Introduction
Despite contemporary surgery, image-guided
radiotherapy and chemotherapy, glioblastoma (GB) persists or
relapses in almost all patients, with tumors almost always
recurring locally (1). The
management of recurrent GB is variable, but approaches include the
best supportive care, second surgery, reirradiation and/or systemic
therapy. Promising novel therapies for GB include temozolomide
(TMZ) (2), stereotactic
radiotherapy [such as Gamma Knife (3) and CyberKnife (4)], immunotherapy (5) and antiangiogenic agents, including
bevacizumab [a monoclonal antibody against vascular endothelial
growth factor (VEGF)] (6). Emerging
data suggests that the use of these therapies alone or in
combination may be safe and effective (7–10).
The current study presents a case of highly
aggressive GB treated with TMZ, CyberKnife radiotherapy and
concurrent autologous formalin-fixed tumor vaccination (AFTV; a
novel tumor vaccine consisting of autologous formalin-fixed tumor
fragments) (11). Following two
years without recurrence, the patient’s condition deteriorated due
to radiation necrosis, which subsequently lead to the initiation of
a bevacizumab infusion, as previous studies have suggested that the
treatment may reduce tumor necrosis (12–15).
The patient’s condition and magnetic resonance imaging (MRI)
results markedly improved and, thus far, the patient has remained
well and without recurrence. Patient provided written informed
consent.
Case report
A 58-year-old female presented with left leg
seizures to the local hospital. The patient had been well prior to
admission. On physical examination, the patient’s vital signs were
normal, as were the results of the laboratory tests.
Gadolinium-enhanced brain MRI revealed a mass (2-cm in diameter)
around the right central sulcus (Fig.
1A). Due to a suspected high-grade glioma, the patient was
transferred to the Moriyama Memorial Hospital (Tokyo, Japan) for
further diagnosis and treatment. As 11C-methionine
positron emission tomography (MET PET) is useful for evaluating
grade, type and proliferative activity of astrocytic tumors
(16), the patient underwent MET
PET, which showed a ‘hot’ lesion (Fig.
1B) and lead to the suspicion of a malignant glioma. The
seizures were intractable and, therefore, antiepileptic drugs were
administered. In addition, a progressive left hemiparesis was
observed. The tumor was highly aggressive and showed rapid growth
in less than one month, as monitored by MRI (Fig. 1C).
The tumor removal by craniotomy was immediately
performed under motor-evoked potential monitoring (MEP). A parietal
midline craniotomy was carried out and the right central sulcus was
identified by N20 phase reversal using sensory-evoked potential.
The corticotomy was performed just behind the sulcus, during which
gray glioma-like tissues were removed and submitted for
pathological analysis. Subtotal removal of the tumor was
accomplished without any MEP abnormalities (Fig. 1D). Additional treatment with TMZ,
CyberKnife radiotherapy and AFTV was also initiated following
surgery, and the patient’s condition remained stable without
recurrence for approximately two years (Fig. 1E).
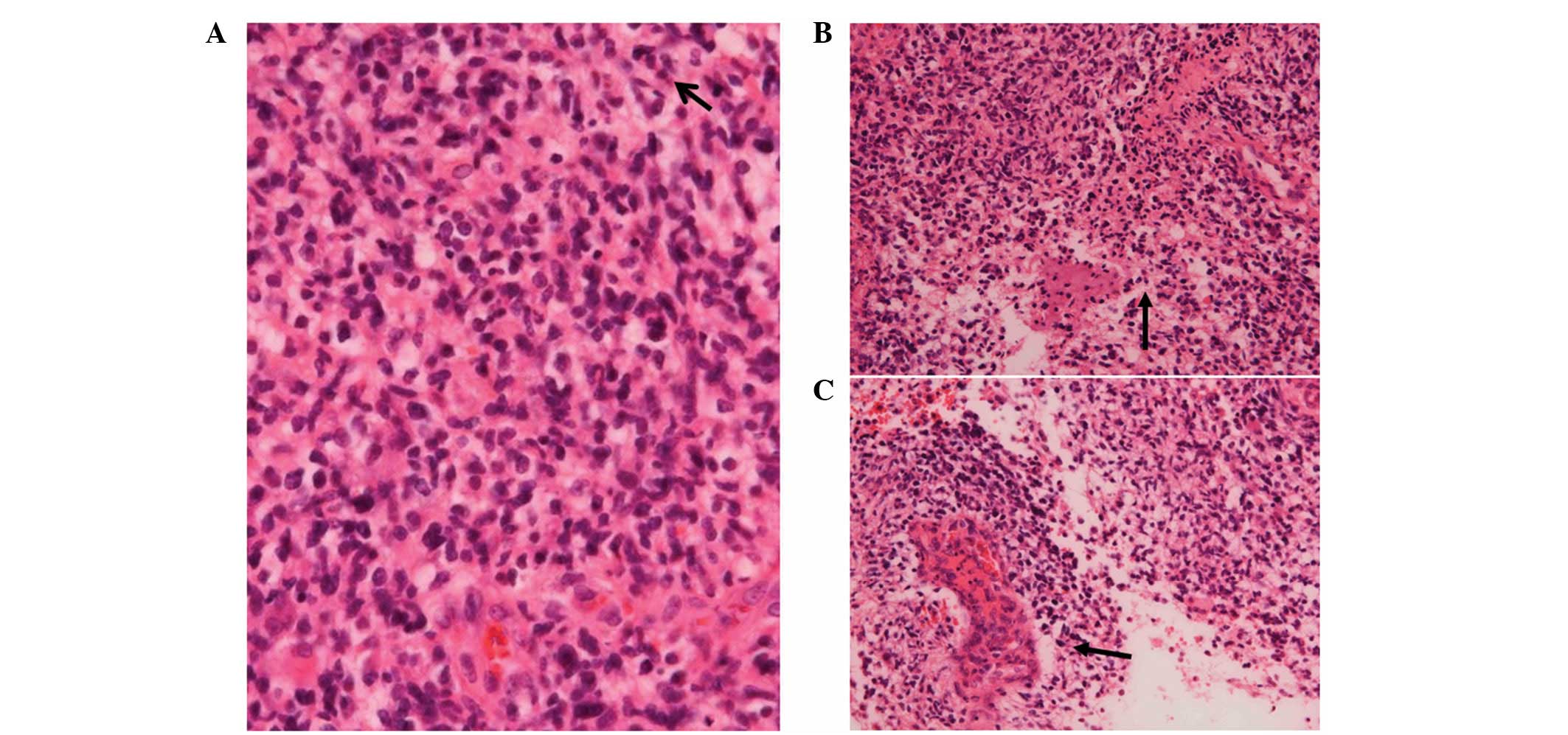
The histopathological analysis of the specimen
revealed that the tumor consisted of atypical glial cells with a
high nuclear to cytoplasmic ratio, proliferating in a fine
fibrillary background (Fig. 2A). A
stream-like arrangement of the spindled neoplastic cells, as well
as a perivascular pseudorosette-like aggregation were also detected
(Fig. 2B). These histological
features indicated astrocytic characteristics. The neoplastic cells
were also small and relatively homogeneous with only mild
pleomorphism, although, the atypia of the neoplastic cells was
high. The mitotic figures (5–6 mitoses/10 high-power fields),
glomeruloid or epithelioid microvascular proliferation (Fig. 2C) and pseudopalisades were also
detected. On the basis of these histological features, a diagnosis
of small cell GB, World Health Organization (WHO) grade IV
(17) and St. Anne-Mayo grade IV
(18), was determined. In addition,
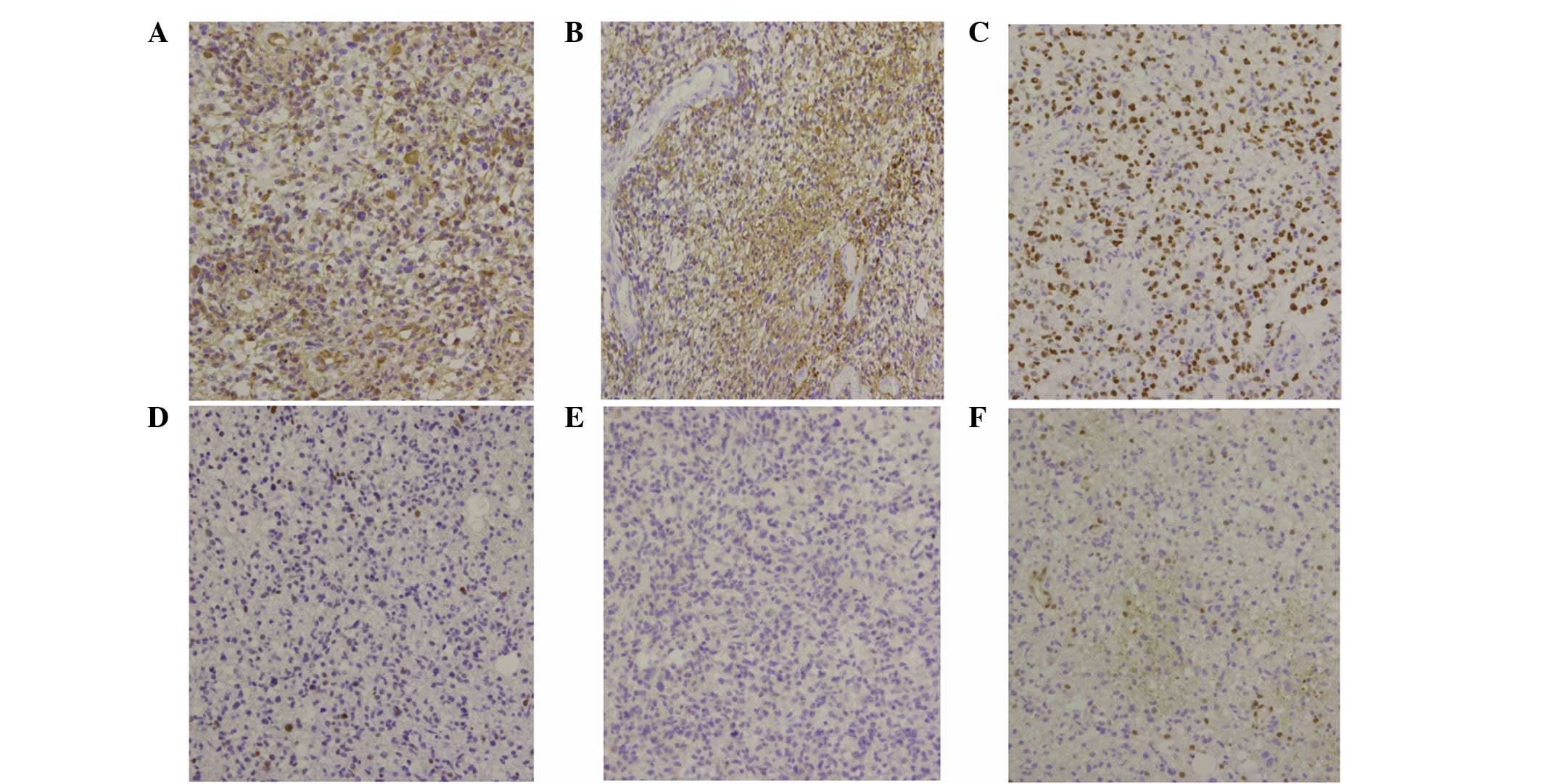
the immunohistochemistry results were consistent with the diagnosis
of GB, revealing strong immunoreactivity for vimentin (Fig. 3A) and moderate positivity for glial
fibrillary acidic protein (Fig. 3B)
and oligodendrocyte lineage transcription factor 2 (Fig. 3C). However, secondary GB could not
be confirmed, as the immunohistochemistry results for p53 and
isocitrate dehydrogenase 1 (IDH1)-R132H were negative (Fig. 3D–E) and the O6-methylguanine-DNA
methyltransferase staining was weak with only <20% of positive
cells (score of 1+; Fig. 3F).
The clinical and pathological observations indicated
a highly aggressive case of GB and, therefore, additional therapies
were required. The patient received CyberKnife radiotherapy (30 Gy
in five fractions for five consecutive days) with TMZ (75
mg/m2/day for 42 consecutive days). The patient was also
administered three courses of AFTV treatment, which was prepared as
previously described (19), with no
adverse events. The patient continued TMZ treatment at 100
mg/m2/day for five days every 28 days; however, the
patient’s lymphocyte count began to decline and subsequently, the
TMZ treatment was discontinued.
Initially, the patient remained well without TMZ
treatment; however, three months later, the patient was transferred
to our hospital due to seizures and aggravation of the left
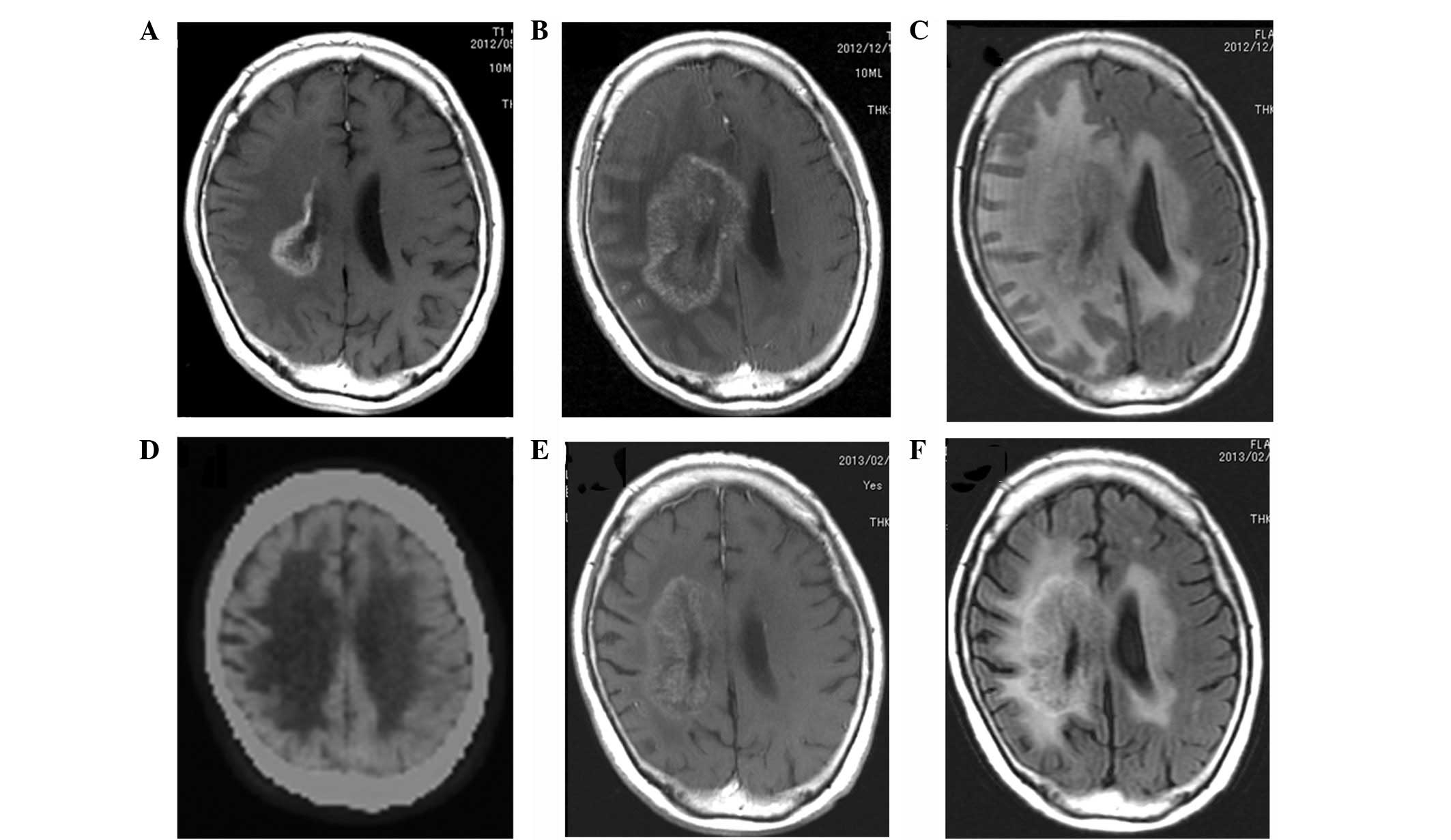
hemiparesis. The MRI studies performed on admission showed an
enhanced lesion caudal to the original lesion, which was considered
to be a recurrence (Fig. 4A).
Subsequently, the patient underwent a second cycle of CyberKnife
radiotherapy, as it was considered to be the best treatment option
at the time. Although the radiotherapy was administered without any
adverse events, following the treatment, the patient’s symptoms
appeared to worsen. Eventually, the patient’s condition declined to
the point where the patient was unable to move unaided and,
therefore, was readmitted to our hospital. Gadolinium-enhanced
brain MRI on admission revealed an increase in the lesion size and
fluid-attenuated inversion recovery image showed outstanding
perifocal edema (Fig. 4B). This
lead to the suspicion that the lesion was not due to tumor
recurrence, but rather radiation necrosis. MET PET was performed
and, similar to the gadolinium-enhanced brain MRI, no hot spot was
detected (Fig. 4C). These results
supported the diagnosis that the lesion was radiation necrosis.
Several reports have suggested that bevacizumab is an effective
treatment for radiation necrosis (12–15)
and, therefore, the patient was enthusiastic to receive this
treatment option. Bevacizumab was administered at a dose of 5 mg/kg
every two weeks and, although the patient became hyperactive
immediately following bevacizumab treatment, no adverse events were
noted. Following three courses of bevacizumab, the MRI revealed a
marked effect (Fig. 4D), which lead
to the administration of three additional cycles of bevacizumab.
Following meticulous rehabilitation, the patient’s condition
continued to improve and, finally, with family support, the patient
was discharged and returned home.
Discussion
The current study presents a case of highly
aggressive GB with a hot spot as visualized by MET PET, highly
mitotic figures as revealed by pathological study and rapid growth
as evaluated by MRI. A multidisciplinary treatment strategy was
used and, three years following treatment, the patient remains well
without recurrence. However, at one point, the patient’s symptoms
did become aggravated due to radiation necrosis, which was
successfully treated using bevacizumab. The standard treatment for
GB is stereotaxic radiotherapy with TMZ and, in the present study,
subtotal removal of the tumor was initially performed, which was
followed by the immediate initiation of CyberKnife radiotherapy
with TMZ. AFTV using paraffin-embedded tissues was also
administered with the predicted outcome of additional antitumor
activity.
Small cell GB is a recognized subtype of GB with a
highly aggressive biology, which is classified as grade IV
according to the WHO grading system. These tumors generally arise
in the cerebral hemispheres of adults (20,21)
and do not normally appear different from ordinary GB. However,
microscopy may reveal features of small cell GB morphology,
including the uniform size of cells with minimal pleomorphism and
monomorphic round to oval nuclei. As with ordinary GB,
microvascular proliferation and pseudopalisading necrosis may also
be detected. In the present study, a number of mitotic figures and
apoptotic cells were also observed, supporting a high proliferative
activity (20,21). The IDH1-R132H mutation is a key
factor in the biology and prognosis of gliomas, and it has been
shown that patients with GBs with the IDH-1-R132H mutation exhibit
improved outcomes compared with patients with wild-type IDH1
(22). It has also been shown that
the IDH-1 mutation is likely to occur during the earlier stages of
glioma tumorigenesis; therefore, a large proportion of low-grade
gliomas possess the IDH-1 mutation. In addition, IDH1-R132H
mutant-type GB may be indicative of a secondary GB progressing from
low-grade glioma (23). In the
present case, the immunohistochemistry results for the IDH1-R132H
mutation were negative, suggesting that the primary small cell GB
carried the wild-type IDH-1. The negative staining for p53
(Fig. 3D) also supported the
diagnosis of primary GB; however, the differences in molecular
abnormalities between small cell and ordinary GB remain undefined.
The results of the current morphological analysis suggest a more
aggressive phenotype for small GB than ordinary GB, but the
prognostic significance of this morphological observation requires
further investigation.
There has been a growing interest in therapeutic
modalities based on tumor-specific immune reactions (5,19) and
AFTV presents as a novel, stable and clinically durable vaccine
that is simple to produce. In comparison with other novel and
promising types of peptide vaccines, such as the Wilms tumor 1
protein vaccine, the use of AFTV does not require a preselection of
patients according to the expression of tumor-associated antigens
(19). The novel AFTV therapy was
applied to the current study with the prospect of an additional
antitumor effect. Although it was not possible to specifically
measure the individual contribution of AFTV to the patient’s
response, no adverse events were attributed to this treatment and
the patient continues to do well.
In the present study, the highly conformal and
accurate CyberKnife radiotherapy was administered to the patient in
fractions. Although numerous studies have reported the use of Gamma
Knife radiotherapy for GB, this approach requires the localization
and immobilization of the target with the attachment of a head
frame to the skull, as well as local anesthesia and the piercing of
the scalp with four screws to secure the frame to the outer table
of the skull. By contrast, CyberKnife radiotherapy does not require
cranial tracking, as it uses the skeletal anatomy to position the
radiation beam and is as precise as frame-based approaches.
Furthermore, by rendering the invasive head frames unnecessary, the
CyberKnife approach facilitates fractionated treatment while
maintaining radiosurgical accuracy (24).
However, reirradiation of the lesion considered to
be recurrent in the current patient using the CyberKnife approach
was found to only aggravate the lesion. Although, further
investigation using MET PET determined the lesion to be radiation
necrosis rather than a recurrence. Various approaches have been
reported for the treatment of radiation necrosis, including
corticosteroids and surgical resection. As radiation necrosis also
includes damage to the vascular endothelial cells and increases in
vascular permeability, the VEGF ligand has been implicated in the
pathogenesis of radiation necrosis, due to its function as a
vascular permeability factor. In addition, antiangiogenic therapy
with bevacizumab, which binds circulating VEGF, has been described
as an effective treatment option for radiation injury (12–15).
At present, GB remains incurable and its median
survival time following diagnosis is approximately one year. In
addition, the ~3–5% of patients who survive for more than three
years are classified as long-term survivors (25). In the present case, the pathological
and clinical characteristics were highly aggressive upon the
initial diagnosis. However, by utilizing a multidisciplinary
treatment strategy, a successful clinical course has been achieved
for three years following the initial diagnosis.
References
|
1
|
Kesari S: Understanding glioblastoma tumor
biology: the potential to improve current diagnosis and treatments.
Semin Oncol. 38(Suppl 4): S2–S10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman HS, McLendon RE, Kerby T, et al:
DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase
analysis and response to Temodal in newly diagnosed malignant
glioma. J Clin Oncol. 16:3851–3857. 1998.
|
|
3
|
Thumma SR, Elaimy AL, Daines N, et al:
Long-term survival after gamma knife radiosurgery in a case of
recurrent glioblastoma multiforme: a case report and review of the
literature. Case Rep Med. 2012:5454922012.
|
|
4
|
Villavicencio AT, Burneikiene S, Romanelli
P, et al: Survival following stereotactic radiosurgery for newly
diagnosed and recurrent glioblastoma multiforme: a multicenter
experience. Neurosurg Rev. 32:417–424. 2009. View Article : Google Scholar
|
|
5
|
Okada H, Kohanbash G, Zhu X, et al:
Immunotherapeutic approaches for glioma. Crit Rev Immunol. 29:1–42.
2009. View Article : Google Scholar
|
|
6
|
Norden AD, Young GS, Setayesh K, et al:
Bevacizumab for recurrent malignant gliomas: efficacy, toxicity,
and patterns of recurrence. Neurology. 70:779–787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park KJ, Kano H, Iyer A, et al: Salvage
gamma knife stereotactic radiosurgery followed by bevacizumab for
recurrent glioblastoma multiforme: a case-control study. J
Neurooncol. 107:323–333. 2012. View Article : Google Scholar
|
|
9
|
Conti A, Pontoriero A, Arpa D, et al:
Efficacy and toxicity of CyberKnife re-irradiation and ‘dose dense’
temozolomide for recurrent gliomas. Acta Neurochir (Wien).
154:203–209. 2012.
|
|
10
|
Cabrera AR, Cuneo KC, Vredenburgh JJ,
Sampson JH and Kirkpatrick JP: Stereotactic radiosurgery and
bevacizumab for recurrent glioblastoma multiforme. J Natl Compr
Canc Netw. 10:695–699. 2012.PubMed/NCBI
|
|
11
|
Ohno T: Autologous formalin-fixed tumor
vaccine. Curr Pharm Des. 11:1181–1188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Midgley R and Kerr D: Bevacizumab--current
status and future directions. Ann Oncol. 16:999–1004. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rahman M and Hoh BL: Avastin in the
treatment for radiation necrosis: exciting results from a recent
randomized trial. World Neurosurg. 75:4–5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong ET, Huberman M, Lu XQ and Mahadevan
A: Bevacizumab reverses cerebral radiation necrosis. Euro J Med
Res. 26:5649–5650. 2008.PubMed/NCBI
|
|
15
|
Furuse M, Kawabata S, Kuroiwa T and
Miyatake S: Repeated treatments with bevacizumab for recurrent
radiation necrosis in patients with malignant brain tumors: a
report of 2 cases. J Neurooncol. 102:471–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kato T, Shinoda J, Nakayama N, et al:
Metabolic assessment of gliomas using 11C-methionine, [18F]
fluorodeoxyglucose, and 11C-choline positron-emission tomography.
AJNR Am J Neuroradiol. 29:1176–1182. 2008.
|
|
17
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Daumas-Duport C, Scheithauer B, O’Fallon J
and Kelly P: Grading of astrocytomas. A simple and reproducible
method. Cancer. 62:2152–2165. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muragaki Y, Maruyama T, Iseki H, et al:
Phase I/IIa trial of autologous formalin-fixed tumor vaccine
concomitant with fractionated radiotherapy for newly diagnosed
glioblastoma. Clinical article. J Neurosurg. 115:248–255. 2011.
View Article : Google Scholar
|
|
20
|
Brat DJ, Parisi JE, Kleinschmidt-DeMasters
BK, et al: Surgical neuropathology update: a review of changes
introduced by the WHO classification of tumours of the central
nervous system, 4th edition. Arch Pathol Lab Med. 132:993–1007.
2008.
|
|
21
|
Burger PC, Pearl DK, Aldape K, et al:
Small cell architecture - a histological equivalent of EGFR
amplification in glioblastoma multiforme? J Neuropathol Exp Neurol.
60:1099–1104. 2001.
|
|
22
|
Yan H, Parsons DW, Jin G, et al: IDH1 and
IDH2 mutations in gliomas. N Engl J Med. 360:765–773. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohgaki H and Kleihues P: Genetic profile
of astrocytic and oligodendroglial gliomas. Brain Tumor Pathol.
28:177–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oermann E, Collins BT, Erickson KT, et al:
CyberKnife enhanced conventionally fractionated chemoradiation for
high grade glioma in close proximity to critical structures. J
Hematol Oncol. 3:222010. View Article : Google Scholar
|
|
25
|
Flechl B, Ackerl M, Sax C, et al:
Neurocognitive and sociodemographic functioning of glioblastoma
long-term survivors. J Neurooncol. 109:331–339. 2012. View Article : Google Scholar
|