Introduction
Pancreatic neoplasms usually display ductal, acinar
or neuroendocrine differentiation. Pancreatic neuroendocrine tumors
(pNETs), previously termed pancreatic endocrine neoplasms, are
relatively uncommon and constitute only 5–8% of all pancreatic
neoplasms. According to The World Health Organization (WHO)
classification (2010), NETs are classed as NET G1 (carcinoid,
mitotic count <2 per 10 high power fields (HPF) and/or ≤2% Ki67
index), NET G2 (mitotic count 2–20 per 10 HPF and/or 3–20% Ki67
index), NET G3 (neuroendocrine carcinoma, mitotic count >20 per
10 HPF and/or >20% Ki67 index), and mixed adenoneuroendocrine
carcinoma (MANEC) (1,2). When neuroendocrine cells comprise
>30% of the tumor, the lesion is classified as MANEC.
The current study presents an extremely rare case of
pancreatic body cancer that was morphologically diagnosed as an
adenocarcinoma, but with a growth and a form of recurrence that
were atypical and indicated an MANEC, as NET components were
detected immunohistochemically.
Case report
The patient was a 61-year-old female found to have a
tumor (2 cm in diameter) in the pancreatic body during
ultrasonography, which was performed for the purpose of monitoring
chronic hepatitis B. The patient was referred to Kanazawa
University Hospital (Kanazawa, Japan) with a presumed diagnosis of
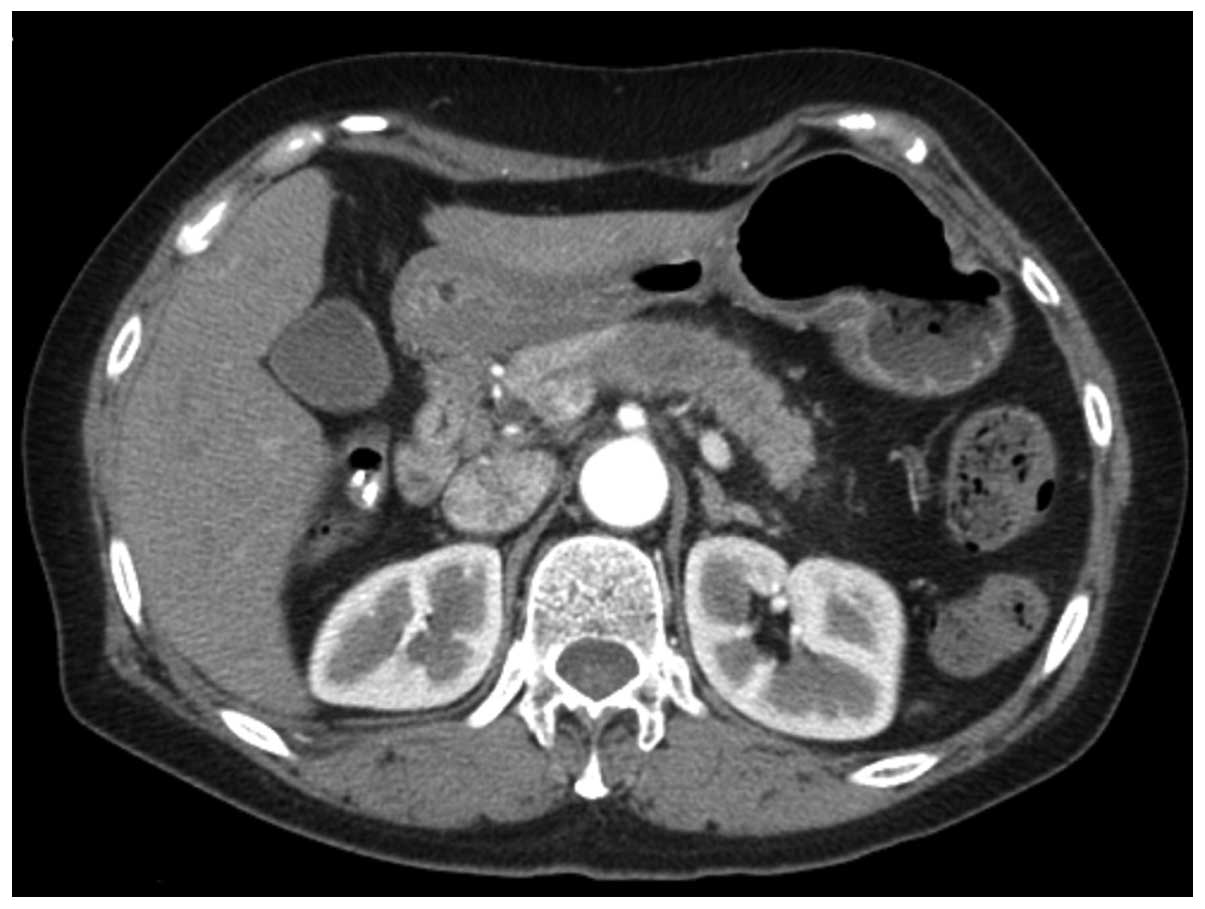
pancreatic body cancer based on computed tomography (CT) imaging
(Fig. 1). Following pre-operative
chemotherapy with gemcitabine (GEM; 800 mg/m2) and oral
S-1 (30 mg/m2), as previously reported (3), the patient underwent a pancreatic body
and tail resection with common hepatic and celiac artery resection,
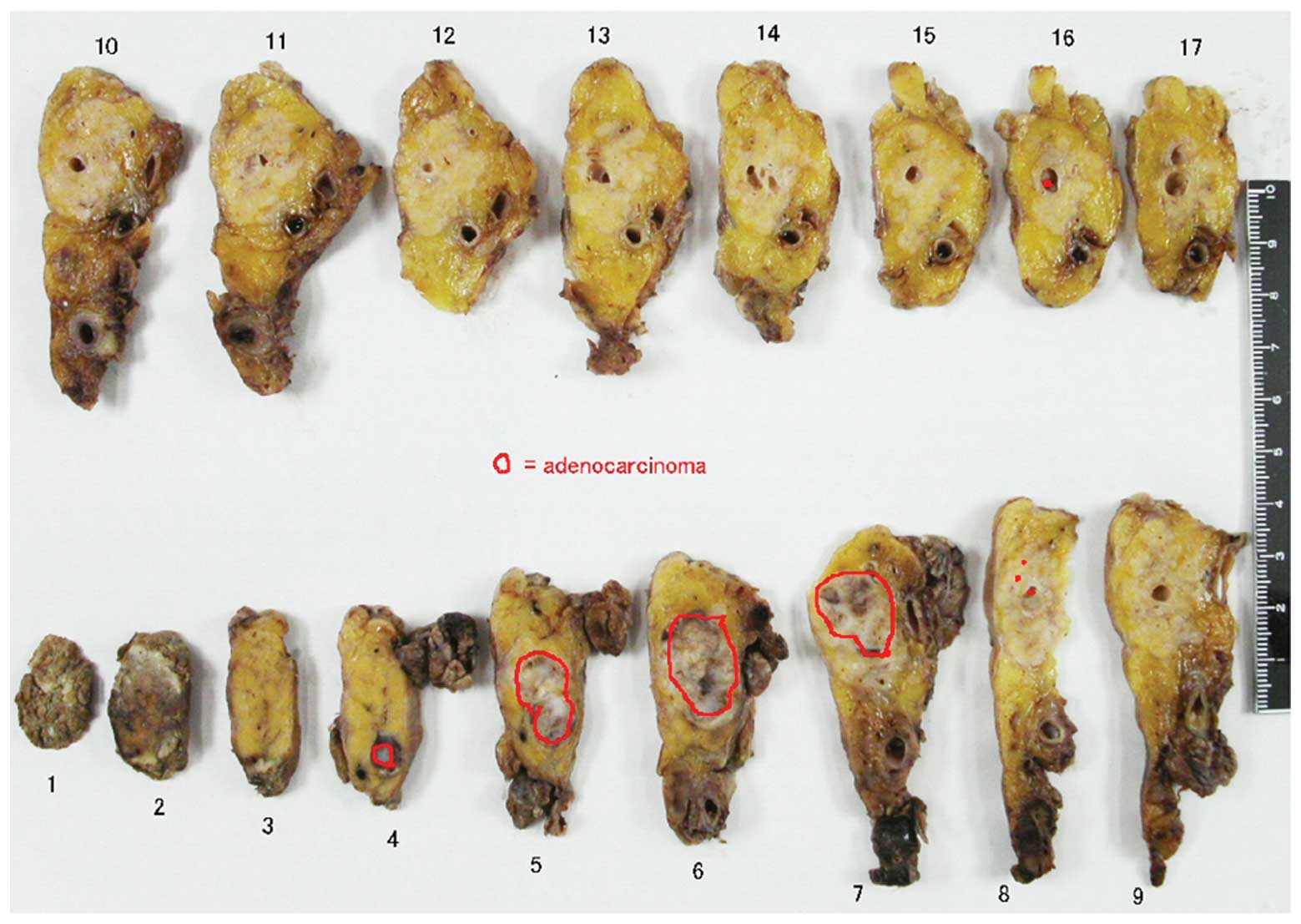
also known as Appleby’s operation. Upon microscopic analysis, the
tumor was diagnosed as a moderately- to poorly-differentiated
adenocarcinoma. Tumor growth filled the dilated main pancreatic
duct (MPD) and infiltrated the surrounding area, but neither
serosal nor retroperitoneal invasion were detected, and there were
no lymph node metastases (Figs. 2
and 3). Furthermore, necrosis was
observed in 1/4 to 1/3 of the tumor cells and was considered to
reflect the low efficacy of pre-operative chemotherapy-compatible
grade IIA tumors, as judged by the Evans staging system (4). A piece of tumor tissue was found in
the MPD on the tail side away from the main tumor, but it was
diagnosed as an artifact at the time of surgery. The pathological
diagnosis was T2, N0, M0, Stage IB, with an R0 resection, and the
post-operative course was uneventful.
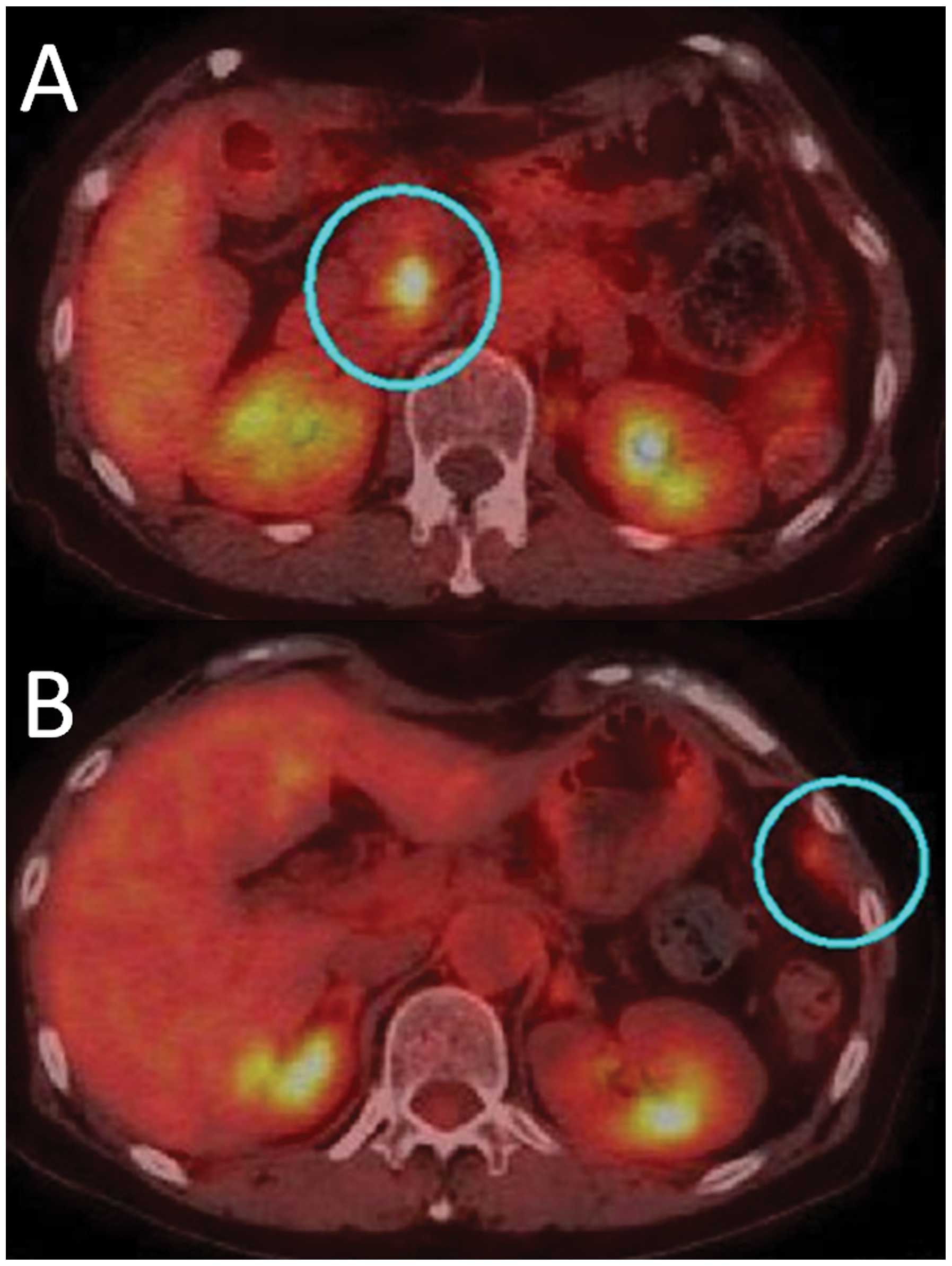
CT and positron emission tomography with
18-fluorodeoxyglucose (FDG-PET) performed 6 months later revealed
metastasis to the left diaphragm and near the MPD in the remaining
pancreatic head (Fig. 4). The
patient underwent GEM and oral S-1 chemoradiotherapy, with the same
ergimen as with the preoperative therapy with 50 Gy radiation to
the pancreatic head, but liver metastases appeared 8 months later.
Subsequently, the chemotherapy regimen was changed to docetaxel (30
mg/m2, biweekly), but the effect was minimal and the
patient succumbed 22 months after the surgery. An autopsy
demonstrated that a moderately- to poorly-differentiated
adenocarcinoma had arisen from the pancreatic head and infiltrated
the duodenum and bile duct. Furthermore, huge liver metastases
formed a subphrenic abscess and there were multiple peritoneal
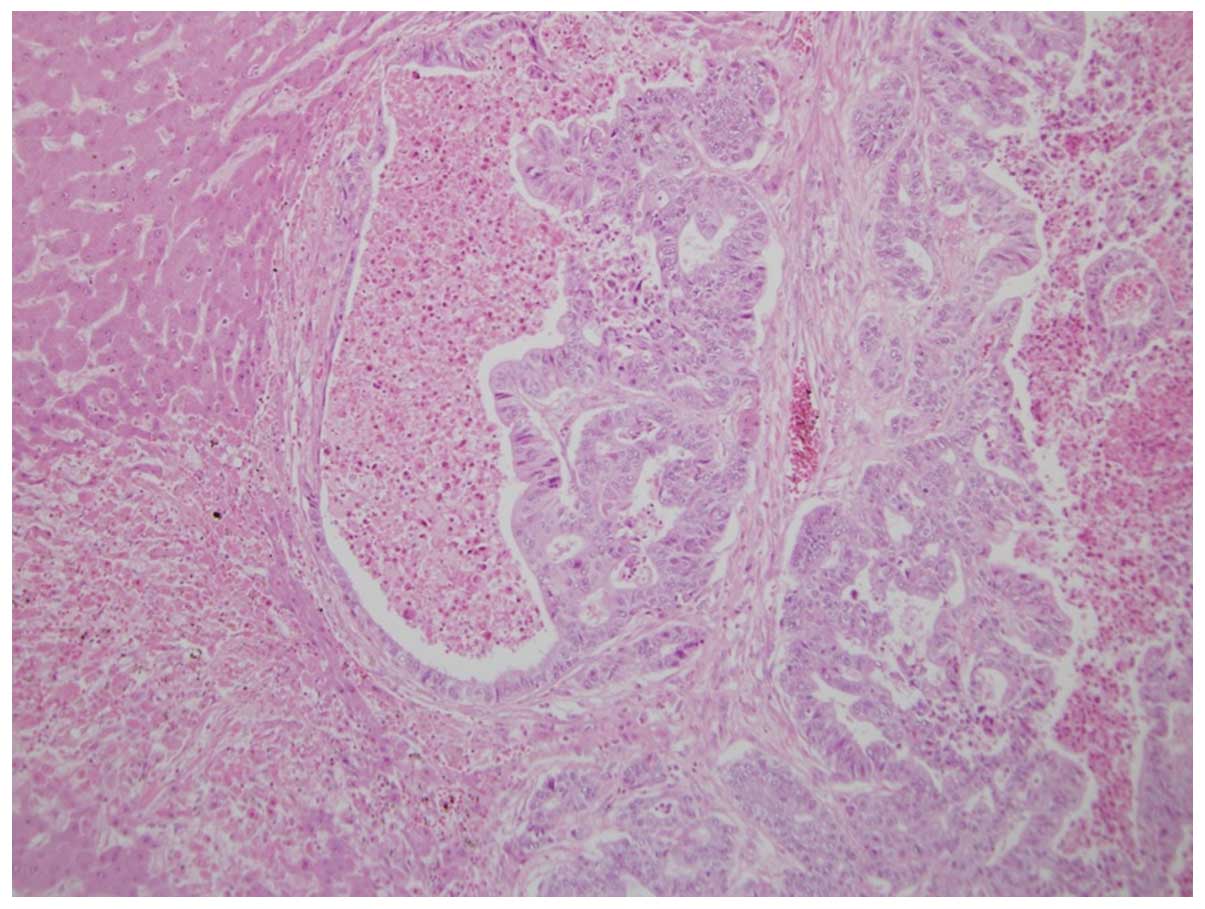
disseminations (Fig. 5). Two
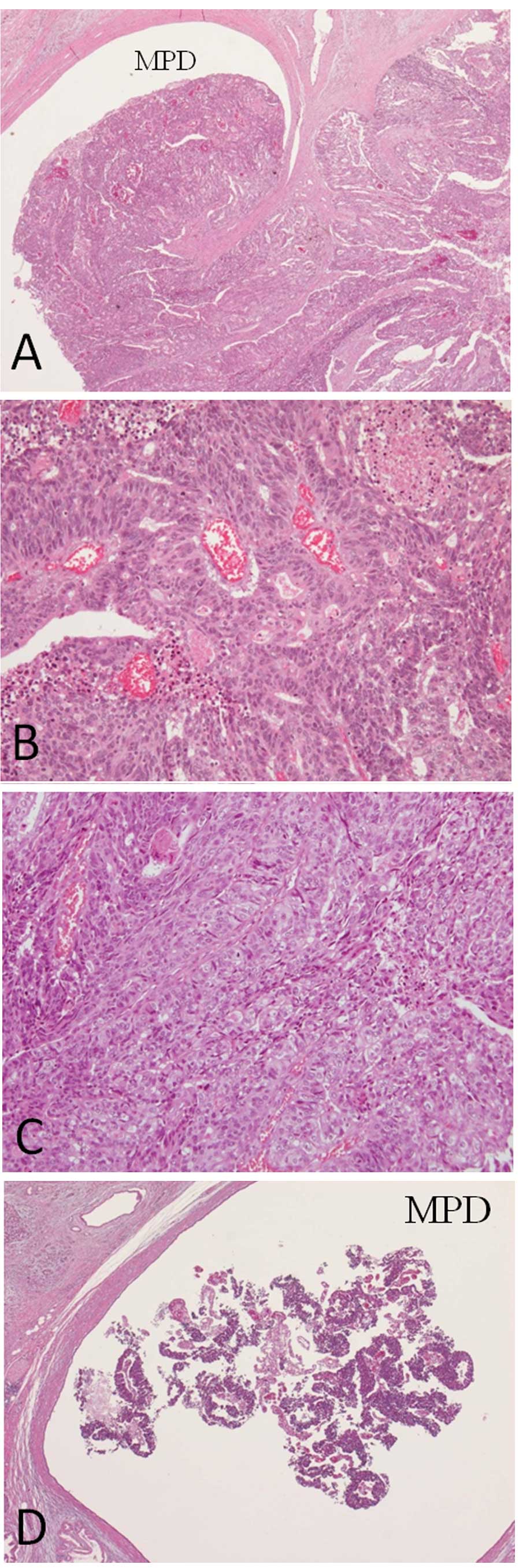
distinct portions were identified microscopically; one portion had
a pseudo-rosette appearance with adenocarcinoma components, while
the other portion showed immunohistochemical characteristics
consistent with a NET, possibly an adenoneuroendocrine carcinoma.
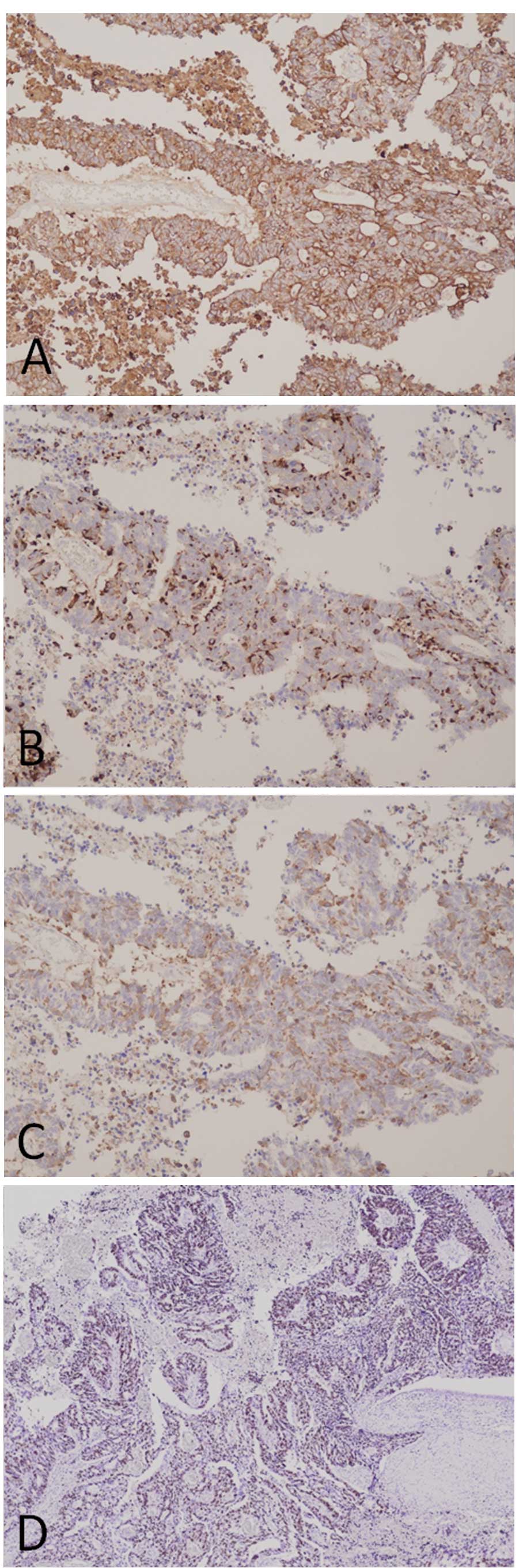
The original surgically-resected tumor also exhibited
adenocarcinoma and NET characteristics immunohistochemically.
Cytokelatin-7, a marker for pancreatic adenocarcinoma, is positive
in almost all tumor cells. Additionally, in the same lesion,
neuroendocrine markers, chromogranin A and synapthophysin, were
positive. Futhermore, the Ki67 index was particularly high
(Fig. 6). There is a possibility,
given the recurrence in the MPD of the remaining pancreatic head
and non-contiguous main tumor growth, that this was either a caudal
pancreatic duct tumor, often pathologically diagnosed for the first
time, or a transitional lesion demonstrating a specific form of
growth. Written informed consent was obtained from the patient’s
family.
Discussion
Neuroendocrine neoplasms, including carcinoid
tumors, are commonly found in several organs, including the
pancreas and gastrointestinal tract. The World Health Organization
(WHO) classification (2000) (2) has
been widely used to categorize NETs for all anatomical sites, and
these tumors are histologically and biologically classified as
well-differentiated NETs (low-grade malignancy),
poorly-differentiated NETs (high-grade malignancy) and MANECs. The
2010 WHO classification strengthened the understanding of three
tumor components; heterogeneity, differentiation and
malignancy.
MANECs are rare and by definition are those
neoplasms in which each component represents ≥30% of the lesion
(1,2). In the 2010 WHO classification of
tumors of the digestive tract, mixed exocrine-neuroendocrine
carcinomas were defined as MANECs. They are morphologically
recognizable as gland-forming epithelial and neuroendocrine
neoplasms, and defined as carcinomas, since their components are
histologically malignant. Notably, adenocarcinomas with scattered
neuroendocrine cells, revealed by immunohistochemistry, cannot be
categorized as MANECs nor neuroendocrine neoplasms with a focal
non-neuroendocrine component. In such cases, almost all the tumor
cells, which were recognized as adenocarcinoma morphologically, had
NEC characteristics immunohistochemically.
For the treatment of unresectable pNETs, the
National Comprehensive Cancer Network (NCCN) guidelines recommend
everolimus, sunitinib, hepatic regional therapy, cytoreductive
surgery, octreotide and cytotoxic chemotherapeutic agents,
including capecitabine, dacarbazine, doxorubicin, 5-FU,
streptozotocin and temozolomide. On the other hand, for
poorly-differentiated NECs of the pancreas, the NCCN guidelines and
North American Neuroendocrine Tumor Society consensus guidelines
(6) recommend combined chemotherapy
with cisplatin and etoposide, one of the standard regimens employed
for the treatment of small cell lung cancer. In addition, the
efficacies of GEM and oral S-1 (7),
bevacizumab (8), folinic acid,
fluorouracil and irinotecan (9),
valproic acid (10) and other
treatments have also been reported.
For the present patient, GEM plus S-1 was
administered pre-operatively, and a modest histopathological effect
was observed. However, this regimen and the use of docetaxel were
ineffective for the recurrent lesions. Therefore, there is a
possibility that the necrosis observed in the resected specimen
reflected properties of the tumor rather than the effects of
pre-operative chemotherapy. Moreover, a small solitary tumor of the
distal MPD, diagnosed as an artifact of the resected specimen, was
recognized as a metastasis in retrospect. Had the tumor been shown
to contain NEC components antemortem, alternative drugs could have
been administered. In conclusion, it is necessary to search for the
presence of NET characteristics in cases of pancreatic
adenocarcinoma with a non-specific form of growth and/or an unusual
clinical course. If the tumor contains NET components, there is a
possibility of using other drugs for treatment.
References
|
1
|
Rindi G, Klimstra DS, Arnold R, et al:
Nomenclature and classification of neuroendocrine neoplasms of the
digestive system. WHO Classification of Tumours of the Digestive
System. Bosman FT, Carneiro F, Hruban RH and Theise ND: 4th
edition. IARC Press; Lyon: pp. 13–14. 2010
|
|
2
|
Komminoth P, Arnold R, Capella C, et al:
Neuroendocrine neoplasms of the gallbladder and extrahepatic bile
duct. WHO Classification of Tumours of the Digestive System. Bosman
FT, Carneiro F, Hruban RH and Theise ND: 4th edition. IARC Press;
Lyon: pp. 274–276. 2010
|
|
3
|
Tajima H, Ohta T, Kitagawa H, et al: Pilot
study of neoadjuvant chemotherapy with gemcitabine and oral S-1 for
resectable pancreatic cancer. Exp Ther Med. 3:787–792.
2012.PubMed/NCBI
|
|
4
|
Evans DB, Rich TA, Byrd DR, et al:
Preoperative chemoradiation and pancreaticoduodenectomy for
adenocarcinoma of the pancreas. Arch Surg. 127:1335–1339. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Capella C, Solcia E, Sobin LH and Arnold
R: Endocrine tumours of the gallbladder and extrahepatic bile
ducts. WHO Classification of Tumours of the Digestive System.
Hamilton SR and Aaltonen LA: IARC Press; Lyon: pp. 214–216.
2000
|
|
6
|
Strosberg JR, Coppola D, Klimstra DS, et
al; North American Neuroendocrine Tumor Society (NANETS). The
NANETS consensus guidelines for the diagnosis and management of
poorly differentiated (high-grade) extrapulmonary neuroendocrine
carcinomas. Pancreas. 39:799–800. 2010. View Article : Google Scholar
|
|
7
|
Yamamoto M, Miyagawa K, Hiura M, et al:
Poorly differentiated neuroendocrine carcinoma of the pancreas
responsive to combination therapy with gemcitabine and S-1.
Internal Med. 51:727–732. 2012. View Article : Google Scholar
|
|
8
|
Kasuya K, Nagakawa Y, Suzuki M, et al:
Anti-vascular endothelial growth factor antibody single therapy for
pancreatic neuroendocrine carcinoma exhibits a marked tumor
growth-inhibitory effect. Exp Ther Med. 2:1047–1052. 2011.
|
|
9
|
Brixi-Benmansour H, Jouve JL, Mitry E, et
al: Phase II study of first-line FOLFIRI for progressive metastatic
well-differentiated pancreatic endocrine carcinoma. Dig Liver Dis.
43:912–916. 2011. View Article : Google Scholar
|
|
10
|
Mohammed TA, Holen KD, Jaskula-Sztul R, et
al: A phase II study of valproic acid for treatment of low-grade
neuroendocrine carcinoma. Oncologist. 16:835–843. 2011. View Article : Google Scholar : PubMed/NCBI
|