Introduction
It has been demonstrated that epithelial ovarian
cancer (EOC) serves as one of the most common types of cancer in
females and is the leading cause of mortality from gynecological
malignancy worldwide (1). In
previous years, a number of patients with EOC have benefited from
refined and more radical surgical techniques, as well as adjuvant
combination chemotherapy. However, the overall 5-year survival rate
of ~70% of patients was low (~33%), as diagnosis was made at the
advanced stages, when extensive cell invasion and migration had
already taken place in the abdominopelvic cavity (2). The potential molecular mechanisms
which cause the initiation, chemotherapy resistance and development
of metastasis in EOC are not well understood. Therefore, it is of
great importance to identify the effector molecules and/or
signaling pathways responsible for EOC pathogenesis and
progression, in order to optimize treatment strategies.
MicroRNAs (miRNAs) are small (20–24 nucleotides)
noncoding RNA gene products that post-transcriptionally regulate
gene expression by negatively modulating the stability or
translational efficiency of their target mRNAs (3). Evidence for the importance of miRNAs
in cancer came from the finding that miRNA genes were specifically
deleted in leukemia (4).
Additionally, miRNAs have been shown to be differentially expressed
in a number of other cancer types (5,6). Taken
together, miRNAs are considered to be the critical factors in
numerous malignancies, acting as tumor suppressors or oncogenes
(7,8). Previous studies have also illustrated
that various miRNAs may lead to invasion and metastasis in EOC
(9–13).
Among the miRNAs, miR-133a is regarded as one of the
major tumor suppressor miRNAs. Aberrant miR-133a expression was
previously reported in human malignancies, including bladder cancer
(5), head and neck cancer (14), rhabdomyosarcoma (15), esophageal cancer (16), colon cancer (17), tongue cancer (18) and renal cell carcinoma (19), using high-throughput technology,
including miRNA oligonucleotide arrays and quantitative polymerase
chain reaction (qPCR). However, it remains unknown whether miR-133a
has a functional role in EOC. Thus, the present study was performed
to investigate the expression of miR-133a in EOC tissues and the
human EOC OVCAR-3 cell line by qPCR. Additionally, the effects of
miR-133a on OVCAR-3 cell proliferation, apoptosis, invasion and
migration were analyzed.
Materials and methods
Human tissues and cell lines
A total of 96 tissue samples, including 70
epithelial ovarian cancer tissues and 26 normal ovarian tissue
sections from the Affiliated Obstetrics and Gynecology Hospital,
Zhejiang University School of Medicine (Hangzhou, China) were
collected between January 2009 and June 2012. Additionally, ovarian
tumor samples from debunking surgery and the corresponding
pathological data were collected. Patients with a previous or
secondary malignancy, or having previously undergone radiation
therapy, chemotherapy, or immunotherapy, were excluded from the
study. The histopathological diagnoses were performed according to
the World Health Organization criteria (20) and the tumor histotypes included 38
serous and 32 non-serous types. All tumor stages were determined
based on the International Federation of Gynecology and Obstetrics
standards (FIGO) (21). The stage
breakdown was as follows: n=8 for stage I, n=12 for stage II, n=35
for stage III and n=15 for stage IV. Samples from patients who had
undergone oophorectomy for benign uterine pathologies were used as
normal control tissues. The study was approved by the Medical
Ethics Committee of the Women’s Hospital, Zhejiang University
(Hangzhou, China) and all patients provided informed consent. All
fresh specimens were initially stored at 4°C for 24 h in RNAlater
(Ambion, Carlsbad, CA, USA) and subsequently at −80°C in liquid
nitrogen until further use. The human EOC OVCAR-3 cell line and
normal human ovarian surface epithelial [OSE(tsT)] cells were
supplied by China Center for Type Culture Collection (Wuhan,
China). Cells were cultured in Dulbecco’s modified Eagle’s medium
(DMEM; Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (Biological Industries, Kibbutz Beit Haemek, Israel)
and incubated at 37°C in 5% CO2.
RNA extraction and qPCR
In order to perform qPCR analysis, total RNA was
first extracted using miRNeasy kit (Qiagen, Hilden, Germany)
following the manufacturer’s instructions. cDNA was synthesized
from total RNA using miR-133a reverse transcription (RT) primer.
The miR-133a RT primer was: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGC
ACTGGATACGACACAGCT-3′. The miR-133a PCR primers were: Forward,
5′-CTGCATTGGTCCCCTTCAAC-3′ and reverse, 5′-CAGTGCAGGGTCCGAGGTAT-3′.
miRNA expression was detected using qPCR with SYBR Green RT-PCR kit
(Takara Bio, Inc., Shiga, Japan). The reaction was incubated at
94°C for 4 min, followed by 35 cycles of 20 sec at 94°C, 30 sec at
60°C and 30 sec at 72°C. U6 small nuclear RNA was used as an
endogenous internal standard control. The threshold cycle (Ct) was
determined as the fractional cycle number at which the fluorescence
passed the fixed threshold. All experiments were repeated twice
and, in each experiment, samples were assayed in triplicate. Data
were expressed as the expression level of miR-133a relative to that
of the internal control U6, using the 2−ΔΔCt method
(22).
miR-133a transfection
miR-133a mimics were obtained from Shanghai
GenePharma Co., Ltd (Shanghai, China). These included synthetic
small duplex sequences of miR-133a-RNA able to be bioprocessed into
mature miR-133a in the cells. The negative control (NC) sequence,
which was not homologous to any human genome sequence, was used to
eliminate any potential non sequence-specific effects. Primers for
miR-133a were as follows: Sense, 5′-UUUGGUCCCCUUCAACCAGCUG-3′ and
antisense, 5′-GCUGGUUGAAGGGGACCAAAUU-3′; NC siRNA sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. The transfection of miR-133a mimics
into cells was carried out using Lipofectamine 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA). The cells were cultured
using complete medium without antibiotics, and Lipofectamine 2000
and miR-133a mimics were diluted with serum-free medium.
Analysis of cell viability in vitro
MTT assay was used to analyze the cell viability of
OVCAR-3 transfected with NC or miR-133a mimics (23). Briefly, cells were seeded into
96-well plates and transfected. In the indicated time periods, 0.1
ml fresh medium containing 0.5 mg/ml MTT was used to replace an
equal volume of spent medium. Following incubation at 37°C for 4 h,
the medium was replaced by 0.1 ml dimethyl sulfoxide
(Sigma-Aldrich, St. Louis, MO, USA) and agitated at room
temperature for 10 min. The absorbance was measured by a
spectrometer at a wavelength of 490 nm.
Detection of apoptosis
OVCAR-3 cells were transfected with NC or miR-133a
mimics for 48 h. Next, cell culture medium was replaced with
serum-free DMEM. At the indicated time periods following serum
deprivation, cells were harvested, washed, resuspended in the
staining buffer and examined with the Vybrant Apoptosis Assay kit
(Invitrogen Life Technologies). Stained cells were identified by
FACSCalibur and data were analyzed with CellQuest software (both
from BD Biosciences, Franklin Lakes, NJ, USA). Annexin V-positive
and propidium iodide-negative cells were considered to be apoptotic
cells.
Invasion assay
Invasion assays were performed using transwell
invasion chambers coated with Matrigel (50 μl per filter; BD
Biosciences) according to the manufacturer’s instructions. OVCAR-3
cells were transfected with NC or miR-133a mimics for 48 h and
transferred onto the top of Matrigel-coated invasion chambers in
serum-free DMEM (1×105 cells per transwell). DMEM
containing 10% fetal calf serum was added to the lower chambers.
Following incubation for 24 h, cells remaining on the top of the
filter were removed and those that migrated to the lower surface
were fixed in 90% alcohol and subjected to crystal violet staining.
The number of migrated cells on the lower surface of the membrane
was counted under a microscope in 10 fields with a magnification of
×400. The invasion assays were carried out in triplicate.
Scratch migration assay
OVCAR-3 cells were transfected with NC or miR-133a
mimics and grown to confluence. Subsequently, a scratch was set
though the dish and cells were cultured under standard conditions
for 24 h. Following several washes, plates were photographed and
the cell migration was evaluated by counting cells that had
migrated from the wound edge.
Statistical analysis
Data are represented as the mean ± standard error of
the mean. Statistical analysis was carried out using a Student’s
t-test. All statistical analyses were performed using SPSS version
17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-133a is downregulated in OVCAR-3
cells and primary EOC tumor samples
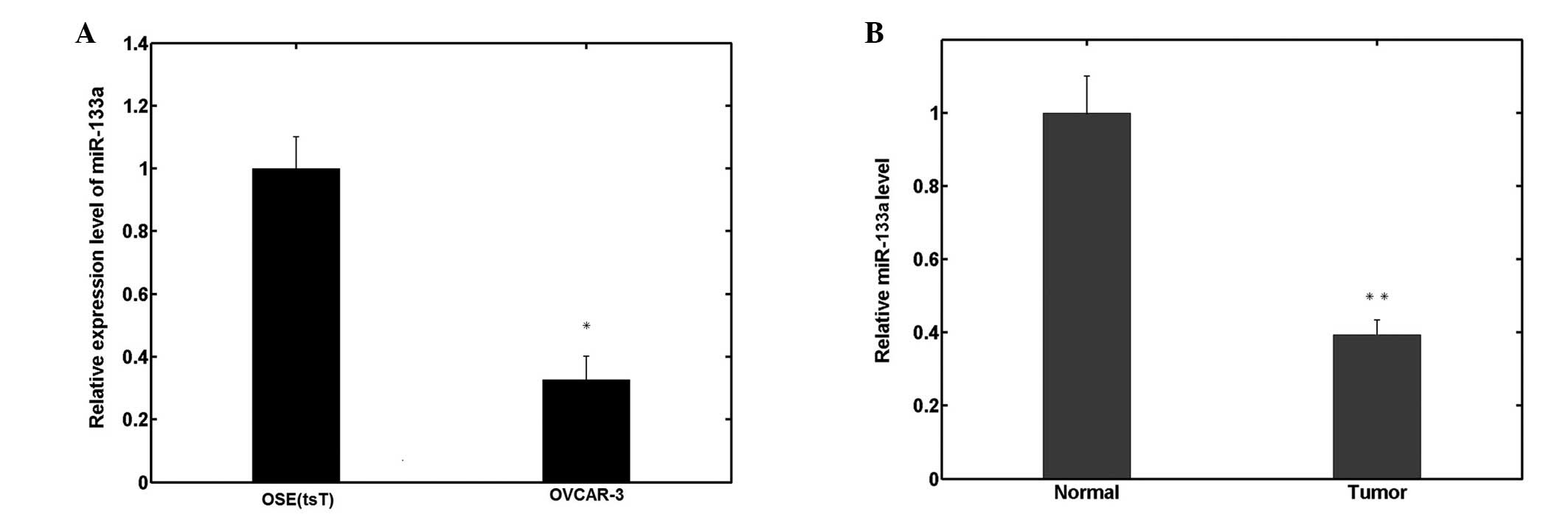
In order to investigate the biological roles of
miR-133a in human EOC pathogenesis and progression, miR-133a
expression was measured by qPCR in human OSE(tsT) and OVCAR-3 EOC
cell lines. As shown in Fig. 1A,
miR-133a expression significantly decreased in OVCAR-3 cells
compared with OSE(tsT) cells. Similarly, among the 70 ovarian
cancer samples analyzed, the relative expression of miR-133a was
also significantly downregulated compared with the 26 normal
ovarian tissues, as shown in Fig.
1B.
Decreased miR-133a expression is
associated with clinicopathological features
Table I shows that
the relative expressions of miR-133a were significantly lower in
advanced stage and grade 3 tumor samples compared with early stage
and grade 1 and 2 tumor samples. The same differences were observed
between the lymph node-positive and -negative group. However, there
were significant differences between serous and non-serous tissue
samples.
 | Table IRelationship between miR-133a
expression level and clinicopathological features. |
Table I
Relationship between miR-133a
expression level and clinicopathological features.
| Factors | Patients, n | miR-133a (average
fold-change ± standard deviation) | P-value |
|---|
| FIGO stage |
| I, II | 20 | 0.59±0.26 | <0.001 |
| III, IV | 50 | 0.16±0.10 | |
| Grade |
| G1, G2 | 28 | 0.45±0.30 | 0.008 |
| G3 | 42 | 0.21±0.17 | |
| Lymph node |
| Negative | 46 | 0.51±0.34 | 0.003 |
| Positive | 24 | 0.18±0.15 | |
| Histotype |
| Serous | 38 | 0.38±0.14 | 0.388 |
| Non-serous | 32 | 0.29±0.19 | |
miR-133a reduces cell viability and
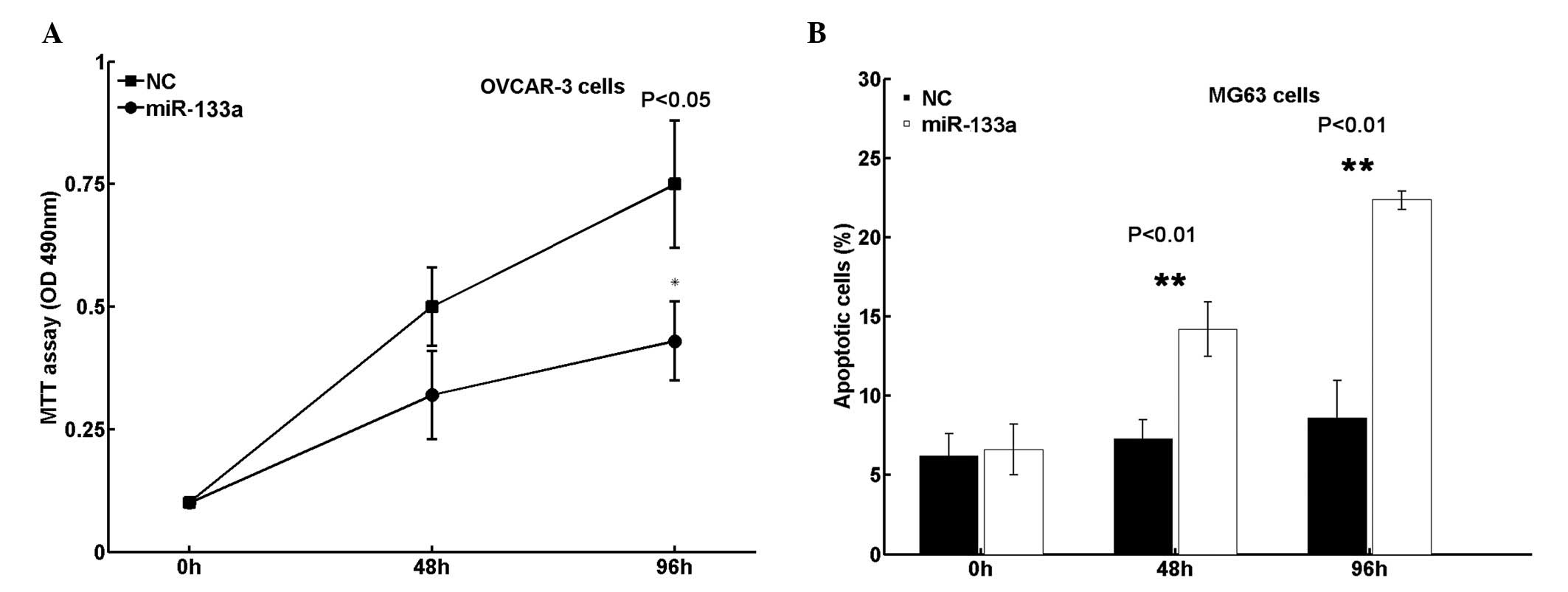
promotes cell apoptosis
In order to determine whether miR-133a functions as
a tumor suppressor in EOC, cell viability and apoptosis were
analyzed in the present study. It was revealed that transfection of
miR-133a mimics significantly reduced cell viability in OVCAR-3
cells (Fig. 2A). Additionally, cell
apoptosis in OVCAR-3 cells increased following restoration of
miR-133a expression (Fig. 2B).
Collectively, these results indicate that miR-133a suppresses EOC
growth in vitro.
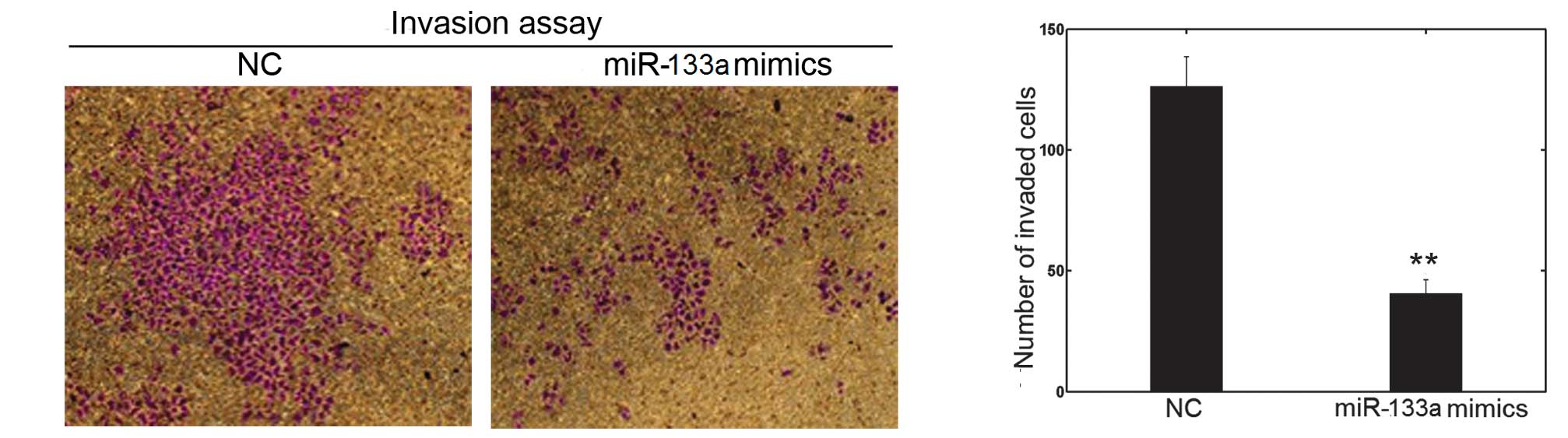
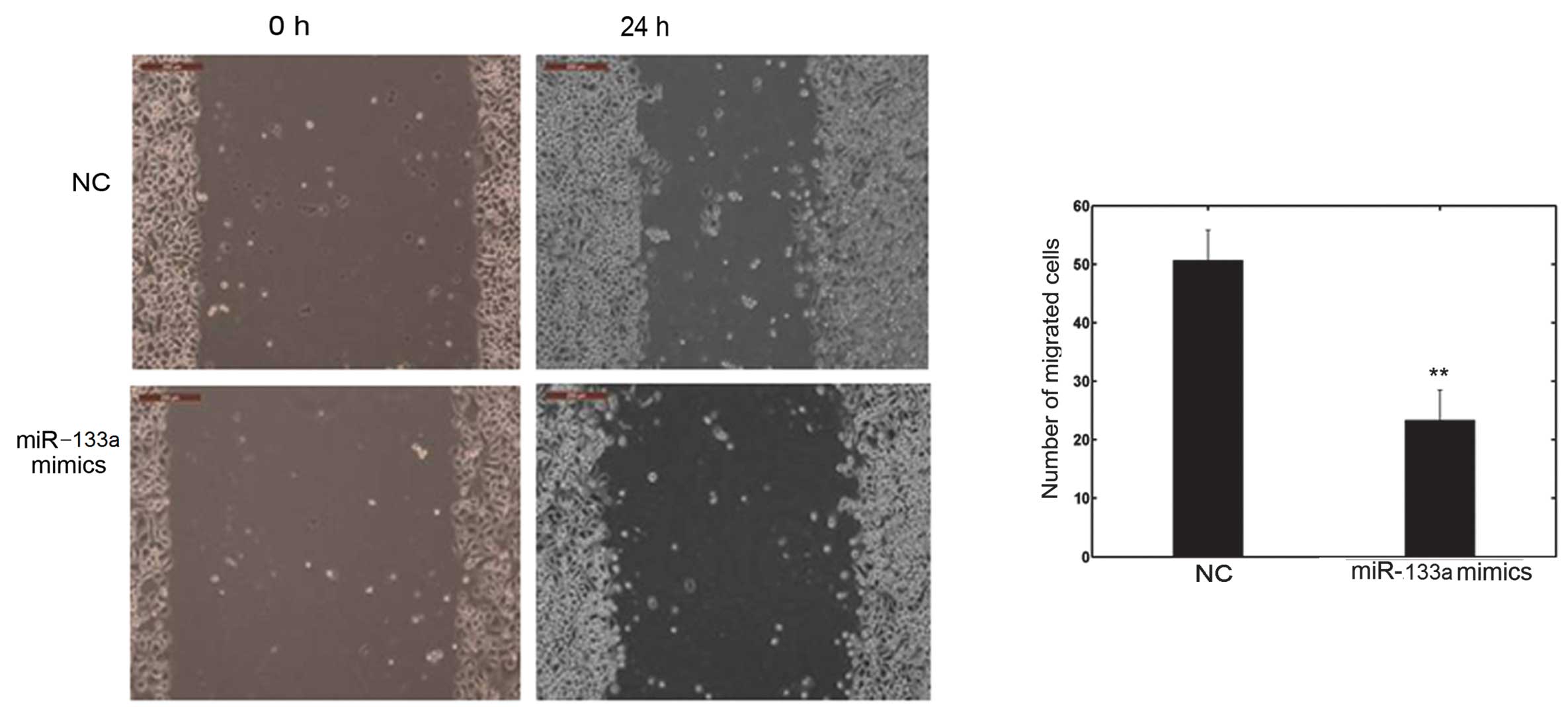
miR-133a affects cell invasion and
migration in vitro
The cell invasion and scratch migration assays were
used to confirm the effects of miR-133a on cell invasion and
migration, respectively. Following transfection with miR-133a
mimics or NC in OVCAR-3, a significant downregulation of invasion
into Matrigel was observed in miR-133a-transfected OVCAR-3 cells
(Fig. 3). Furthermore, the number
of migrated cells transfected with miR-133a mimics was
significantly lower than with the NC, as shown in Fig. 4. These observations confirm the
function of miR-133a in the invasion and metastasis of OVCAR-3
cells.
Discussion
It is well known that ovarian cancer is a common
gynecological malignancy and a leading cause of cancer mortality
among females worldwide. Although progress has been made in ovarian
cancer diagnosis and treatment, there are still numerous unexplored
areas, and patients with metastasis or recurrent diseases continue
to have a poor prognosis. miRNAs are important regulatory factors
and are involved in a number of biological processes, including
cell cycle regulation, cell growth, apoptosis, cell differentiation
and stress response (24). Notably,
miRNAs may have a modulatory role in oncogenic and tumor suppressor
pathways (25). Investigation of
miRNA activity in the human body may further develop the new and
promising therapies for the treatment and management of human
malignancies, including EOC.
The present study has demonstrated for the first
time that miR-133a is downregulated in the OVCAR-3 ovarian cancer
cell line and primary tumor samples. In addition, miR-133a was
found to reduce OVCAR-3 cell viability, promote cell apoptosis and
affect cell invasion and migration. Furthermore, the expression
levels of miR-133a were markedly associated with clinical and
pathological features, including tumor stage and grade, and lymph
node metastasis. These findings suggest that miR-133a may be a
useful target for therapeutic intervention and a biomarker for the
prediction of EOC progression and prognosis.
Previous studies have illustrated that miR-133a
plays a suppressive role in tumors. For example, ectopic miR-133a
has been reported to suppress cell growth in lung cancer (26), maxillary sinus squamous cell
carcinoma (27), tongue cancer
(18), esophageal cancer (16), prostate cancer (28), bladder cancer (29) and renal cell carcinoma (19). Additionally, miR-133a was found to
induce apoptosis in maxillary sinus squamous cell carcinoma
(27), tongue cancer (18), bladder cancer (29) and renal cell carcinoma (19). miR-133a may also inhibit cell
migration and invasion activities in esophageal cancer (16), prostate cancer (28), bladder cancer (29) and renal cell carcinoma (19). Furthermore, Wu et al revealed
that loss of expression of miR-133a was markedly associated with
tumor lymph node metastasis, advanced clinical stages and shortened
relapse-free survival in patients with breast cancer (30). Collectively, these results suggest
that miR-133a may be of vital importance in tumor initiation and in
the development and progression of malignancy.
Several targets of miR-133a have recently been
identified, including fascin homologue 1 (31), transgelin 2 (19,27),
purine nucleoside phosphorylase (27), actin-related protein 2/3 complex
subunit 5 (26), glutathione
S-transferase π 1 (26),
caveolin-1 (14), LASP1 (32) and pyruvate kinase M2 isoform
(18), using qPCR, western
blotting, reporter assays and bioinformatic prediction programs.
However, the molecular genetic basis of carcinogenesis and cancer
progression remains unclear. miRNAs may have a functional role,
according to a combinatorial circuit model, in which a single miRNA
may be used as the target of multiple mRNAs, and several
coexpressed miRNAs may target a single mRNA. Studies are likely to
remain far from unveiling all miR-133a targets, and the roles of
some of the potential targets in EOC carcinogenesis and progression
are poorly understood. Therefore, future research is required to
identify the targetome and the exhaustive roles of miR-133a in
ovarian cancer, based on this assumption.
In conclusion, the present study has demonstrated
that miR-133a is downregulated in epithelial ovarian cancer and
that decreased miR-133a expression is associated with advanced
clinical stage, poor histological differentiation and lymph node
metastasis. In addition, miR-133a plays a critical role in the cell
viability, apoptosis, invasion and migration of ovarian cancer
OVCAR-3 cells. These findings suggest that miR-133a may be a useful
biomarker for the prediction of ovarian cancer progression and a
potential promising target for gene therapy.
References
|
1
|
Hoskins WJ: Prospective on ovarian cancer:
why prevent? J Cell Biochem Suppl. 23:189–199. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clarke-Pearson DL: Clinical practice.
Screening for ovarian cancer. N Engl J Med. 361:170–177. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambros V: MicroRNA pathways in flies and
worms: growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar
|
|
4
|
Calin GA, Dumitru CD, Shimizu M, et al:
Frequent deletions and down-regulation of micro- RNA genes miR15
and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad
Sci USA. 99:15524–15529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song T, Xia W, Shao N, et al: Differential
miRNA expression profiles in bladder urothelial carcinomas. Asian
Pac J Cancer Prev. 11:905–911. 2010.PubMed/NCBI
|
|
6
|
Calin GA, Sevignani C, Dumitru CD, et al:
Human microRNA genes are frequently located at fragile sites and
genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar
|
|
7
|
Liu ZY, Zhang GL, Wang MM, Xiong YN and
Cui HQ: MicroRNA-663 targets TGFB1 and regulates lung cancer
proliferation. Asian Pac J Cancer Prev. 12:2819–2823.
2011.PubMed/NCBI
|
|
8
|
Fabian MR and Sonenberg N: The mechanics
of miRNA-mediated gene silencing: a look under the hood of miRISC.
Nat Struct Mol Biol. 19:586–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lou Y, Cui Z, Wang F, Yang X and Qian J:
miR-21 down-regulation promotes apoptosis and inhibits invasion and
migration abilities of OVCAR3 cells. Clin Invest Med.
34:E2812011.PubMed/NCBI
|
|
10
|
Kan CW, Hahn MA, Gard GB, et al: Elevated
levels of circulating microRNA-200 family members correlate with
serous epithelial ovarian cancer. BMC Cancer. 12:6272012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flavin R, Smyth P, Barrett C, et al:
miR-29b expression is associated with disease-free survival in
patients with ovarian serous carcinoma. Int J Gynecol Cancer.
19:641–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lou Y, Yang X, Wang F, Cui Z and Huang Y:
MicroRNA-21 promotes the cell proliferation, invasion and migration
abilities in ovarian epithelial carcinomas through inhibiting the
expression of PTEN protein. Int J Mol Med. 26:819–827.
2010.PubMed/NCBI
|
|
13
|
Yeh YM, Chuang CM, Chao KC and Wang LH:
MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis
by targeting SOX4 and HIF-1α. Int J Cancer. 15:867–878.
2013.PubMed/NCBI
|
|
14
|
Nohata N, Hanazawa T, Kikkawa N, et al:
Caveolin-1 mediates tumor cell migration and invasion and its
regulation by miR-133a in head and neck squamous cell carcinoma.
Int J Oncol. 38:209–217. 2011.PubMed/NCBI
|
|
15
|
Rao PK, Missiaglia E, Shields L, et al:
Distinct roles for miR-1 and miR-133a in the proliferation and
differentiation of rhabdomyosarcoma cells. FASEB J. 24:3427–3437.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kano M, Seki N, Kikkawa N, et al: miR-145,
miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in
esophageal squamous cell carcinoma. Int J Cancer. 127:2804–2814.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sarver AL, French AJ, Borralho PM, et al:
Human colon cancer profiles show differential microRNA expression
depending on mismatch repair status and are characteristic of
undifferentiated proliferative states. BMC Cancer. 9:4012009.
View Article : Google Scholar
|
|
18
|
Wong TS, Liu XB, Chung-Wai Ho A, Po-Wing
Yuen A, Wai-Man Ng R and Ignace Wei W: Identification of pyruvate
kinase type M2 as potential oncoprotein in squamous cell carcinoma
of tongue through microRNA profiling. Int J Cancer. 123:251–257.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawakami K, Enokida H, Chiyomaru T, et al:
The functional significance of miR-1 and miR-133a in renal cell
carcinoma. Eur J Cancer. 48:827–836. 2012. View Article : Google Scholar
|
|
20
|
Karseladze AI: WHO histological
classification of ovarian tumors. Geneva: 1999, Scully RE and Sobin
LH: Arkh Patol Suppl. pp. 1–64. 2005, (In Russian).
|
|
21
|
Odicino F, Pecorelli S, Zigliani L and
Creasman WT: History of the FIGO cancer staging system. Int J
Gynaecol Obstet. 101:205–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alley MC, Scudiero DA, Monks A, et al:
Feasibility of drug screening with panels of human tumor cell lines
using a microculture tetrazolium assay. Cancer Res. 48:589–601.
1988.
|
|
24
|
Jovanovic M and Hengartner MO: miRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Casalini P and Iorio MV: MicroRNAs and
future therapeutic applications in cancer. J BUON. 14(Suppl 1):
S17–S22. 2009.PubMed/NCBI
|
|
26
|
Moriya Y, Nohata N, Kinoshita T, et al:
Tumor suppressive microRNA-133a regulates novel molecular networks
in lung squamous cell carcinoma. J Hum Genet. 57:38–45. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nohata N, Hanazawa T, Kikkawa N, et al:
Identification of novel molecular targets regulated by tumor
suppressive miR-1/miR-133a in maxillary sinus squamous cell
carcinoma. Int J Oncol. 39:1099–1107. 2011.PubMed/NCBI
|
|
28
|
Kojima S, Chiyomaru T, Kawakami K, et al:
Tumour suppressors miR-1 and miR-133a target the oncogenic function
of purine nucleoside phosphorylase (PNP) in prostate cancer. Br J
Cancer. 106:405–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshino H, Chiyomaru T, Enokida H, et al:
The tumour-suppressive function of miR-1 and miR-133a targeting
TAGLN2 in bladder cancer. Br J Cancer. 104:808–818. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu ZS, Wang CQ, Xiang R, et al: Loss of
miR-133a expression associated with poor survival of breast cancer
and restoration of miR-133a expression inhibited breast cancer cell
growth and invasion. BMC Cancer. 12:512012. View Article : Google Scholar
|
|
31
|
Chiyomaru T, Enokida H, Tatarano S, et al:
miR-145 and miR-133a function as tumour suppressors and directly
regulate FSCN1 expression in bladder cancer. Br J Cancer.
102:883–891. 2010. View Article : Google Scholar
|
|
32
|
Chiyomaru T, Enokida H, Kawakami K, et al:
Functional role of LASP1 in cell viability and its regulation by
microRNAs in bladder cancer. Urol Oncol. 30:434–443. 2012.
View Article : Google Scholar : PubMed/NCBI
|