1. Introduction
Autophagy is a conserved intracellular degradation
process in which cellular organelles, proteins and invading
microbes are degraded by lysosomes. According to the routines of
target cargos delivered to lysosome, there are three types of
autophagy: macroautophagy, mitoautophagy and chaperone-mediated
autophagy (1). This review focused
on macroautophagy, hereafter referred to as autophagy.
Autophagy is a multifaceted process, consisting of
sequential stages, including initiation, elongation, maturation and
degradation, which are regulated by a series of highly conserved
autophagy-related genes (Atgs) involved in various signaling
pathways (2–4). Autophagy is characterized by the
formation of double-membrane vesicles, known as autophagosomes,
which are engulfed by cytoplasmic molecules. Subsequently, the
autophagosome fuses with the lysosome, which provides hydrolases
and the sequestered contents undergo degradation and recycling.
Autophagy contributes to the pathogenesis of diverse diseases, such
as neuronal degeneration, inflammatory bowel disease, aging and
cancer (5–8). Autophagy is involved in tumor
development and progression, however, its exact role remains to be
elucidated. Based on current information, autophagy plays a dual
role in cancer initiation and development. First, autophagy
eliminates senescent and injured cells, thereby limiting
chromosomal instability and suppresses tumor initiation. Deletion
of Atgs in mice results in a high incidence of spontaneous tumors
(9). Second, autophagy could
provide energy by recycling damaged organelles, DNA, aggregated
proteins and pathogens to maintain energy balance, which promotes
cancer cell survival. As a result, the inhibition of autophagy may
be a novel strategy to improve the efficacy of anti-cancer
therapy.
Primary liver cancer is the fifth most common cancer
worldwide and the third most common cause of cancer-related
mortality (10). Hepatocarcinoma
(HCC) is the most common primary malignancy of hepatocytes which
accounts for ~90% of primary liver cancers (11). Most cases of HCC (~80%) are
associated with chronic hepatitis B virus (HBV) or hepatitis C
virus (HCV) infection (11–13). In addition, non-alcoholic and
alcoholic fatty liver disease contribute to the development of HCC
(14). Surgical resection or liver
transplantation remains the mainstay of treatment for early HCC
patients. However, the majority of patients present at an advanced
stage, and only a few newly diagnosed HCC patients are eligible for
chemotherapy, targeted therapy, transcatheter arterial
chemoembolization (TACE) or radiofrequency ablation.
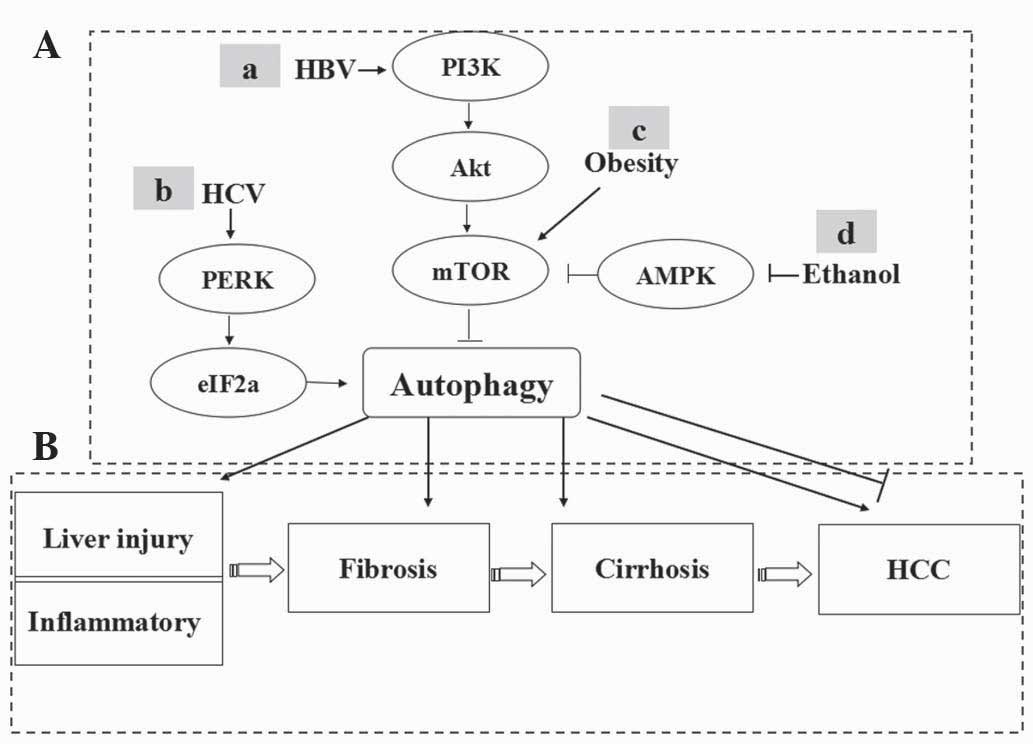
Autophagy in HCC has been previously investigated.
Dysregulation of autophagy is involved in hepatitis, fibrosis,
cirrhosis and HCC (15–17). Modulation of autophagy can affect
the efficacy of anti-HCC therapy. Therefore, it is crucial to
understand the potential mechanisms underlying the involvement of
autophagy in the development, progression and anti-cancer therapy
of HCC, which may lead to novel therapeutic approaches for liver
cancer. This review aimed to provide an overview of current
available information regarding the role of autophagy in the
development of HCC and the effect of autophagy in anti-HCC
therapy.
2. The role of autophagy in HCC
Since autophagy is a stress response, it is
associated with the development of HCC (16). Thus, understanding the role and
potential molecular mechanisms underlying the involvement of
autophagy in HCC formation and development, which may provide novel
therapeutic strategies for HCC, is crucial.
Autophagy is involved in the formation of
HCC
The formation of HCC is a multi-stage process, which
frequently develops in patients suffering from chronic liver injury
caused by chronic alcohol consumption and hepatitis B or C
infections (18). These conditions
result in the death of healthy liver cells and the initiation of an
inflammatory response that sequentially induces liver cell
proliferation, subsequently compensating cirrhosis and eventually
the development of HCC.
Recent studies have demonstrated that almost all
factors leading to chronic liver injury or inflammation were
capable of inducing autophagy. Autophagy is involved in hepatic
lipid and alcohol metabolism (19,20).
In Atg7-specific knockdown mice, lipid was markedly deposited in
hepatocytes (19). Ding et
al (21) found that acute
ethanol administration promoted the removal of lipid droplets and
damaged mitochondria by the induction of autophagy in mouse
hepatocytes. Suppression of autophagy exacerbated alcoholic liver
injury.
Epidemiological, clinical and experimental studies
have demonstrated that the relative risk of HCC in HBsAg carriers
is >200 times that in matched non-carriers (22,23).
HBV can enhance autophagy in Huh7 and HepG2 cells in mouse
orthotopic liver cancer models (24,25).
The HBV X protein sensitizes hepatoma cells to starvation-induced
autophagy via the upregulation of Beclin-1 expression (24,26).
In addition, HBV promotes viral replication by the binding of HBx
and PI3KC3 (26). Recent findings
suggest that autophagy is involved in HCV infection (27–29).
Inhibition of autophagy abrogates the replication of HCV by
siRNA-targeting Atgs (30). HCV
induces the accumulation of autophagosome in hepatoma cells by
unfolded protein response (UPR) (27).
Liver fibrosis is the final result of liver injury
or chronic liver disease, which ultimately progresses to liver
cirrhosis and cancer. Induction of autophagy promotes hepatic
stellate cell (HSC) proliferation or activation, which is transited
to myofibroblast when it is activated under the conditions of liver
hepatitis, alcohol or non-alcohol liver diseases (31). Pharmacological inhibitors baflomycin
A1, 3-methyladenine (3-MA) or chloroquine (CQ) suppress the
activation and proliferation of HSC in vitro and in
vivo.
Collectively, autophagy is involved in chronic liver
disease caused by non-alcoholic and alcohol factors, as well as HBV
or HCV infection. Various potential signaling pathways are involved
(Fig. 1).
Autophagy plays a dual role in
hepatocarcinogenesis
Despite the literature available, the role of
autophagy in hepatocarcinogenesis remains controversial. Since
autophagy is a stress response and survival mechanism, mouting
evidence demonstrates that autophagy contributes to the survival of
cancer (8,32). It has been reported that autophagy
was increased in tumor interior rather than in cancer margins,
contributing to the survival of interior tumor cells under an
hypoxic-ischemic environment (32).
Microtubule-associated protein light chain 3 (LC3) was
significantly highly expressed in HCC compared with non-cancerous
tissues, and was also significantly correlated with tumor size. In
addition, LC3 was an independent predictor of HCC recurrence after
surgery only in the context of large tumors (33). Similarly, increased levels of LC3-II
were observed in HCC tissues with low glucose uptake and a high
K-Ras expression (34).
Collectively, these data support the hypothesis that autophagy
serves to maintain tumor survival.
As an essential regulator for cellular homeostasis,
autophagy plays an important role in carcinogenesis. It has been
well-documented that autophagy is a tumor suppressor that acts as a
housekeeping gene (35). Mice with
homozygous Beclin-1 knockout have a high incidence of spontaneous
tumors, such as HCC (36).
Similarly, the deletion of Atg5 or Atg7 in liver, two key elements
of autophagy elongation, resulted in the increasing incidence of
HCC (37). The expression and
activity of Atg5 or Atg7 are reduced in HCC cell lines compared
with normal hepatocytes in vitro (38). Kotsafti et al (37) found that the decreased expression of
Beclin-1 was observed in human HCC tissue and was correlated with
recurrent disease and free-disease survival (37). These findings establish a role for
autophagy as a suppressor in HCC.
Autophagy is known to suppress tumorigenesis in
healthy cells, albeit it contributes to the survival of an
established tumor (Fig. 2).
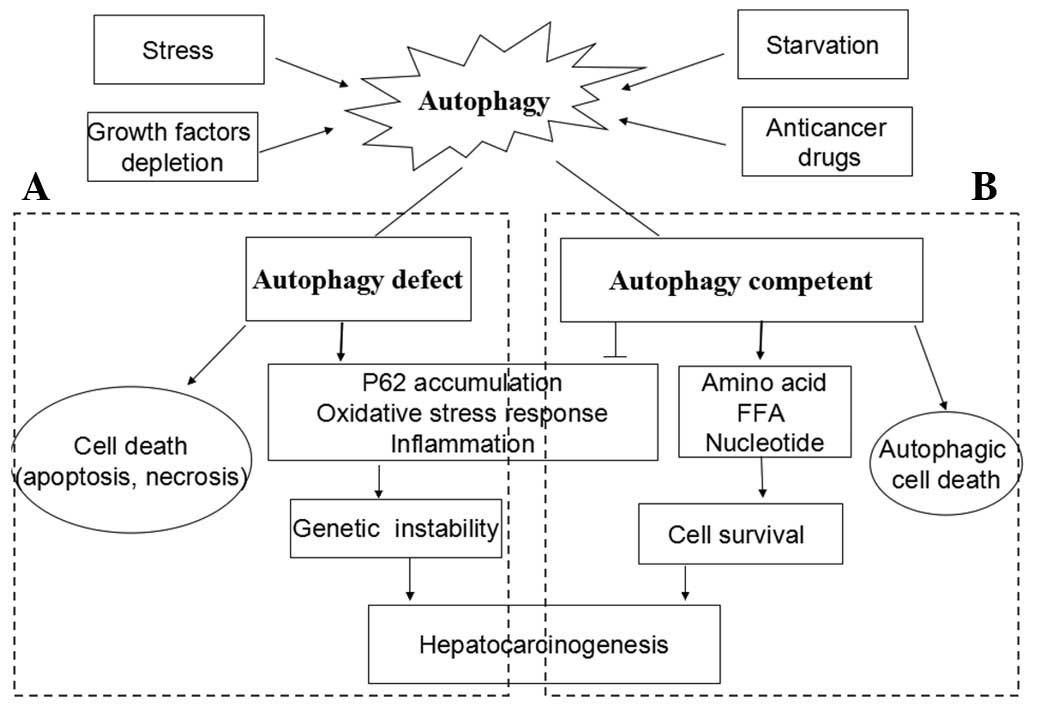 | Figure 2The dual role of autophagy in the
development of hepatocarcinoma (HCC). Autophagy is activated as a
response to stress, growth factors depletion, starvation and
anti-tumor treatment. (A) Under autophagy-deficient conditions,
cells succumb to death when challenged with death stimuli. Thus,
autophagy acts as a tumor suppressor. On the other hand, proteins
scavenged by autophagy accumulate and result in genetic
instability, which in turn promote hepatocarcinogenesis. (B) Under
autophagy-competent conditions, cells succumb to survival when
challenged with death stimuli. Autophagy removes damaged
organelles, misfolded and aggregated proteins, both of which
generate free fatty acids and amino acids that can provide energy
to facilitate hepatocarcinogenesis. However, the sustained
activation of autophagy leads to autophagic cell death, termed as
type II programmed cell death. FFA, free fatty acids. |
3. Autophagy and anti-HCC therapy
Due to the controversial role it plays in the
initiation and development of HCC, autophagy has become an emerging
and noteworthy field of study for identifying treatment for HCC.
Appreciation of the function of autophagy in cancer treatment is
critical, because anticancer therapies have been shown to initiate
autophagy in vitro and in vivo.
Autophagy in chemotherapy
Currently, chemotherapy is almost ineffective for
HCC because of the inherent or acquired chemoresistance and
limitation of liver function. Autophagy is known to promote cancer
resistance to chemotherapy. Guo et al (39) reported that cisplatin or 5-FU
induced autophagy in HepG2, SMMC-7721 and Hep3B cells. Autophagy
inhibition by 3-MA or the siRNA targeting Beclin-1 increased
chemotherapy-induced apoptosis by causing significant damage of
mitochondrial membrane in vitro and in vivo.
Oxaliplatin-based combination chemotherapy has shown promising
anti-tumor activities in patients with HCC (40). Ding et al (41) found that autophagy was activated by
oxaliplatin in the HCC cells. Suppression of autophagy with
pharmacologic inhibitors or siRNAs targeting essential autophagic
genes enhanced cell death induced by oxaliplatin in HCC cells,
which correlated with the generation of reactive oxygen
species.
However, adriamycin, which is routinely used as a
monotherapy for advanced HCC, induced autophagic cell death rather
than cytoprotective autophagy in Hep3B cells (42). It is known that the MAPK/ERK
pathway, which is upregulated in HCC, can regulate autophagy
(43). The potential mechanism of
autophagic cell death induced by adriamycin is present in the
sustained activation of the MAPK/ERK pathway, which leads to
autophagic progression, followed by an irreversible stage and
ultimately cell death.
Autophagy is known to serve as a protective
mechanism under chemotherapeutics (39–41).
Although autophagic cell death has been reported, this should be
defined carefully in its particular context and the results should
be elucidated prudently.
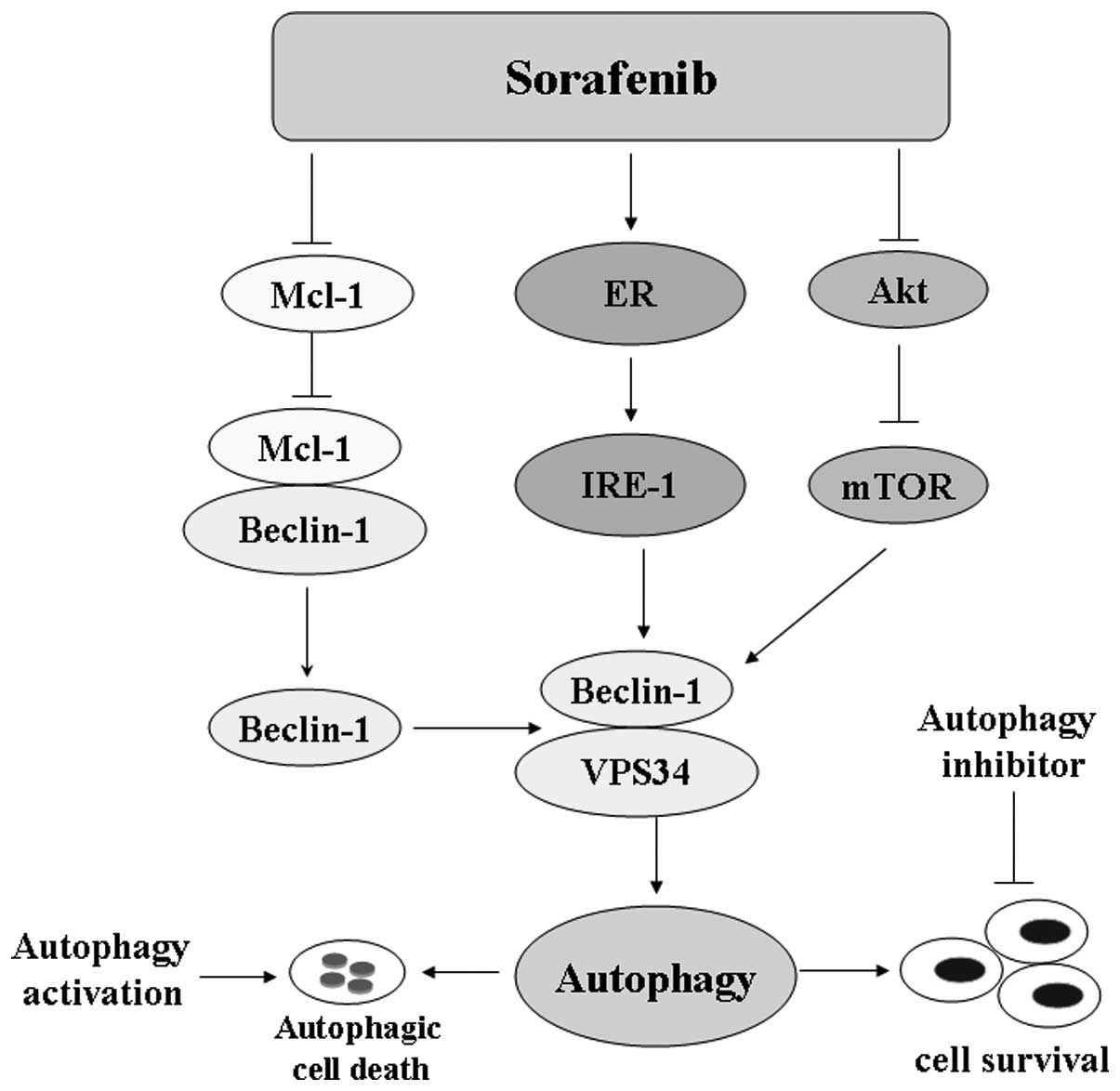
Autophagy in molecular-targeted
therapy
Molecular-targeted therapy is critical for advanced
or recurrent HCC. Sorafenib, a multi-targeted receptor tyrosine
kinase inhibitor (TKI) that targets Ras, VEGFR and PDGFR, was
approved as the standard therapy for advanced unresectable HCC
(44). However, sorafenib only
provides modest effects, prolonging survival in patients with HCC
from a median of 7.9 to 10.7 months (45,46).
Sorafenib induced the accumulation of autophagosomes in HCC cells
through inhibition of the mTORC1 pathway (47,48).
The underlying molecular mechanisms of this process are: i) The
PI3K/Akt/mTOR signaling pathway is capable of regulating autophagy.
Besides the Raf/MEK/MAPK pathway, sorafenib inhibits activation of
the mTORC1 pathway, which ultimately stimulates a series of signals
to induce autophagy. ii) The endoplasmic reticulum (ER) is an
essential intracellular organelle required for the synthesis and
quality control of proteins. Findings of recent studies have
demonstrated that autophagosome membranes originate from ER,
thereby suggesting a direct connection between the ER and autophagy
(49). Shi et al (48) reported that sorafenib significantly
increased the mRNA and protein expression levels of the UPR target
genes IRE-1 and CHOP as well as eIF2α phosphorylation. Thus,
sorafenib-triggered ER stress is critical for autophagy activation.
Briefly, autophagy conferred a survival advantage for sorafenib
treatment in HCC, which may be an attractive strategy for HCC
treatment. Similarly, autophagy exerts a cytoprotective effect in
HCC cell lines treated with proteasome inhibitor
carbobenzoxy-Leu-Leu-leucinal (MG-132), bortezomib, or bevacizumab,
a humanized monoclonal antibody that binds VEGF-A (50–52).
It has, however, been demonstrated that autophagic
cell death was a major contributor to molecular-targeted therapies
associated with the anti-proliferative effect on tumor cells. Tai
et al (53) found that
sorafenib and SC-59, a novel sorafenib derivative, disrupt myeloid
cell leukemia-1 (Mcl-1) associated with Beclin-1 and promote
significant autophagic cell death. OSU-03012, a highly selective
COX-2 inhibitor, induced reactive oxygen species-related autophagy
to inhibit HCC cell proliferation (54). Nilotinib, a second-generation TKI
for leukemia, induced autophagic cell death in HCC by deactivating
phosphatase PP2A and increasing AMPK phosphorylation. Autophagy
inhibition by hydroxychloroquine (HCQ) reduced the effect of
nilotinib in vivo (55).
Collectively, molecular-targeted therapy activates
autophagy in HCC cells and autophagy can function to promote either
tumor cell survival or cell death.
Autophagy in radiotherapy
Conformal radiotherapy (RT) and stereotactic body
radiation therapy are used to treat single or solitary liver
metastases or unresectable HCC in some preferential patients. A
phase II trial demonstrated that 48% of patients who had HCC or
local metastases, unsuitable for or refractory to standard therapy
and received palliative RT, exhibited improvement in symptoms such
as pain, abdominal discomfort, nausea, or fatigue (56). In recent studies, it was shown that
genetic or pharmacological interference with autophagy can enhance
the response to radiation in renal cell carcinoma, breast cancer,
head and neck squamous cell carcinoma and glioblastoma (57–59).
Iron radiation contributed to a cytoprotective autophagy that could
be inhibited by CQ or by the silencing of autophagy-regulatory
genes, with the consequent enhancement of radiation sensitivity in
breast cancer (59,60). By contrast, Altmeyer et al
(61) found that irradiation with
fast neutrons, which are high-linear energy transfer (LET)
particles, induced autophagic cell death in the human HCC SK-Hep1
cells (61). Furthermore, autophagy
plays a pivotal role in cell death after high-LET irradiation in
orthotopic human hepatocellular carcinoma (62). Briefly, autophagy can be induced by
radiation therapy, which functions to protect or promote cell
death. However, the potential mechanism underlying this role
remains to be determined.
Autophagy in TACE or photodynamic
therapy
TACE is used in unresectable HCC, as well as pre- or
post-operative adjuvant therapy in patients with resectable HCC to
improve survival. Studies have shown that LC3 expression was
significantly higher after TACE compared to tumors that had not
undergone treatment in human HCC tissue samples (41). Autophagy inhibitor CQ combined with
TACE represented better outcomes compared to TACE alone in a rabbit
VX2 liver tumor model (63).
Photodynamic therapy (PDT) is a process whereby the
interaction between photodynamic agents localized in neoplastic
tissues and oxygens in tissues was initiated by irradiation at
appropriate wavelength (64). Using
a murine hepatoma 1c1c7 model, Andrzejak et al (65) found PDT-induced autophagy was
cytoprotective since PDT efficacy was significantly enhanced in
Atg7-knockdown cells.
Autophagy in immunotherapy
During the process of tumor development, tumor
antigens are not visible to T cells thereby escaping immune
surveillance (66). Thus,
immunotherapy is considered a promising therapeutic option with the
aim of inducing or increasing HCC-specific immune response and
overcoming immune escape, demonstrating the importance of autophagy
in central aspects of the immune response, making it an attractive
target for cancer therapy. Cytokines such as IFN-γ, IL-12 and
TNF-β, are important effector components in the immune response
(67). IFN-γ, which plays an
important role in HCC immunotherapy, inhibited liver cancer cell
growth by the induction of autophagic cell death. Knockdown of the
Beclin-1 or Atg5 attenuated the inhibitory effect of IFN-γ
(68). IL-2, a major regulator of
immunotherapy that was approved for advanced renal cancer and
melanoma, can increase autophagy flux in murine liver (67). The combination of IL-2 with CQ
prolonged survival in a murine metastatic liver tumor model. The
potential mechanism involved is that CQ inhibited oxidative
phosphorylation and ATP production and promoted apoptosis of cancer
cells (67). Li et al
(68) reported that toll-like
receptor-2 (TLR-2) deletion sensitized liver cancer cells to
diethylnitrosamine, a genotoxic carcinogen that can induce HCC.
TLR-2 deficiency caused a decrease in the expression of IFN-γ, IL-6
as well as suppression of the autophagic flux. Restoring autophagic
flux by treating TLR2-deficient mice with IFN-γ, a T-helper 1 (Th1)
cytokine and positive modulator of autophagy, attenuated the
carcinogenesis and progression of HCC in TLR2-deficient mice
(68). Recently, a cancer vaccine
originating from tumor cell-derived autophagosomes (DRibbles)
combined with dendritic cells (DCs) has shown a specific T-cell
response against HCC and resulted in the significant inhibition of
tumor growth compared to mice treated with DCs alone (69).
Autophagy in liver transplantation
Liver transplantation is a widely accepted treatment
for HCC patients and is the best available option for early HCC.
Ischemia/reperfusion (I/R) injury occurs during the procedure of
liver transplantation, which is the main cause of initial
deficiencies and primary dysfunction of liver grafts (70). Accumulating evidence suggests that
CQ administration worsens I/R injury via autophagy inhibition in
kidney and heart after ischemia (71,72).
It was also demonstrated that CQ treatment ameliorated liver I/R
injury in the early phase of reperfusion but worsened liver I/R
injury in the late phase via inhibition of autophagy on rat hepatic
I/R injury (32). Hepatocytes that
possessed abundant autophagosomes often underwent autophagic cell
death which triggered liver graft dysfunction (73).
By contrast, Degli Esposti et al (74) demonstrated that ischemic
preconditioning induces autophagy in human steatotic liver grafts
and reduces rejection in recipients. Rapamycin, a key
immunosuppressive drug and autophagy inducer, improved the survival
of HCC patients with liver transplantation (75).
Thus, whether autophagy functions in cell survival
or death in anti-HCC therapy is highly dependent on the cell type,
mechanisms of agents and the signaling pathways (Table I).
 | Table IThe functional status of autophagy in
hepatocarcinoma treated with different agents in experiments. |
Table I
The functional status of autophagy in
hepatocarcinoma treated with different agents in experiments.
| Agents | Autophagy | Function | (Refs.) |
|---|
| Chemotherapy |
| Cisplatin | ↑ | Cell survival | (39) |
| 5-FU | ↑ | Cell survival | (39) |
| Oxaliplatin | ↑ | Cell survival | (41) |
| Adriamycin | ↑ | Cell death | (42) |
| Targeting
therapy |
| Sorafenib | ↑ | Cell survival | (47,48) |
| ↑ | Cell death | (53) |
| MG-132 | ↑ | Cell survival | (50) |
| Bevacizumab | ↑ | Cell survival | (51) |
| Bortezomib | ↑ | Cell survival | (52) |
| OSU-03012 | ↑ | Cell death | (54) |
| Nilotinib | ↑ | Cell death | (55) |
| Immunotherapy |
| IFN-γ | ↑ | Cell death | (68) |
| IL-2 | ↑ | Cell death | (67) |
| DRibble | ↑ | Cell survival | (69) |
4. Autophagy modulation and anti-HCC
therapy
Although the role of autophagy in
hepatocarcinogenesis and treatment thereof has been outlined in
detail, autophagy modulation based on its function may provide
novel opportunities for HCC treatment. Autophagy inhibition is an
emerging strategy that enhances cytotoxicity in combination with
anti-HCC therapies in the prosurvival function of autophagy. By
contrast, the activation of autophagy is another method to
facilitate the anti-tumor effect combination with current
therapeutic methods in autophagic cell death.
Inhibiting autophagy in anti-HCC
therapy
Recent studies have reported that genetic or
pharmacological interference with autophagy can enhance the
response to chemotherapy, molecular-targeted and radiation therapy
(50,52,59).
CQ and HCQ, which are used in malaria, are commonly used as
autophagy inhibitors in various tumor experiment models (76). In pre-clinical and clinical trials
conducted, the role of autophagy inhibition through pharmacologic
inhibitors such as CQ and HCQ was examined in various tumors
(www.clinicaltrials.gov). As mentioned above,
autophagy inhibitors can enhance the effectiveness of oxaliplatin,
cisplatin, 5-FU and sorafenib in HCC models (39,41,47).
The coadministration of oxaliplatin and CQ induced a marked
decrease in tumor volume compared with either agent alone in HCC
xenografts (41). CQ interacted
synergistically with bortezomib to suppress tumor growth to a
greater extent in HCC experimental models (51). The concomitant inhibition of
autophagy by CQ or genetic knockdown Atg7 sensitized hepatoma cells
to sorafenib (47). Similarly,
autophagy suppression by means of 3-MA and inactive Atg4B inhibited
proliferation in Huh7 cells (77).
Thus, autophagy inhibition is an attractive strategy for overcoming
therapeutic resistance in the protective functions of
autophagy.
Inducing autophagic cell death in
anti-HCC therapy
Sustained activation of autophagy may kill cancer
cells with a high apoptotic defect, termed autophagic cell death
(78). Autophagic cell death has
been observed in malignant glioma cells treated with arsenic
trioxide or sodium selenite (79,80).
Vorinostat, a histone deacetylase inhibitor, induced autophagic
cell death in the U937 hematological cell line (81). Autophagy activation may serve as an
alternative strategy for eliminating cancer cells, especially HCC
cells with apoptotic defect. As discussed above, sorafenib induced
autophagic cell death through the Mcl-1 signaling pathway (53). Under context-specific conditions,
the sustained upregulation of autophagy may benefit from sorafenib
treatment. However, evidence from in vivo studies and
clinical trials are relatively limited and whether the induction of
autophagic cell death in tumor cell death can be sensitized to HCC
therapy remains unclear.
Taken together, although connections between
autophagy and anti-HCC therapies have been suggested, autophagy
modulation provides new prospects in anti-HCC therapy. The
complexity of autophagy in hepatocarcinogenesis and anti-HCC
therapies, however, makes it difficult to define how to regulate
autophagy (inhibition or activation) in order to ensure maximum
therapeutic advantage. A typical example is that sorafenib-induced
autophagy is particularly context-dependent and exhibits an
opposite function through different signaling pathways (Fig. 3).
5. Conclusions
The role of autophagy in cancer remains
controversial and highly context-dependent. As outlined in this
review, autophagy plays a dual role in multiple aspects to the
sequential process of liver cancer initiation, promotion,
progression and metastasis. In addition to this, autophagy is
induced through various types of anti-HCC therapies. Previous
studies (34,37) have demonstrated that autophagy plays
an anti-tumor effect in suppression of the formation of HCC, while
serving as a pro-survival mechanism to promote liver cancer
development, and results in resistance to anti-HCC therapy. Thus,
targeting autophagy is a promising strategy for liver cancer
therapy.
Limitations to the clinical application of autophagy
in anti-HCC therapy should first be overcome. Although it is widely
accepted that anti-tumor treatment induces autophagy, it remains to
be determined whether this activation promotes cell survival as a
response to stress, or leads to cell death under the condition of
apoptotic defects. Therefore, obtaining the function status of
autophagy in anti-HCC treatment may contribute to devising a
rationale for the treatment of HCC. Additionally, whether autophagy
modulation (inhibition or activation) increased the susceptibility
to treatment in healthy cells or eradicated the balance of
homeostasis should be clarified. As an autophagy inhibitor, CQ
sensitizes the normal renal proximal tubular cells to cisplatin
administration (82). Rapamycin, an
autophagy inducer, is also an immunosuppressor (83). Selection of a suitable drug that
targets autophagy in order to enhance the efficacy of anti-HCC
therapy remains a challenge. Subsequently, coadministration of the
autophagy regulator with anti-HCC therapy may also aid in the
elucidation of the antistatic effect. Thus, targeting autophagy
remains a promising interventional strategy for the treatment of
HCC.
Acknowledgements
The authors would like to thank Meng Sun for kindly
assisting with language on the manuscript. This study was supported
by the National Natural Science Foundation of China (grant no.
81272593, www.nsfc.gov.cn) to H. Pan, the Fundamental
Research Funds for the Central Universities and the National
Natural Science Foundation of China (grant no. 81372621, www.nsfc.gov.cn) to W. Han.
References
|
1
|
Degenhardt K, Mathew R, Beaudoin B, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation, and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kroemer G, Marino G and Levine B:
Autophagy and the integrated stress response. Mol Cell. 40:280–293.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosenfeldt MT and Ryan KM: The multiple
roles of autophagy in cancer. Carcinogenesis. 32:955–963. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mizushima N: Autophagy: process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar
|
|
5
|
Yue Z: Regulation of neuronal autophagy in
axon: implication of autophagy in axonal function and
dysfunction/degeneration. Autophagy. 3:139–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scharl M and Rogler G: Inflammatory bowel
disease: dysfunction of autophagy? Dig Dis. 30(Suppl 3): 12–19.
2012. View Article : Google Scholar
|
|
7
|
Yamaguchi O and Otsu K: Role of autophagy
in aging. J Cardiovasc Pharmacol. 60:242–247. 2012. View Article : Google Scholar
|
|
8
|
Eskelinen EL: The dual role of autophagy
in cancer. Curr Opin Pharmacol. 11:294–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang C and Jung JU: Autophagy genes as
tumor suppressors. Curr Opin Cell Biol. 22:226–233. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fares N and Peron JM: Epidemiology,
natural history, and risk factors of hepatocellular carcinoma. Rev
Prat. 63:216–217. 2013.(In French).
|
|
12
|
Guerrieri F, Belloni L, Pediconi N, et al:
Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin
Liver Dis. 33:147–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamazaki K, Masugi Y and Sakamoto M:
Molecular pathogenesis of hepatocellular carcinoma: altering
transforming growth factor-beta signaling in hepatocarcinogenesis.
Dig Dis. 29:284–288. 2011. View Article : Google Scholar
|
|
14
|
Maillard E: Epidemiology, natural history
and pathogenesis of hepatocellular carcinoma. Cancer Radiother.
15:3–6. 2011.(In French).
|
|
15
|
Ni HM, Williams JA, Yang H, et al:
Targeting autophagy for the treatment of liver diseases. Pharmacol
Res. 66:463–474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui J, Gong Z and Shen HM: The role of
autophagy in liver cancer: molecular mechanisms and potential
therapeutic targets. Biochim Biophys Acta. 1836:15–26.
2013.PubMed/NCBI
|
|
17
|
Rautou PE, Mansouri A, Lebrec D, et al:
Autophagy in liver diseases. J Hepatol. 53:1123–1134. 2010.
View Article : Google Scholar
|
|
18
|
Cabibbo G, Maida M, Genco C, et al:
Natural history of untreatable hepatocellular carcinoma: a
retrospective cohort study. World J Hepatol. 4:256–261. 2012.
View Article : Google Scholar
|
|
19
|
Singh R, Kaushik S, Wang Y, et al:
Autophagy regulates lipid metabolism. Nature. 458:1131–1135. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dolganiuc A, Thomes PG, Ding WX, et al:
Autophagy in alcohol-induced liver diseases. Alcohol Clin Exp Res.
36:1301–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding WX, Li M and Yin XM: Selective taste
of ethanol-induced autophagy for mitochondria and lipid droplets.
Autophagy. 7:248–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lavanchy D: Hepatitis B virus
epidemiology, disease burden, treatment, and current and emerging
prevention and control measures. J Viral Hepat. 11:97–107. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beasley RP: Hepatitis B virus. The major
etiology of hepatocellular carcinoma. Cancer. 61:1942–1956. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sir D, Tian Y, Chen WL, et al: The early
autophagic pathway is activated by hepatitis B virus and required
for viral DNA replication. Proc Natl Acad Sci USA. 107:4383–4388.
2010. View Article : Google Scholar
|
|
25
|
Tian Y, Sir D, Kuo CF, et al: Autophagy
required for hepatitis B virus replication in transgenic mice. J
Virol. 85:13453–13456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang H, Da L, Mao Y, et al: Hepatitis B
virus X protein sensitizes cells to starvation-induced autophagy
via up-regulation of beclin 1 expression. Hepatology. 49:60–71.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shinohara Y, Imajo K, Yoneda M, et al:
Unfolded protein response pathways regulate Hepatitis C virus
replication via modulation of autophagy. Biochem Biophys Res
Commun. 432:326–332. 2013. View Article : Google Scholar
|
|
28
|
Sir D, Kuo CF, Tian Y, et al: Replication
of hepatitis C virus RNA on autophagosomal membranes. J Biol Chem.
287:18036–18043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shrivastava S, Bhanja Chowdhury J, Steele
R, et al: Hepatitis C virus upregulates Beclin1 for induction of
autophagy and activates mTOR signaling. J Virol. 86:8705–8712.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dreux M, Gastaminza P, Wieland SF and
Chisari FV: The autophagy machinery is required to initiate
hepatitis C virus replication. Proc Natl Acad Sci USA.
106:14046–14051. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thoen LF, Guimaraes EL, Dolle L, et al: A
role for autophagy during hepatic stellate cell activation. J
Hepatol. 55:1353–1360. 2011. View Article : Google Scholar
|
|
32
|
Fang H, Liu A, Dahmen U and Dirsch O: Dual
role of chloroquine in liver ischemia reperfusion injury: reduction
of liver damage in early phase, but aggravation in late phase. Cell
Death Dis. 4:e6942013. View Article : Google Scholar
|
|
33
|
Yang JD, Seol SY, Leem SH, et al: Genes
associated with recurrence of hepatocellular carcinoma: integrated
analysis by gene expression and methylation profiling. J Korean Med
Sci. 26:1428–1438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JH, Kim HY, Lee YK, et al: Involvement
of mitophagy in oncogenic K-Ras-induced transformation: overcoming
a cellular energy deficit from glucose deficiency. Autophagy.
7:1187–1198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosenfeldt MT and Ryan KM: The role of
autophagy in tumour development and cancer therapy. Expert Rev Mol
Med. 11:e362009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qu X, Yu J, Bhagat G, et al: Promotion of
tumorigenesis by heterozygous disruption of the beclin 1 autophagy
gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kotsafti A, Farinati F, Cardin R, et al:
Autophagy and apoptosis-related genes in chronic liver disease and
hepatocellular carcinoma. BMC Gastroenterol. 12:1182012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takamura A, Komatsu M, Hara T, et al:
Autophagy-deficient mice develop multiple liver tumors. Genes Dev.
25:795–800. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo XL, Li D, Hu F, et al: Targeting
autophagy potentiates chemotherapy-induced apoptosis and
proliferation inhibition in hepatocarcinoma cells. Cancer Lett.
320:171–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Uhm JE, Park JO, Lee J, et al: A phase II
study of oxaliplatin in combination with doxorubicin as first-line
systemic chemotherapy in patients with inoperable hepatocellular
carcinoma. Cancer Chemother Pharmacol. 63:929–935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ding ZB, Hui B, Shi YH, et al: Autophagy
activation in hepatocellular carcinoma contributes to the tolerance
of oxaliplatin via reactive oxygen species modulation. Clin Cancer
Res. 17:6229–6238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Manov I, Pollak Y, Broneshter R and Iancu
TC: Inhibition of doxorubicin-induced autophagy in hepatocellular
carcinoma Hep3B cells by sorafenib - the role of extracellular
signal-regulated kinase counteraction. FEBS J. 278:3494–3507. 2011.
View Article : Google Scholar
|
|
43
|
Huynh H, Nguyen TT, Chow KH, et al:
Over-expression of the mitogen-activated protein kinase (MAPK)
kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor
progression and apoptosis. BMC Gastroenterol. 3:192003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wilhelm S, Carter C, Lynch M, et al:
Discovery and development of sorafenib: a multikinase inhibitor for
treating cancer. Nat Rev Drug Discov. 5:835–844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang X, Yang XR, Huang XW, et al:
Sorafenib in treatment of patients with advanced hepatocellular
carcinoma: a systematic review. Hepatobiliary Pancreat Dis Int.
11:458–466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xie B, Wang DH and Spechler SJ: Sorafenib
for treatment of hepatocellular carcinoma: a systematic review. Dig
Dis Sci. 57:1122–1129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shimizu S, Takehara T, Hikita H, et al:
Inhibition of autophagy potentiates the antitumor effect of the
multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J
Cancer. 131:548–557. 2012. View Article : Google Scholar
|
|
48
|
Shi YH, Ding ZB, Zhou J, et al: Targeting
autophagy enhances sorafenib lethality for hepatocellular carcinoma
via ER stress-related apoptosis. Autophagy. 7:1159–1172. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hayashi-Nishino M, Fujita N, Noda T, et
al: A subdomain of the endoplasmic reticulum forms a cradle for
autophagosome formation. Nat Cell Biol. 11:1433–1437. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hui B, Shi YH, Ding ZB, et al: Proteasome
inhibitor interacts synergistically with autophagy inhibitor to
suppress proliferation and induce apoptosis in hepatocellular
carcinoma. Cancer. 118:5560–5571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu HC, Hou DR, Liu CY, et al: Cancerous
inhibitor of protein phosphatase 2A mediates bortezomib-induced
autophagy in hepatocellular carcinoma independent of proteasome.
PLoS One. 8:e557052013. View Article : Google Scholar
|
|
52
|
Guo XL, Li D, Sun K, et al: Inhibition of
autophagy enhances anticancer effects of bevacizumab in
hepatocarcinoma. J Mol Med (Berl). 91:473–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tai WT, Shiau CW, Chen HL, et al:
Mcl-1-dependent activation of Beclin 1 mediates autophagic cell
death induced by sorafenib and SC-59 in hepatocellular carcinoma
cells. Cell Death Dis. 4:e4852013. View Article : Google Scholar
|
|
54
|
Gao M, Yeh PY, Lu YS, et al: OSU-03012, a
novel celecoxib derivative, induces reactive oxygen species-related
autophagy in hepatocellular carcinoma. Cancer Res. 68:9348–9357.
2008. View Article : Google Scholar
|
|
55
|
Yu HC, Lin CS, Tai WT, et al: Nilotinib
induces autophagy in hepatocellular carcinoma through AMPK
activation. J Biol Chem. 288:18249–18259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Soliman H, Ringash J, Jiang H, et al:
Phase II trial of palliative radiotherapy for hepatocellular
carcinoma and liver metastases. J Clin Oncol. 31:3980–3986. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Anbalagan S, Pires IM, Blick C, et al:
Radiosensitization of renal cell carcinoma in vitro through the
induction of autophagy. Radiother Oncol. 103:388–393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cerniglia GJ, Karar J, Tyagi S, et al:
Inhibition of autophagy as a strategy to augment radiosensitization
by the dual phosphatidylinositol 3-kinase/mammalian target of
rapamycin inhibitor NVP-BEZ235. Mol Pharmacol. 82:1230–1240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bristol ML, Di X, Beckman MJ, et al: Dual
functions of autophagy in the response of breast tumor cells to
radiation: cytoprotective autophagy with radiation alone and
cytotoxic autophagy in radiosensitization by vitamin D 3.
Autophagy. 8:739–753. 2012. View Article : Google Scholar
|
|
60
|
Wilson EN, Bristol ML, Di X, et al: A
switch between cytoprotective and cytotoxic autophagy in the
radiosensitization of breast tumor cells by chloroquine and vitamin
D. Horm Cancer. 2:272–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Altmeyer A, Jung AC, Ignat M, et al:
Pharmacological enhancement of autophagy induced in a
hepatocellular carcinoma cell line by high-LET radiation.
Anticancer Res. 30:303–310. 2010.PubMed/NCBI
|
|
62
|
Altmeyer A, Ignat M, Denis JM, et al: Cell
death after high-LET irradiation in orthotopic human hepatocellular
carcinoma in vivo. In Vivo. 25:1–9. 2011.
|
|
63
|
Gao L, Song JR, Zhang JW, et al:
Chloroquine promotes the anticancer effect of TACE in a rabbit VX2
liver tumor model. Int J Biol Sci. 9:322–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ochsner M: Photophysical and
photobiological processes in the photodynamic therapy of tumours. J
Photochem Photobiol B. 39:1–18. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Andrzejak M, Price M and Kessel DH:
Apoptotic and autophagic responses to photodynamic therapy in 1c1c7
murine hepatoma cells. Autophagy. 7:979–984. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Arum CJ, Anderssen E, Viset T, et al:
Cancer immunoediting from immunosurveillance to tumor escape in
microvillus-formed niche: a study of syngeneic orthotopic rat
bladder cancer model in comparison with human bladder cancer.
Neoplasia. 12:434–442. 2010.
|
|
67
|
Liang X, De Vera ME, Buchser WJ, et al:
Inhibiting systemic autophagy during interleukin 2 immunotherapy
promotes long-term tumor regression. Cancer Res. 72:2791–2801.
2012. View Article : Google Scholar
|
|
68
|
Li P, Du Q, Cao Z, et al: Interferon-gamma
induces autophagy with growth inhibition and cell death in human
hepatocellular carcinoma (HCC) cells through interferon-regulatory
factor-1 (IRF-1). Cancer Lett. 314:213–222. 2012. View Article : Google Scholar
|
|
69
|
Su S, Zhou H, Xue M, et al: Anti-tumor
efficacy of a hepatocellular carcinoma vaccine based on dendritic
cells combined with tumor-derived autophagosomes in murine models.
Asian Pac J Cancer Prev. 14:3109–3116. 2013. View Article : Google Scholar
|
|
70
|
Leithead JA, Armstrong MJ, Corbett C, et
al: Hepatic ischemia reperfusion injury is associated with acute
kidney injury following donation after brain death liver
transplantation. Transpl Int. 26:1116–1125. 2013. View Article : Google Scholar
|
|
71
|
Yasuda H, Leelahavanichkul A, Tsunoda S,
et al: Chloroquine and inhibition of Toll-like receptor 9 protect
from sepsis-induced acute kidney injury. Am J Physiol Renal
Physiol. 294:F1050–F1058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hoshino A, Matoba S, Iwai-Kanai E, et al:
p53-TIGAR axis attenuates mitophagy to exacerbate cardiac damage
after ischemia. J Mol Cell Cardiol. 52:175–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gotoh K, Lu Z, Morita M, et al:
Participation of autophagy in the initiation of graft dysfunction
after rat liver transplantation. Autophagy. 5:351–360. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Degli Esposti D, Sebagh M, Pham P, et al:
Ischemic preconditioning induces autophagy and limits necrosis in
human recipients of fatty liver grafts, decreasing the incidence of
rejection episodes. Cell Death Dis. 2:e1112011.
|
|
75
|
Toso C, Merani S, Bigam DL, et al:
Sirolimus-based immunosuppression is associated with increased
survival after liver transplantation for hepatocellular carcinoma.
Hepatology. 51:1237–1243. 2010. View Article : Google Scholar
|
|
76
|
Yang ZJ, Chee CE, Huang S and Sinicrope
FA: The role of autophagy in cancer: therapeutic implications. Mol
Cancer Ther. 10:1533–1541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Toshima T, Shirabe K, Matsumoto Y, et al:
Autophagy enhances hepatocellular carcinoma progression by
activation of mitochondrial beta-oxidation. J Gastroenterol. May
24–2013.(Epub ahead of print).
|
|
78
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kanzawa T, Kondo Y, Ito H, et al:
Induction of autophagic cell death in malignant glioma cells by
arsenic trioxide. Cancer Res. 63:2103–2108. 2003.
|
|
80
|
Kim EH, Sohn S, Kwon HJ, et al: Sodium
selenite induces superoxide-mediated mitochondrial damage and
subsequent autophagic cell death in malignant glioma cells. Cancer
Res. 67:6314–6324. 2007. View Article : Google Scholar
|
|
81
|
Dupere-Richer D, Kinal M, Menasche V, et
al: Vorinostat-induced autophagy switches from a death-promoting to
a cytoprotective signal to drive acquired resistance. Cell Death
Dis. 4:e4862013. View Article : Google Scholar
|
|
82
|
Takahashi A, Kimura T, Takabatake Y, et
al: Autophagy guards against cisplatin-induced acute kidney injury.
Am J Pathol. 180:517–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ching JK and Weihl CC: Rapamycin-induced
autophagy aggravates pathology and weakness in a mouse model of
VCP-associated myopathy. Autophagy. 9:799–800. 2013. View Article : Google Scholar
|