Introduction
Low-grade fibromyxoid sarcoma (LGFMS) is a
distinctive variant of fibrosarcoma (1). LGFMS is a rare soft tissue tumor that
tends to develop in the deep soft tissue of young adults and has
the potential for local recurrence or distant metastasis (2). The majority of LGFMSs occur in a
subfascial location, however, on rare occasions the subcutis or
dermis may be affected (3). These
fibroblastic tumors typically occur in the lower limb/groin area,
however, sporadically occur in other deep soft tissues. In a
previous study by Evans (4), there
was an even distribution of male and female patients, yet a marked
male preponderance was observed. The ages ranged between six and 51
years, although the majority were young adults (ages, 25–46 years).
The majority of the LGFMSs were well-circumscribed but not
encapsulated, therefore, the resections were often incomplete
(5). This study was approved by the
ethics committee of Vivekanand Polyclinic and Institute of Medical
Sciences (Lucknow, India).
Case report
A 22-year-old male was admitted to the Department of
Orthopaedics (Vivekanand Polyclinic and Institute of Medical
Sciences, Lucknow, India) complaining of a slow growing painless
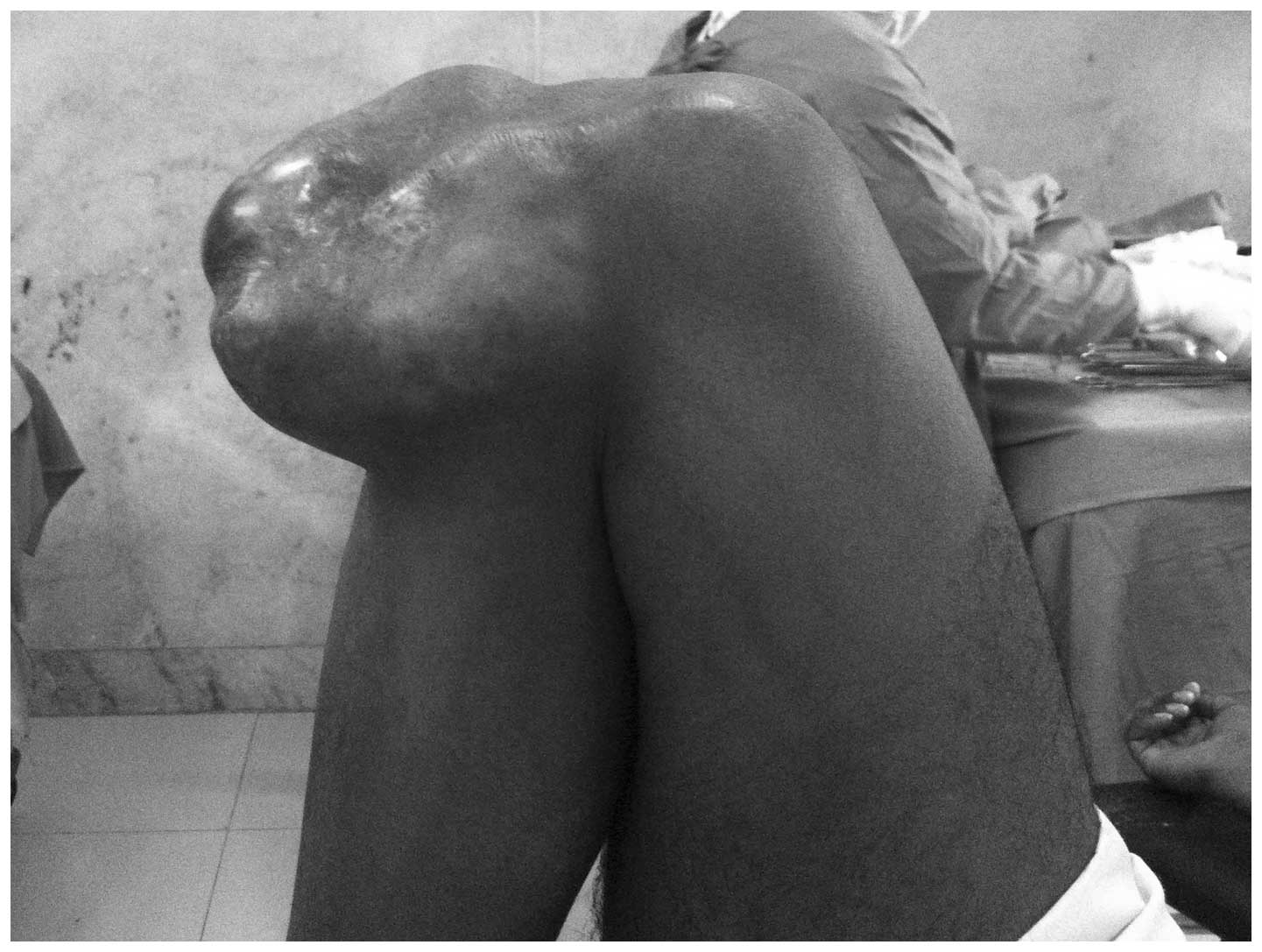
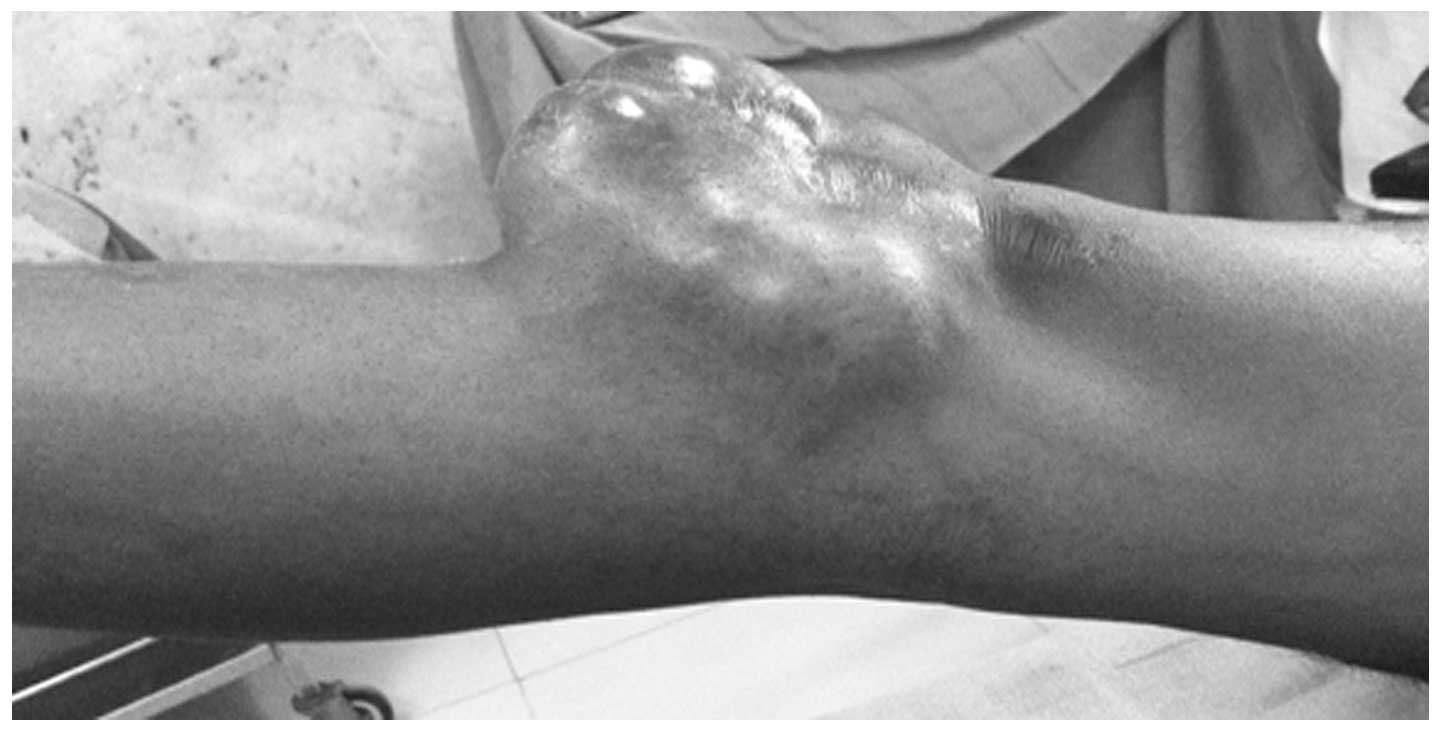
mass in the right knee that had developed over a period of 10
years. Physical examination demonstrated a mass on the anterior
medial aspect of the right knee, which was not tender or mobile,
however was rubbery and hard in consistency. Full flexion and
extension was observed without any restriction of joint movement
(Figs. 1 and 2).
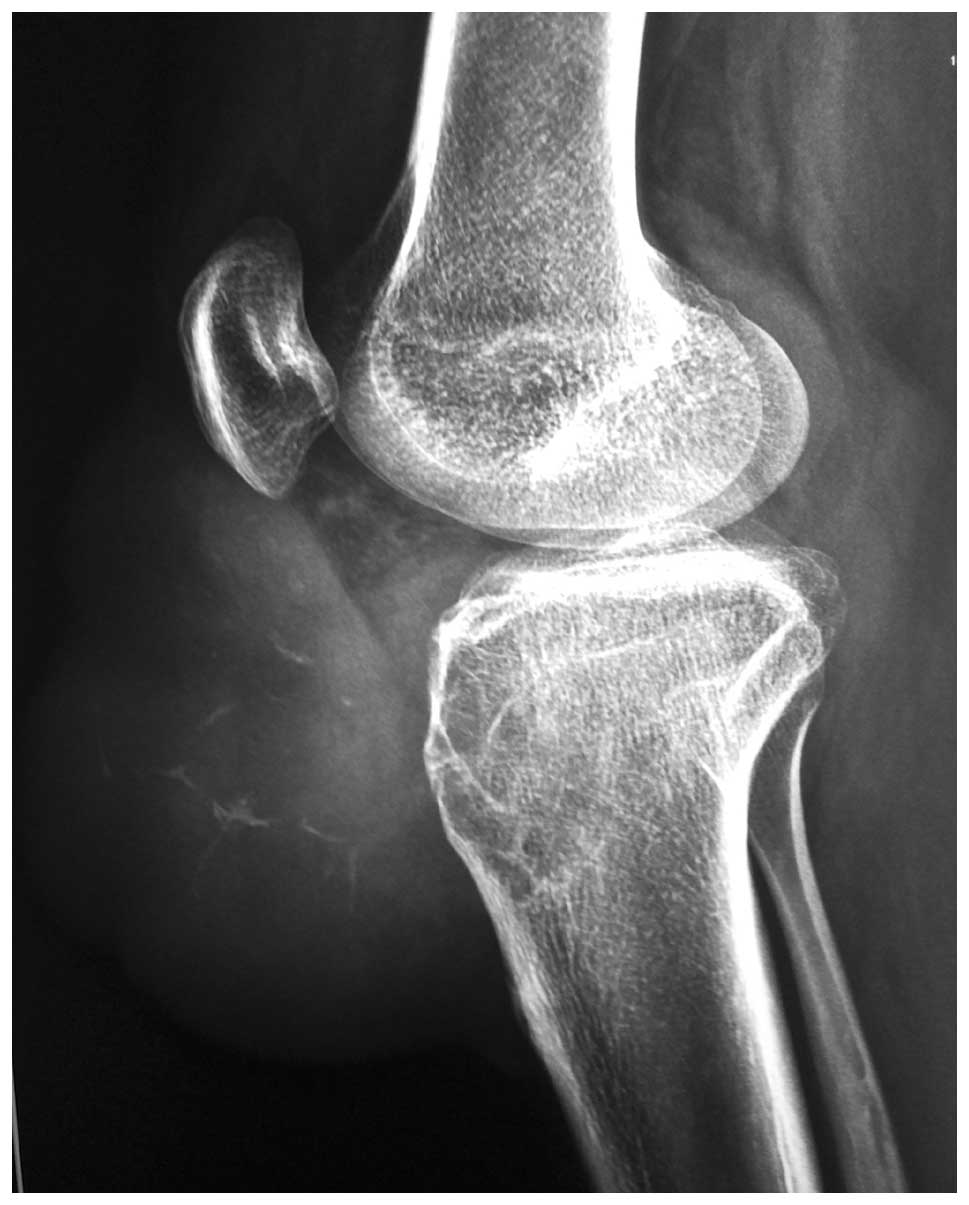
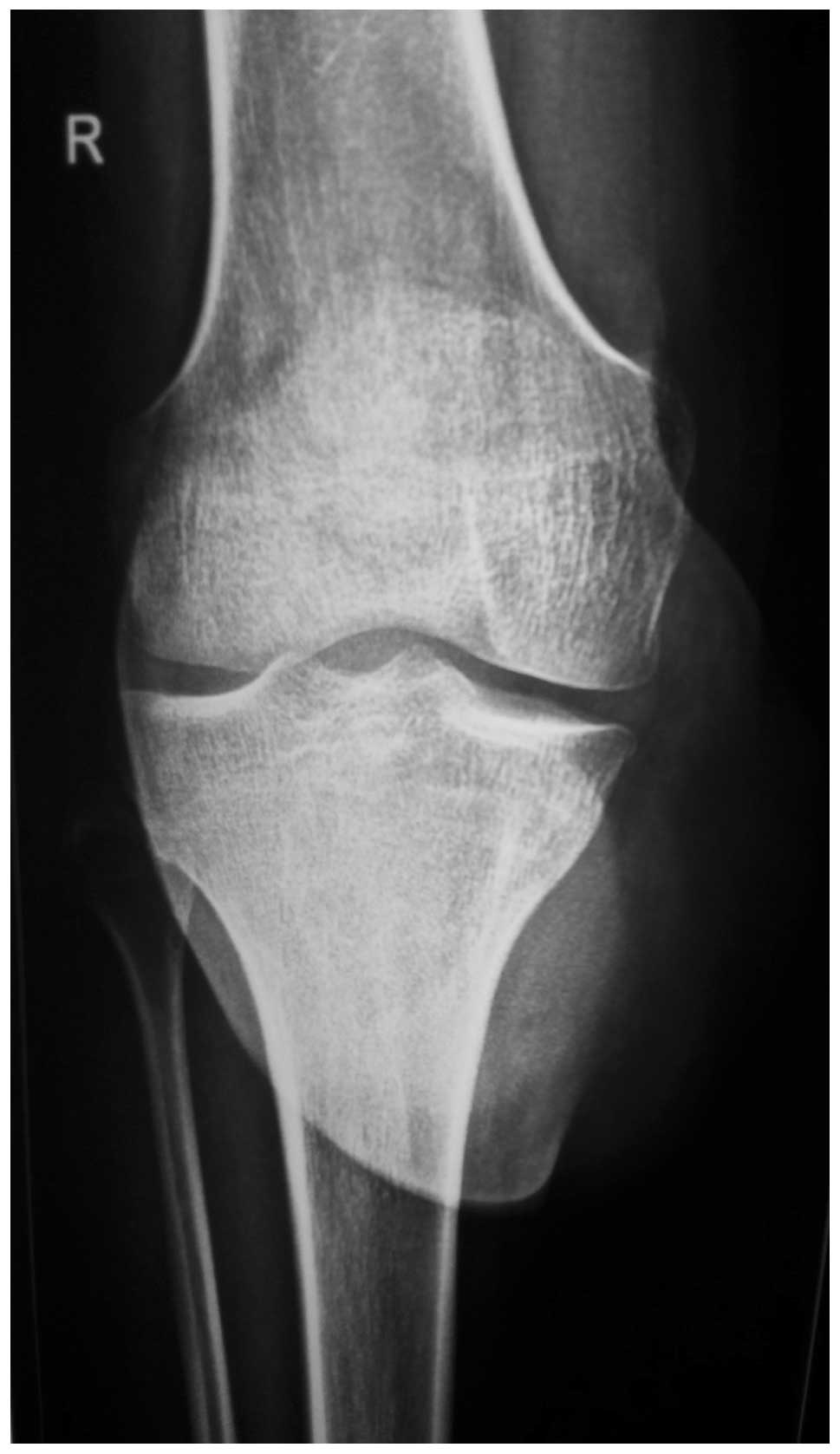
The laboratory investigations were unremarkable. A
plain radiograph showed a lytic lesion in the anteromedial proximal
tibia and a large soft tissue shadow presenting with slight
calcification (Figs. 3 and 4).
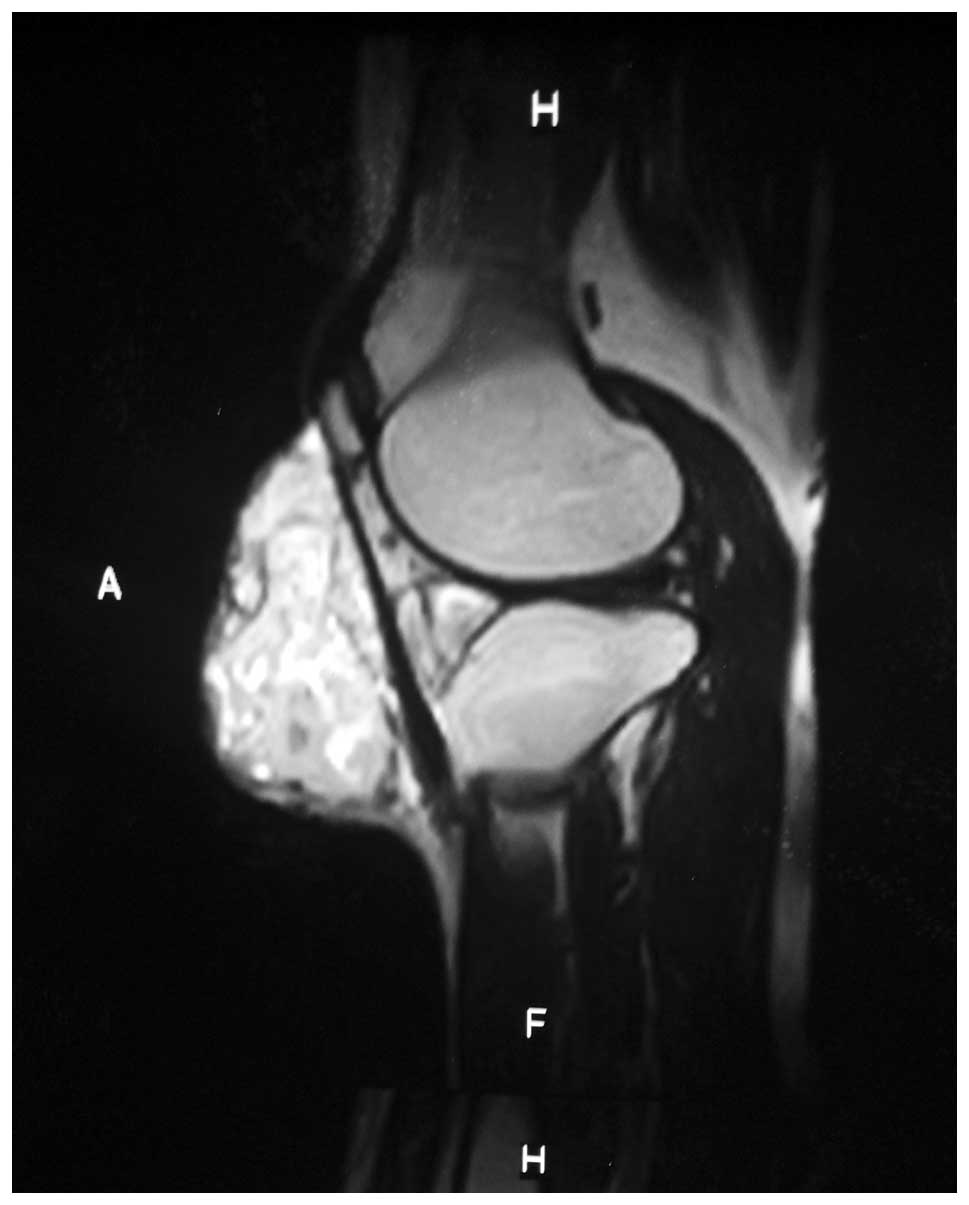
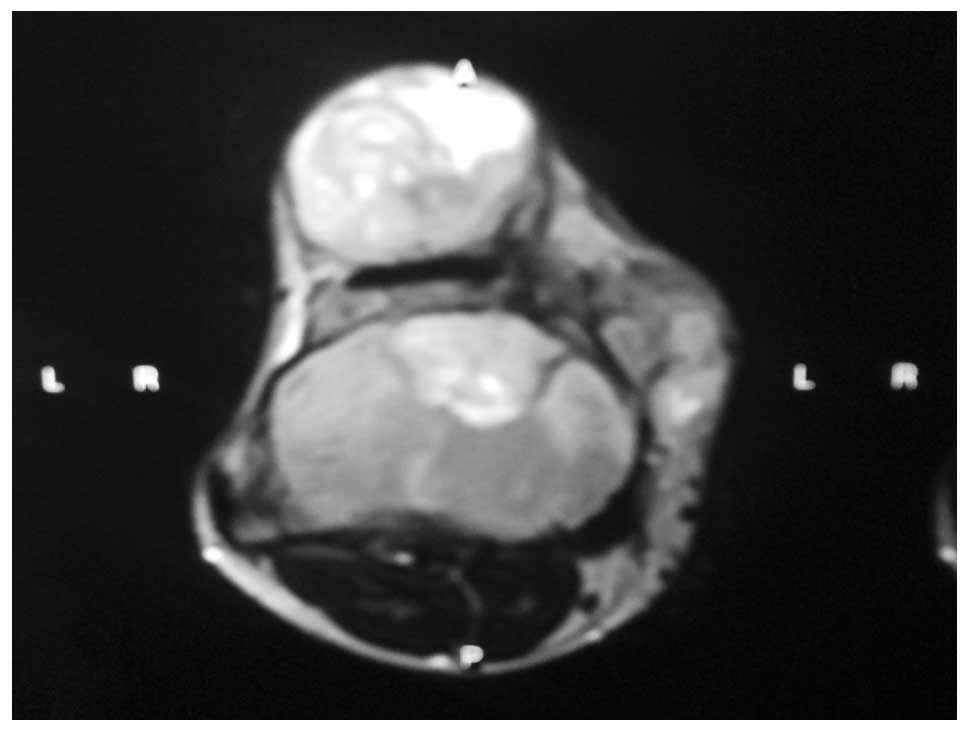
Magnetic resonance imaging (MRI) revealed that the
tumor was 14×12×9 cm in size, incorporated the periosteum and
anteromedial aspect of the tibia, and was beneath the synovium and
periosteum of the anterior margin of the patella. The MRI
evaluation of the area also demonstrated a mixed myxoid and fibrous
pattern within the tumor. The tumor matrix was partially calcified
and relatively well-defined with an irregular low signal intensity
on the T1-weighted image and heterogeneous low and high signals on
the T2-weighted image (Figs. 5 and
6).
Subsequently, the patient underwent a wide biopsy to
acquire additional information prior to surgical resection of the
mass. Histopathological and immunohistochemical evaluations were
performed to examine the neoplastic cells and a diagnosis was
confirmed. As a result of the clinical and radiographic
observations, malignancy was not ruled out and a low-grade
mesenchymal neoplasm was considered.
A wide excision of the tumor was performed four
weeks later. The mass was well-circumscribed, however, it was not
encapsulated, thus, there was uncertainty with regard to the
surgical margins of the resection. Thus, 5-cm excisions of the
proximal tibia from the tumor margins, patella, synovium, muscles
and patellar tendon surrounding the tumor mass were obtained, and
sent for histopathological examination.
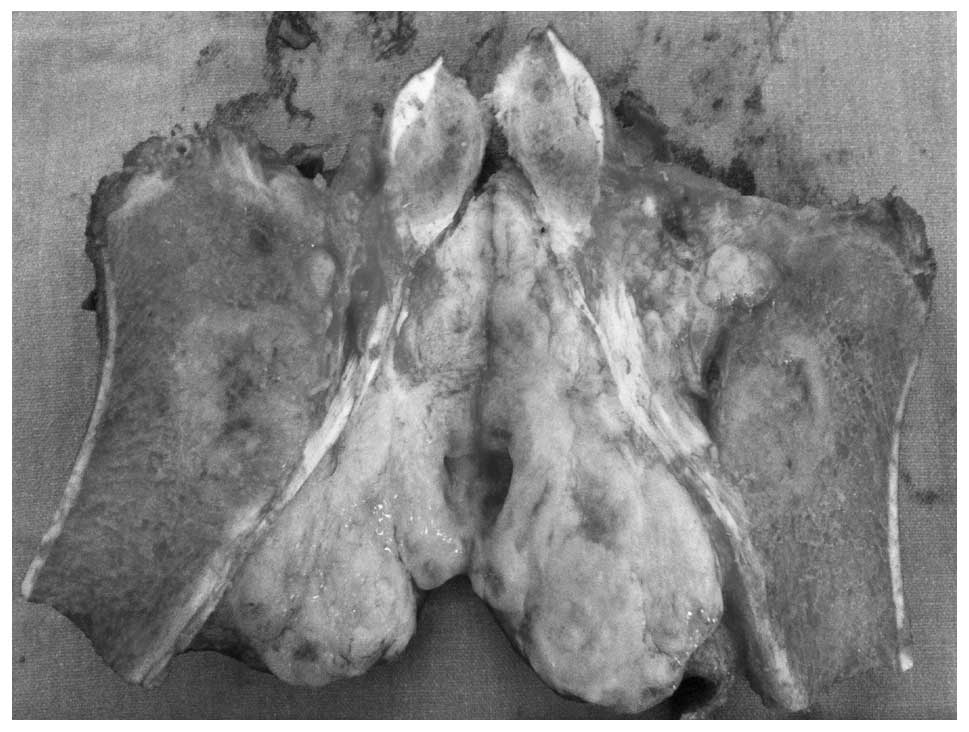
The cut (excised) section of the gross tumor showed
a well-circumscribed, lobulated, round and firm mass. The excised
surface tissue was yellow-white in color with a focal gelatinous
appearance and an elastic consistency; furthermore there was a
viscous seromucinous fluid. In addition, the excised surface was
fibrous to myxoid without any areas of hemorrhaging or necrosis. A
gritty sensation was experienced while cutting the tumor, which
invoved the periosteum, the antero-medial aspect of proximal tibia
and the inferior pole of patella (Fig.
7).
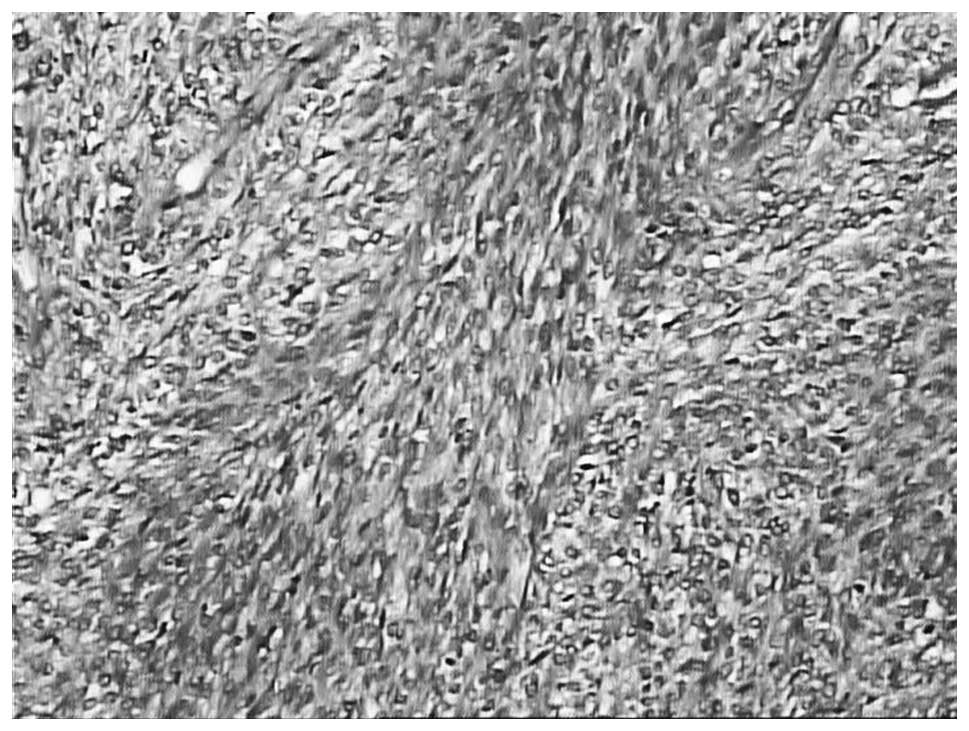
Microscopic examination of the tumor identified
alternating fibrous and myxoid components; the myxoid area
demonstrated tumor cells in a whirling growth pattern with
capillary-sized blood vessels (Fig.
8).
The proximal fibula (~6 cm) was removed as docking
was to be performed on the distal femur, for which a notch was
created in the intercondylar area to accommodate the tibia. The
distal femur and tibia were stabilized using two Kirschner-wires,
and bone grafting was performed using the femoral condyles
following the removal of the articular cartilage.
The limb was stabilized via Ilizarov fixation
followed by corticotomy of the distal tibia with distal
fibulectomy, as shortening of >6 cm was identified following
arthrodesis of the knee joint and limb length preservation was a
predominant concern for the patient. No perforation of the vessels
or nerves was evident during the mass removal. Following surgery,
the patient experienced no major complications and the
neurovascular status of the patient remained intact. At present,
the patient has been regularly followed up for five years; every
three months for the first year, six monthly for the following two
years and annually for the last two years. No clinical or
radiological evidence has been identified indicating recurrence or
metastasis.
Discussion
LGFMS is a distinctive variant of fibrosarcoma with
a high metastasizing potential and, occasionally, long intervals
between tumor presentation and metastasis are observed (1). LGFMS is recognized as an uncommon soft
tissue neoplasm (6). These tumors
have previously been described in numerous locations, including the
chest wall, axilla, shoulder, inguinal region, buttocks, neck and
thigh (7). Although a large LGFMS
has been reported to have arisen from the abdominal wall of the
falciform ligament (8), LGFMS has
not previously been reported around the knee, and involved the
proximal end of the tibia, synovium, periosteum of the patella and
patellar tendon.
Originally, LGFMS was reported by Evans in 1987
(1), who subsequently described 10
additional cases in 1993 (4). Thus
far, a few sporadic case reports and series have been reported
(9). The predominant pathologies to
be considered in a differential diagnosis include desmoid
fibromatosis, peripheral nerve sheath tumor myxoid liposarcoma,
spindle cell liposarcoma and low-grade myxofibrosarcoma (MFS)
(5).
Macroscopically, LGFMS is a well-circumscribed and
encapsulated mass, indicative of a benign lesion. The excised
surfaces of such tumors exhibit a firm, fibrous, yellow-white
appearance with glistening areas secondary to the accumulation of a
myxoid ground substance (1).
The microscopic appearance of LGFMS is somewhat
variable, as the name indicates, consisting of bland fibroblasts
with a whorled or linear arrangement, alternating between less
cellular areas and myxoid stroma. The tumor cells tend to be small,
with poorly defined, pale eosinophilic cytoplasms and round or
ovoid nuclei. Nucleoli are absent or indistinct and the mitotic
figures tend to be absent or sparse. In addition, nuclear anaplasia
and necrosis are generally absent, although, one case in a study by
Evans were identified as ‘dedifferentiated’, in a 30-year
follow-up, with sheets of anaplastic rounded cells (4).
Immunohistochemical staining for LGFMS is only
positive for vimentin and is negative for a variety of antibodies,
including desmin, keratin, S100 protein, epithelial membrane
antigen, CD34 and CD31. Muscle-specific actin is positive in the
wall of small vessels within the tumor and markedly positive in the
peripheral fibrous layer (11). Ten
years after the first description of LGFMS by Evans (1), a similar pathological entity,
hyalinizing fibrosarcoma with giant rosettes (HSTGR), was
identified. In addition, a following cytogenetic report
demonstrated an identical recurrent t(7;16)(q34;p11) translocation
and fusion between the FUS and CREB3L2 genes in LGFMS and HSTGR,
which was confirmed by multiple studies regarding these subtypes of
fibrosarcoma, thereby providing genetic proof that these two tumors
are variants of the same entity (12). Furthermore, the FUS/CREB3L1 fusion
transcripts of LGFMS may be reliably detected in paraffin-embedded
tissues using reverse transcription-polymerase chain reaction
(13).
LGMFS must be distinguished from MFS, the most
common sarcoma affecting the limbs of elderly patients and its
high-grade end of the spectrum is considered to be a myxoid variant
of malignant fibrous histiocytomas (MFH) by previous authors
(14,15). MFS comprises of a wide morphological
spectrum; high-grade lesions tend to form solid sections with a
continuous transition to a storiform-pleomorphic type MFH.
Continuity between high- and low-grade areas of MFS has previously
been indicated by the presence of solid high-grade components
within low-grade tumors, as well as the progression of a subset of
low-grade MFS into high-grade tumors in local recurrences (16). Owing to its ultrastructural
features, which closely resemble ordinary fibroblasts, previous
studies have favored the nomenclature of MFS instead of myxoid MFH.
MFS was regarded as a distinct fibroblastic neoplasm that is
characterized by a myxoid nodular appearance, and curvilinear
vasculatures with a considerably broad spectrum of nuclear
pleomorphism, cellularity and mitoses (17).
Diagnosis of LGFMS or HSTGR is not difficult if the
tumor is removed completely, and sent for histopathological and
immunohistochemical staining, demonstrating characteristic
morphological and immunophenotypic features as previously
described. For an exact diagnosis, it is recommended that the
patient receives an open or excisional biopsy as occasionally,
material obtained from a fine-needle aspiration or core biopsy is
not sufficient and leads to misdiagnosis. If a myxoid pattern is
present, the patient must be referred to a cytogenetics department
to exclude rare LGFMS (3).
Although imaging observations of LGFMS are
non-specific, certain computed tomography (CT) and MRI observations
have previously been described. On non-contrast CT images, the
fibrous component of these tumors has been described as isodense or
muscle tissue and the myxoid component has been described as
hypodense (18). Several previous
case reports described the variable MRI observations in patients
with LGFMS (19). In general,
fibrous tissue components appear hypointense on T1- and T2-weighted
images and show marginal enhancement. Myxoid tissue components are
hypointense on T1-weighted images, hyperintense on T2-weighted
images and show variable enhancement on contrast-enhanced
T1-weighted images (18).
Occasionally, calcification may also be identified within the
tumors (11). Fibromatosis is
usually hypointense on T2-weighted images, however, may also show
heterogeneous hyperintensity, reflecting marked cellularity or
myxoid tissue. In addition, neurogenic tumors are characterized by
the entry and exit of nerves (19).
The clinical presentation of LGFMS is usually
long-standing and is predominantly associated with the anatomic
location of the mass. LGFMS usually presents as a painless soft
tissue mass with a prebiopsy duration of more than five years in
15% of patients that are diagnosed with benign lesions and local
recurrence in 68%, metastasis in 41% and mortality in 18% of the
patients (20). In an additional
study, the patients exhibited a recurrence rate of 54%, metastasis
rate of 6% and mortality rate of 2%. In the same study, the
presence of focal areas of high cellularity, nuclear enlargement,
increased mitotic activity and necrosis were found to be of no
prognostic significance for recurrence or metastasis (21).
LGFMS has previously been reported to recur and
metastasize, and has been found to recur as early as six months to
as late as 50 years in 65% of patients. Metastasis has also been
reported to occur frequently, with the lungs as a common site.
These tumors have a protracted course even subsequent to metastasis
(8).
Once the diagnosis of LGFMS or HSTGR has been
determined, a full oncological assessment is required. This must
include a CT scan of the chest as metastasis to the lung is the
most common scenario. Due to the high risk of late metastasis, a
clinical follow-up and chest imaging must be performed for an
extended period of time. However, how regularly imaging of the
chest must be repeated remains unclear (3). The current study presented a case of
rare LGFMS around the knee, involving the proximal end of the
tibia, synovium, periosteum of the inferior pole of the patella and
the patellar tendon. The patient was treated with wide excision (5
cm) of the proximal tibia and fibula from the tumor margin, along
with the patella and synovium. This was followed by docking of the
tibia, for which a notch was created in the intercondylar area of
the distal femur. In addition, Ilizarov fixation was used to
stabilize the limb. Fibulectomy and corticotomy was performed to
maintain limb length.
In conclusion, little is known concerning LGFMS, its
occurrence in and around the joints, the involvement of the bone
and its definitive treatment. However, the current case report
presents further information regarding the diagnosis, imaging and
management of LGFMS. There is no dedicated protocol for the early
diagnosis, treatment and follow-up of LGFMS, however, it is
associated with a high incidence of recurrence and metastasis after
a long duration. Following a long latent period, all LGFMS must be
treated as a malignant tumor and, thus, undergo a wide excision,
followed by a full oncological assessment and follow-up. This must
include a CT scan of the chest, as metastasis to the lung is the
most common scenario. Additionally, due to a high risk of
metastasis, a clinical follow-up and chest CT scan must be
performed over an extended period, as it remains unclear how
frequently chest CT scans must be repeated.
References
|
1
|
Evans HL: Low-grade fibromyxoid sarcoma. A
report of two metastasizing neoplasms having a deceptively benign
appearance. Am J Clin Pathol. 88:615–619. 1987.
|
|
2
|
Lee BJ, Park WS, Jin JM, Ha CW and Lee SH:
Low grade fibromyxoid sarcoma in thigh. Clin Orthop Surg.
1:240–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu X, Petrovic V, Torode IP and Chow CW:
Low grade fibromyxoid sarcoma: problems in the diagnosis and
management of a malignant tumour with bland histological
appearance. Pathology. 41:155–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Evans HL: Low-grade fibromyxoid sarcoma. A
report of 12 cases. Am J Surg Pathol. 17:595–600. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goodlad JR, Mentzel T and Fletcher CD: Low
grade fibromyxoid sarcoma: clinicopathological analysis of eleven
new cases in support of a distinct entity. Histopathology.
26:229–237. 1995. View Article : Google Scholar
|
|
6
|
Devaney DM, Dervan P, O’Neill S, Carney D
and Leader M: Low-grade fibromyxoid sarcoma. Histopathology.
17:463–465. 1990. View Article : Google Scholar
|
|
7
|
Husek K, Janícek P and Jelínek O: Low
grade malignant fibromyxoid sarcoma. Cesk Patol. 34:139–141.
1998.(In Czech).
|
|
8
|
Harish K, Ashok AC and Alva NK: Low grade
fibromyxoid sarcoma of the falciform ligament: a case report. BMC
Surgery. 3:72003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SS, Song C, Sun DH and Moon MS: Low
grade fibromyxoid sarcoma in shoulder: one case report. J Korean
Bone Joint Tumor Soc. 10:130–133. 2004.
|
|
10
|
Vernon SE and Bejarano PA: Low-grade
fibromyxoid sarcoma: a brief review. Arch Pathol Lab Med.
130:1358–1360. 2006.PubMed/NCBI
|
|
11
|
Arnaoutoglou C, Lykissas MG, Gelalis ID,
Batistatou A, Goussia A, Doukas M and Xenaki TA: Low grade
fibromyxoid sarcoma: a case report and review of the literature. J
Orthop Surg Res. 5:492010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reid R, de Silva MV, Paterson L, et al:
Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor
with giant rosettes share a common t(7;16)(q34;p11) translocation.
Am J Surg Pathol. 27:1229–1236. 2003. View Article : Google Scholar
|
|
13
|
Guillou L, Benhattar J, Gengler C, et al:
Translocation-positive low-grade fibromyxoid sarcoma:
clinicopathologic and molecular analysis of a series expanding the
morphologic spectrum and suggesting potential relationship to
sclerosing epithelioid fibrosarcoma: a study from the French
Sarcoma Group. Am J Surg Pathol. 31:1387–1402. 2007.
|
|
14
|
Angervall L, Kindblom LG and Merck C:
Myxofibrosarcoma. A study of 30 cases. Acta Pathol Microbiol Scand
A. 85A:127–140. 1977.PubMed/NCBI
|
|
15
|
Weiss SW and Enzinger FM: Myxoid variant
of malignant fibrous histiocytoma. Cancer. 39:1672–1685. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Merck C, Angervall L, Kindblom LG and Odén
A: Myxofibrosarcoma. A malignant soft tissue tumor of
fibroblastic-histiocytic origin A clinicopathologic and prognostic
study of 110 cases using multivariate analysis. Acta Pathol
Microbiol Immunol Scand Suppl. 282:1–40. 1983.
|
|
17
|
Kindblom LG, Merck C and Angervall L: The
ultrastructure of myxofibrosarcoma. A study of 11 cases. Virchows
Arch A Pathol Anat Histol. 381:121–139. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyake M, Tateishi U, Maeda T, Arai Y,
Seki K, Hasegawa T and Sugimura K: CT and MRI features of low-grade
fibromyxoid sarcoma in the shoulder of a pediatric patient. Radiat
Med. 24:511–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SK, Jee WH, Lee AW and Chung YG:
Haemorrhagic low-grade fibromyxoid sarcoma: MR findings in two
young women. Br J Radiol. 84:146–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Folpe A, van den Berg E and Molenaar WM:
Low grade fibromyxoid sarcoma. World Health Organization
Classification of Tumours. Pathology and Genetics of Tumours of
Soft Tissue and Bone. Fletcher CDM, Unni KK and Mertens F: IARC
Press; Lyon: pp. 104–105. 2002
|
|
21
|
Folpe AL, Lane KL, Paull G and Weiss SW:
Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor
with giant rosettes: a clinicopathologic study of 73 cases
supporting their identity and assessing the impact of high-grade
areas. Am J Surg Pathol. 24:1353–1360. 2000. View Article : Google Scholar
|