld N, et al:
MicroRNA-137 is downregulated in glioblastoma and inhibits the
stemness of glioma stem cells by targeting RTVP-1. Oncotarget.
4:665–676. 2013.PubMed/NCBI
|
|
16
|
Ren C, Li L, Goltsov AA, et al: mRTVP-1, a
novel p53 target gene with proapoptotic activities. Mol Cell Biol.
22:3345–3357. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Fattah EA, Cao G, et al: Glioma
pathogenesis-related protein 1 exerts tumor suppressor activities
through proapoptotic reactive oxygen species-c-Jun-NH2
kinase signaling. Cancer Res. 68:434–443. 2008. View Article : Google Scholar
|
|
18
|
Thompson TC: Glioma pathogenesis-related
protein 1: tumor-suppressor activities and therapeutic potential.
Yonsei Med J. 51:479–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong SM, Pang JC, Poon WS, et al:
Concurrent hypermethylation of multiple genes is associated with
grade of oligodendroglial tumors. J Neuropathol Exp Neurol.
60:808–816. 2001.PubMed/NCBI
|
|
20
|
Wolter M, Reifenberger J, Blaschke B, et
al: Oligodendroglial tumors frequently demonstrate hypermethylation
of the CDKN2A (MTS1, p16INK4a), p14ARF, and CDKN2B (MTS2, p15INK4b)
tumor suppressor genes. J Neuropathol Exp Neurol. 60:1170–1180.
2001.
|
|
21
|
Noushmehr H, Weisenberger DJ, Diefes K, et
al: Identification of a CpG island methylator phenotype that
defines a distinct subgroup of glioma. Cancer Cell. 17:510–522.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Everhard S, Tost J, El Abdalaoui H, et al:
Identification of regions correlating MGMT promoter methylation and
gene expression in glioblastomas. Neuro Oncol. 11:348–356. 2009.
View Article : Google Scholar : PubMed/NCBI
|