Introduction
Acute lymphoblastic leukemia (ALL) is a malignancy
of hematopoietic stem cell origin with distinctive clinical,
immunophenotypic, cytogenetic and molecular biological
characteristics. ALL is the most common malignancy in children and
has five-year event-free survival rates ranging between 76 and 86%
in patients who receive protocol-based therapy. In adults, ALL is a
common disease and is generally associated with a worse prognosis.
Although intensified chemotherapy protocols may result in 70–90%
remission in adult ALL patients, the long-term survival rate is
extremely low and the probability of survival is <30–40%
(1–9). Consequently, further improvements in
the outcome of ALL therapy require the development of novel,
targeted and less toxic therapies.
The solute carrier (SLC) family of membrane
transport proteins include >300 members organized into >50
families (10). Among these, SLC25
is notable as it is localized on the inner mitochondrial membrane
and is, therefore, referred to as a mitochondrial carrier (11). SLC25 is a family of structurally and
functionally related proteins that contain six α-helical
membrane-spanning regions (12).
SLC25 member proteins are encoded by nuclear genes and are
synthesized in the cytosol. Newly synthesized proteins subsequently
translocate into the inner membranes of mitochondria, where they
transport various substrates, such as metabolites, nucleotides and
cofactors between the cytoplasm and the mitochondrial matrix
(13). SLC25A38 belongs to the
SLC25 family (13) and previous
studies have found that variations in the SLC25A38 gene, which is
located on chromosome 3p22, are responsible for severe
pyridoxine-refractory congenital sideroblastic anemia (14–16).
The gene encodes a mitochondrial carrier protein required for
erythropoiesis and it may act by importing glycine into
mitochondria or may act as a transporter of
glycine/5-aminolevulinic acid (ALA) across the mitochondrial inner
membrane (14–16). Transport of glycine/ALA across the
mitochondria is a crucial and rate-limiting step for the synthesis
of heme, which is essential in various biological processes, such
as respiration, detoxification and signal transduction (17,18).
SLC25A38 mutations can lead to glycine/ALA transport disorder, thus
affecting the synthesis of heme.
Notch signaling pathway activation is known to
contribute to the pathogenesis of a spectrum of human malignancies,
including T-cell lymphoblastic leukemia and multiple myeloma (MM)
(19–20). The Notch family of proteins is a
group of four highly conserved receptors (Notch 1–4) that are
expressed on the cell surface and directly regulate gene
transcription. It has been reported that Notch1 and its ligand
Jagged1 are proteins with important roles in the growth of leukemia
cells. High levels of Notch1 and Jagged1 are common in acute
myeloid leukemia and chronic lymphocytic leukemia samples from
patients, and leukemia cell lines (21). Notch activation is associated with
an improved early therapeutic response and increased sensitivity to
glucocorticoids (22). Therefore,
it is important to investigate the correlation between SLC25 and
Notch protein expression in ALL patients.
Recently, our laboratory found that SLC25A38 is a
general pro-apoptotic protein that regulates intrinsic
caspase-dependent apoptosis by modulating heme biosynthesis. It was
demonstrated that SLC25A38 is abundantly expressed in the liver
during early embryonic development, thus, indicating the
involvement of SLC25A38 in hematopoiesis. In addition, it was found
that SLC25A38 was able to form homodimers (23) and an earlier report demonstrated
that SLC25A38 is highly expressed in erythroid cells (10). Therefore, we hypothesized that the
SLC25A38 protein may be significant in the pathological changes
associated with leukemia. To further clarify the significance of
SLC25A38 protein expression in ALL, samples were collected from
patients with different types of lymphoblastic leukemia, and the
abnormal expression of SLC25A38 protein and its impact on the
prognosis of ALL was observed.
Materials and methods
Cells and antibodies
RPMI 8226 and U266 (the MM cell lines), and Molt-4
and Jurkat (leukemia cell lines) cells were obtained from the
Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, China).
All the cell lines were seeded at 0.3×106/ml RPMI-1640
medium, supplemented with 10% fetal bovine serum (FBS) and
penicillin/streptomycin, and maintained at 37°C in a humidified
atmosphere containing 5% CO2.
Rabbit anti-SLC25A38 and anti-Notch1 antibodies were
obtained from Abcam (Cambridge, MA, USA), rabbit
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies
were obtained from Cell Signaling Technology, Inc. (Beverly, MA,
USA) and fluorescence-conjugated secondary antibodies were obtained
from Pierce Biotechnology, Inc. (Rockford, IL, USA).
Patient samples
Fifty-five bone marrow samples were collected at
diagnosis from 32 adult patients with ALL (median age, 29 years;
range 18–74 years), 23 infant ALL patients (median age, 4 years;
range between 10 months and 12 years). Twelve peripheral blood
samples were collected from healthy volunteers (median age, 28
years; range, 22–35 years). The patients provided written informed
consent for the bone marrow collection for diagnostic and research
purposes according to the Declaration of Helsinki and the study was
approved by the ethics committee of the Zhongshan Hospital of
Xiamen University (Xiamen, China). The leukemia diagnosis was
established according to French-American-British (24) and World Health Organization
(25) classifications and
immunophenotyping was conducted by flow cytometry (equipped with
488 nm and other wavelength excitation lasers; 1×106
cells per tube; BD FACSCalibur Flow Cytometer, BD Biosciences,
Franklin Lakes, NJ, USA) using a panel of monoclonal antibodies.
Reverse transcription-polymerase chain reaction analysis for
BCR-ABL and MLL-AF4 was performed at the time of diagnosis.
Sample preparation
Bone marrow mononuclear cells were isolated by
FICOLL gradient centrifugation with lymphocyte cell separation
medium (Tianjin Hao Yang Biological Manufacture Co., Ltd., Tianjin,
China) at 350 × g for 30 min at room temperature and cultured in
RPMI-1640 containing 10% FBS, l-glutamine and
penicillin/streptomycin. The lymphocytes and cell lines were lysed
for 30 min on ice in lysis buffer containing freshly prepared
protease inhibitors. The cell debris was removed by centrifugation
(14,000 × g for 15 min at 4°C). The protein contents of the lysates
were determined using the Bio-Rad Protein assay kit (Bio-Rad,
Hercules, CA, USA) and a BSA standard.
Western blot analysis
Equal amounts of protein lysates (40 μg) were
analyzed by SDS-PAGE and were electrotransferred to polyvinylidene
difluoride membranes. The membranes were washed with 1X
Tris-buffered saline 1% Tween-20 (TBST), blocked for 1 h at room
temperature (RT) in 5% milk/TBST, and immunoblotted with specific
antibodies, which included the rabbit anti-SLC25A38, anti-Notch1
(Abcam) and anti-GAPDH (Cell Signaling Technology, Inc.) monoclonal
antibodies. GAPDH served as a loading control. On the subsequent
day, the membranes were washed with 1X TBST and incubated with goat
anti-rabbit horseradish peroxidase-conjugated secondary antibodies
diluted to 1:4,000 in 5% milk/TBST for 1 h at RT. The antibodies
were detected using an enhanced chemiluminescence detection kit
(Vazyme Biotech Co., Ltd., Nanjing, China). The relative densities
of the presenting quantity of target proteins were assessed using
Quantity One software (Bio-Rad) followed by normalization against
the housekeeping protein, GAPDH.
Statistical analysis
Data are summarized as means ± standard deviation
and comparisons of the means between groups were performed using
Student’s t-test. SPSS software for Windows 13.0 was used to
determine the P-values and P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of SLC25A38 protein in
leukemia cell lines
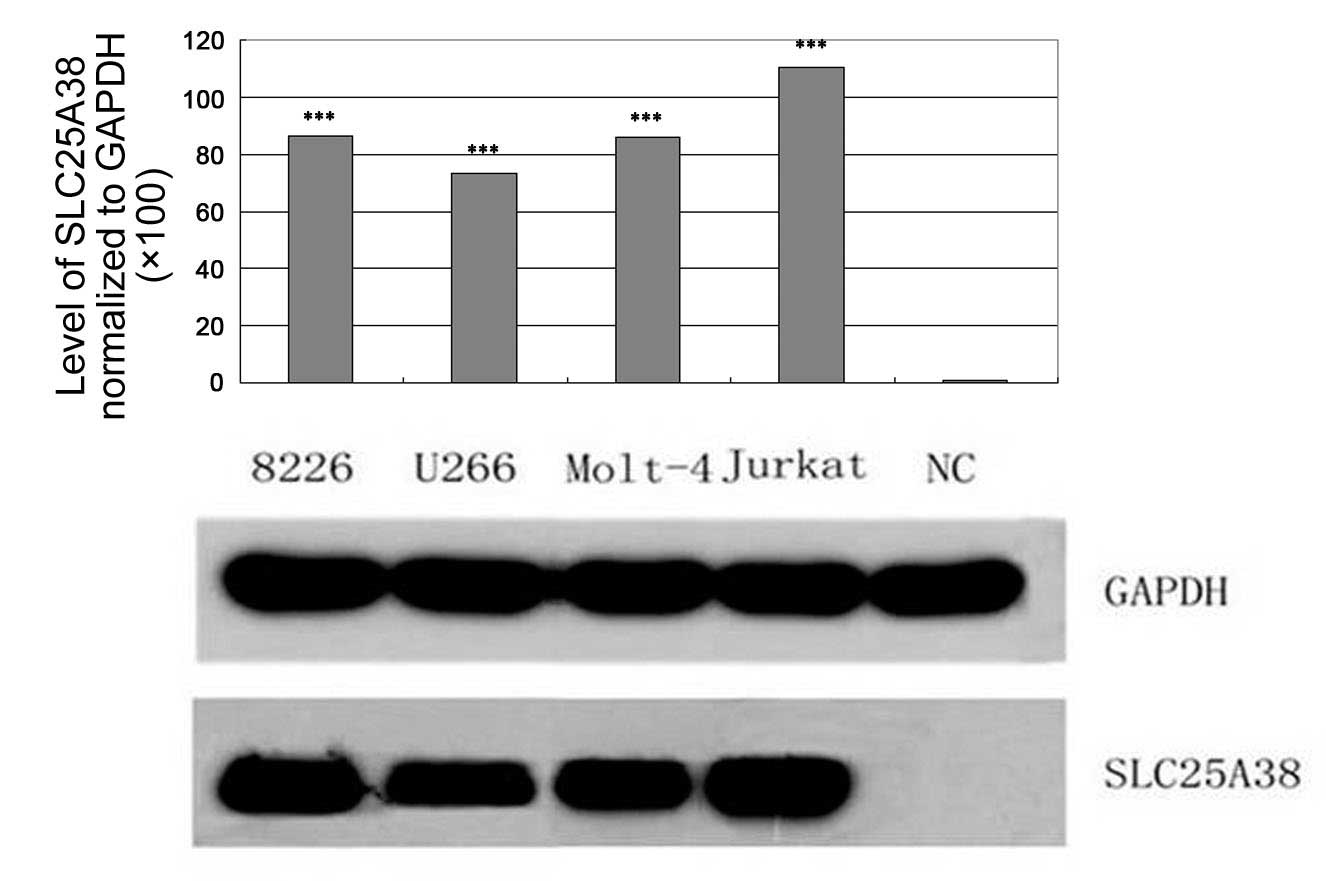
Western blot analysis was used to analyze the
expression of SLC25A38 protein in various MM and leukemia cell
lines. As shown in Fig. 1, an
overexpression of SLC25A38 protein was observed in four cell lines.
There was no SLC25A38 protein expression detected in the normal
lymphocyte cells from the healthy volunteers.
SLC25A38 protein expression in ALL
patients
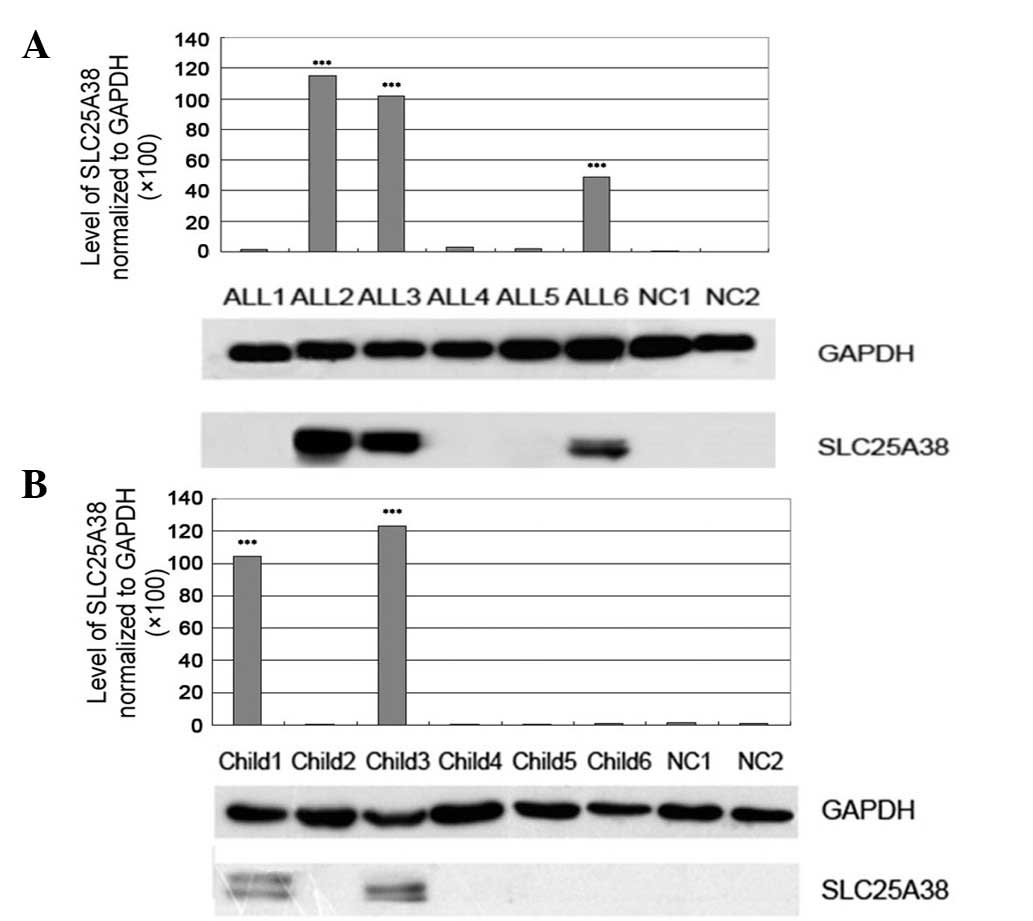
The expression of SLC25A38 protein was detected in
the 55 leukemia patients and the results showed that a high
expression of SLC25A38 was common in adult (15/32, 46.9%) and
infant (7/23, 30.4%) ALL patients (Fig.
2A and B).
Expression level of SLC25A38 protein
reflects the tumor burden
To obtain a more reliable conclusion, two adult ALL
patients with positive SLC25A38 were observed. It was found that
the SLC25A38 expression level significantly reduced or disappeared
after receiving combined chemotherapy, however, the expression
returned on ALL recurrence (Fig. 3A and
B). The results also indicated that the SLC25A38 expression
level was associated with the proportion of blast cells within the
bone marrow.
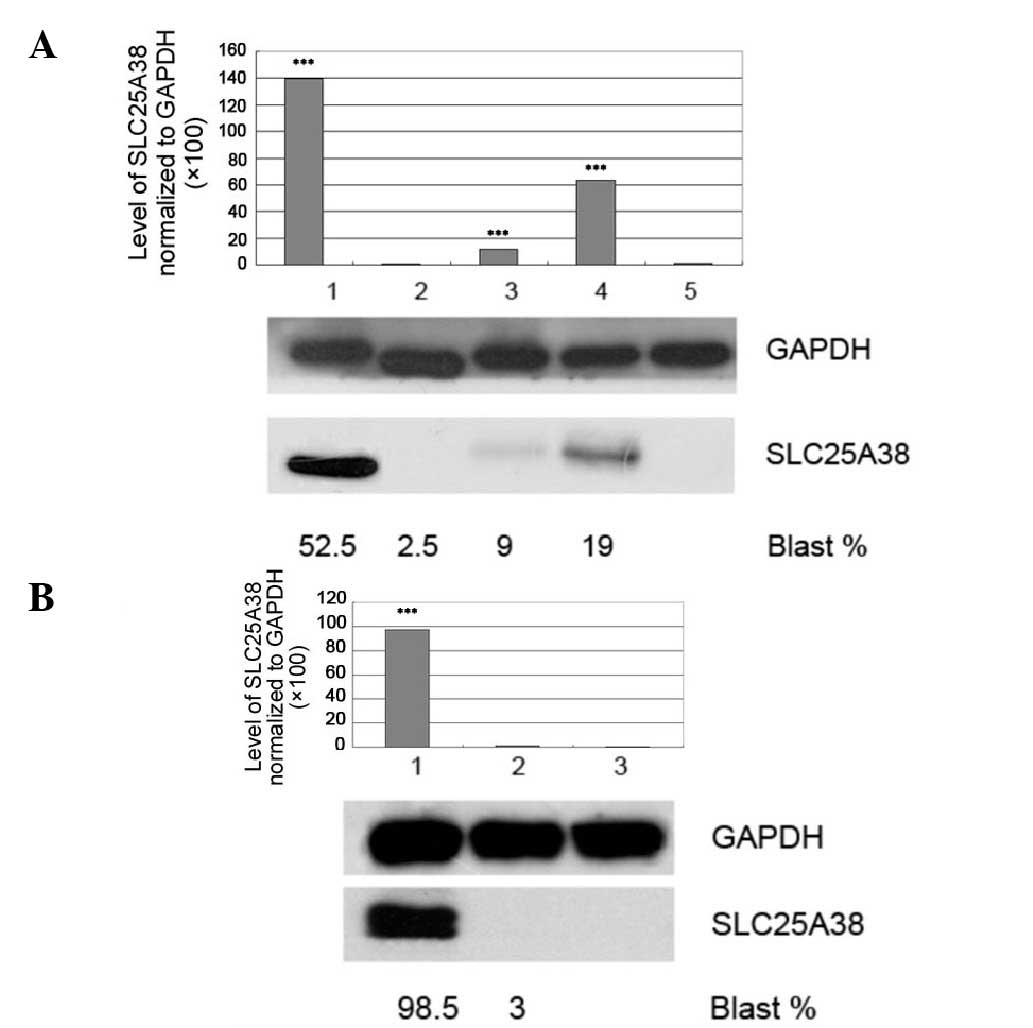 | Figure 3Expression of SLC25A38 protein was
associated with the blast cell burden. Two adult ALL patients with
SLC25A38-protein positive expression were observed. Bone marrow
mononuclear cells were collected from the patients at different
phases (including newly diagnosed, remission and relapse). The
results showed that the level of SLC25A38 protein expression was
associated with the blast cell proportion in the bone marrow.
During complete remission, SLC25A38 protein expression ceased,
however, it was observed during ALL relapse. (A) The level of
SLC25A38 protein expression with varying blast cell proportions in
the bone marrow: Lanes 1–4, adult ALL patient no. 1; lane 5, NC.
(B) The level of SLC25A38 protein expression with varying blast
cell proportions in the bone marrow: Lanes 1–2, adult ALL patient
no. 2; lane 3, NC. Data are presented relative to the expression of
GAPDH. ***P<0.0001 compared with the NC. GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; ALL, acute lymphoblastic
leukemia; NC, normal control. |
Notch1 protein expression on
SLC25A38-positive cells
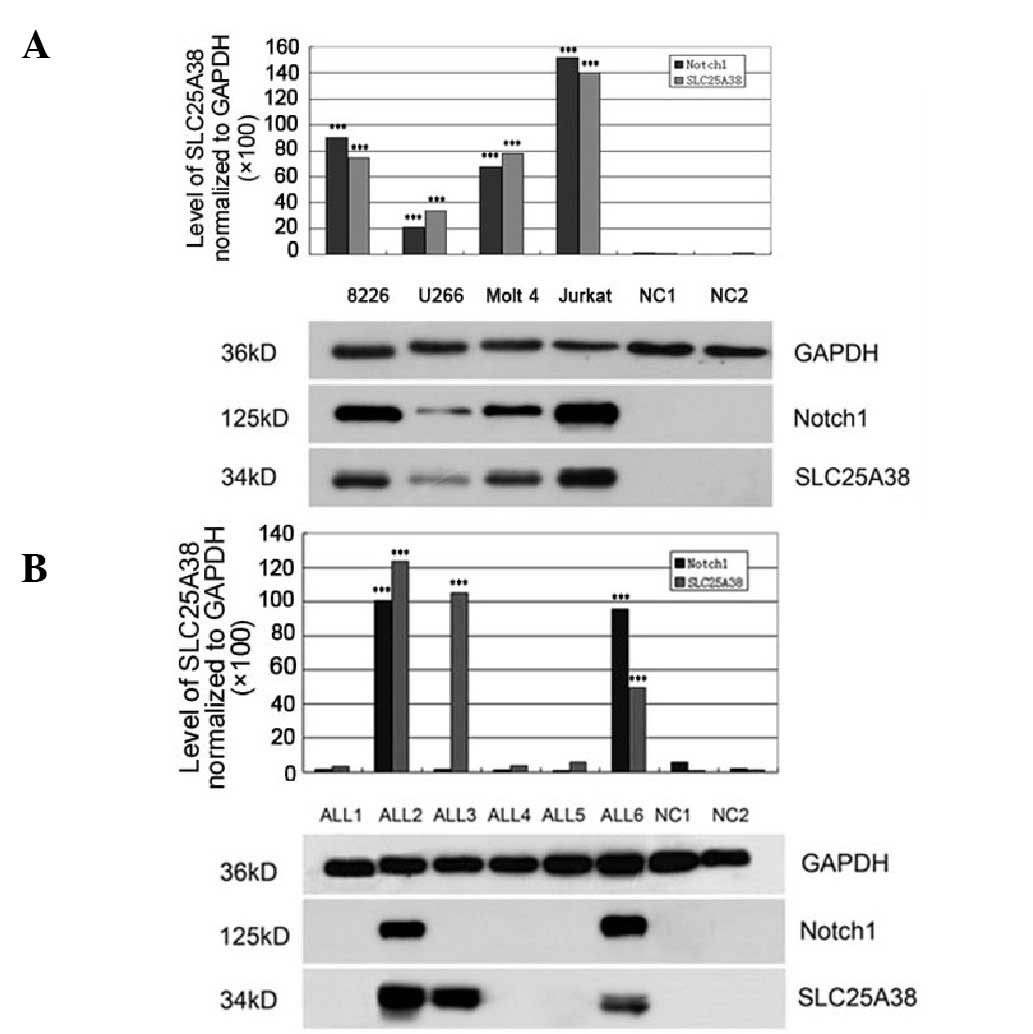
In the present study, Notch1 protein expression was
analyzed in the cell lines and the patient samples. As shown in
Fig. 4A, U266 cells marginally
expressed the Notch1 protein; RPMI 8226, Molt-4 and Jurkat cells
expressed Notch1, with the highest levels observed in the Jurkat
cells. In addition, Notch1 and SLC25A38 proteins were co-expressed
in the cell lines. Notch1 overexpression was detected in more than
half of the adult ALL patients who were SLC25A38-positive (8/15 ALL
patients, 53.3%); however, Notch1 protein expression was not
detected in the patients with negative SLC25A38 protein expression
(Fig. 4B).
Discussion
ALL is a genetically heterogeneous disease in which
different genetic alterations cooperate to promote the uncontrolled
clonal proliferation and survival of leukemic lymphoblasts
(26–29). The outcome of ALL patients has
improved significantly over the last two decades as a result of
combination chemotherapy. However, patients with several ALL
subtypes continue to show a poor prognosis, and treatments are
responsible for the short and long-term toxicities experienced by
certain long-term surviving patients. Unfortunately, the majority
of patients eventually experience relapse or succumb to their
disease after developing drug resistance (30). However, monoclonal antibodies, gene
inhibitors and upregulation of microRNAs (31–33)
may provide promising tools in the search for ALL-targeted
therapy.
As it was discovered recently, the normal
physiological function of SLC25A38 protein remains obscure. Similar
to the observations of the present study, a study by Guernsey et
al (14) indicates that the
SLC25A38 protein is related to heme synthesis and oxygen
transportation in cells. Downregulation of SLC25A38 resulted in a
significant decrease in the levels of cellular heme, which affects
mitochondrial respiration and oxidative phosphorylation. To the
best of our knowledge, this is the first study to report SLC25A38
protein expression in ALL and preliminarily identify that a high
expression of SLC25A38 is a common phenomenon in ALL, with certain
clinical significance. In cellular experiments, it was found that
four cell lines expressed high levels of SLC25A38. Additionally,
SLC25A38 expression was observed to be common in adult ALL patients
(15/32, 46.9%) and in infant ALL patients (7/23, 30.4%).
Notch signaling affects multiple processes that
govern normal morphogenesis, programmed cell death and cellular
proliferation. Altered Notch signaling has been associated with
various malignancies, including pancreatic, breast, leukemia and
lymphoma (34). Activating
mutations in the Notch1 gene are present in >50% of human T
cell-ALL (T-ALL) cases making Notch1 the most prominent oncogene,
which is specifically involved in the pathogenesis of this disease,
and defining T-ALL as a disease that is primarily characterized by
aberrant Notch1 activation (35–38).
In the present study, the expression of the SLC25A38 and Notch1
proteins was identified to be common in cell lines as well as in
the majority of ALL patients exhibiting positive SLC25A38
expression. Therefore, it was hypothesized that the overexpression
of SLC25A38 may be connected to the activation of the Notch
signaling pathway.
In conclusion, in the present study the
overexpression of SLC25A38 protein in the cell lines and in ALL
patients was observed. The data indicates that future studies are
required to determine the role of SLC25A38 in the pathogenesis of
leukemia, and establish whether overexpression of this protein
regulates the proliferation, differentiation and apoptosis of
leukemia cells. The present findings are considered to be important
for determining novel leukemia markers and molecular targets for
the treatment of leukemia.
Acknowledgements
The authors would like to thank Dr Jiangning Zhao,
Dr Jiasheng Hu and Dr Yanhong Zhuang, who provided the bone marrow
samples for the study and medical care for the patients at
Zhongshan Hospital of Xiamen University (Xiamen, China). The
present study was partially supported by the National Natural
Science Fund (grant no. 81172246) and the ‘985’ Program of the
Medical College of Xiamen University.
References
|
1
|
Barrett AJ, Horowitz MM, Pollock BH, et
al: Bone marrow transplants from HLA-identical siblings as compared
with chemotherapy for children with acute lymphoblastic leukemia in
a second remission. N Engl J Med. 331:1253–1258. 1994. View Article : Google Scholar
|
|
2
|
Schroeder H, Gustafsson G,
Saarinen-Pihkala UM, et al: Allogeneic bone marrow transplantation
in second remission of childhood acute lymphoblastic leukemia: a
population-based case control study from the Nordic countries. Bone
Marrow Transpl. 23:555–560. 1999. View Article : Google Scholar
|
|
3
|
McNeil DE, Coté TR, Clegg L and Mauer A:
SEER update of incidence and trends in pediatric malignancies:
acute lymphoblastic leukemia. Med Pediatr Oncol. 39:554–557. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bassan R: Evolving strategies for the
management of high-risk adult acute lymphoblastic leukemia.
Haematologica. 90:12992005.PubMed/NCBI
|
|
5
|
Pui CH and Evans WE: Treatment of actue
lymphoblastic leukemia. N Engl J Med. 354:166–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pullarkat V, Slovak ML, Kopecky KJ, et al:
Impact of cytogenetics on the outcome of adult actue lymphoblastic
leukemia: results of Southwest Oncology Group 9400 study. Blood.
111:2563–2572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marks DI, Paietta EM, Moorman AV, et al:
T-cell acute lymphoblastic leukemia in adults: clinical features,
immunophenotype, cytpgenetics, and outcome from the large
randomized prospective trial (UKALLXII/ECOG 2993). Blood.
114:5136–5145. 2009. View Article : Google Scholar
|
|
9
|
Pui CH, Pei D, Sandlund JT, et al:
Long-term results of St Jude Total Therapy Studies 11, 12, 13A,
13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia.
24:371–382. 2009.
|
|
10
|
Hediger MA, Romero MF, Peng JB, et al: The
ABCs of solute carriers: physiological, pathological and
therapeutic implications of human membrane transport proteins.
Pflugers Arch. 447:465–468. 2004. View Article : Google Scholar
|
|
11
|
Palmieri F: The mitochondrial transporter
family (SLC25): physiological and pathological implications.
Pflugers Arch. 447:689–709. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pebay-Peyroula E, Dahout-Gonzalez C, Kahn
R, et al: Structure of mitochondrial ADP/ATP carrier in complex
with carboxyatractyloside. Nature. 426:39–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haitina T, Lindblom J, Renström T and
Fredriksson R: Fourteen novel human members of mitochondrial solute
carrier family 25 (SLC25) widely expressed in the central nervous
system. Genomics. 88:779–790. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guernsey DL, Jiang H, Campagna DR, et al:
Mutations in mitochondrial carrier family gene SLC25A38 cause
nonsyndromic autosomal recessive congenital sideroblastic anemia.
Nat Genet. 41:651–653. 2009. View
Article : Google Scholar
|
|
15
|
Bergmann AK, Campagna DR, Mcloughlin EM,
et al: Systematic molecular genetic analysis of congenital
sideroblastic anemia: evidence for genetic heterogeneity and
identification of novel mutations. Pediatr Blood Cancer.
54:273–278. 2010.
|
|
16
|
Kannengiesser C, Sanchez M, Sweeney M, et
al: Missense SLC25A38 variations play an important role in
autosomal recessive inherited sideroblastic anemia. Haematoligica.
96:33242011.
|
|
17
|
Atamna H: Heme, iron, and the
mitochondrial decay of ageing. Ageing Res Rev. 3:303–318. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryter SW and Tyrrell RM: The heme
synthesis and degradation pathways: role in oxidant sensitivity.
Heme oxygenase has both pro- and antioxidant properties. Free
Radical Biol Med. 28:289–309. 2000. View Article : Google Scholar
|
|
19
|
Leong KG and Karsan A: Recent insights
into the role of Notch signaling in tumorigenesis. Blood.
107:2223–2233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aster JC, Blacklow SC and Pear WS: Notch
signalling in T-cell lymphoblastic leukaemia/lymphoma and other
haematological malignancies. J Pathol. 223:262–273. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanamori E, Itoh M, Tojo N, et al: Flow
cytometric analysis of Notch1 and Jagged1 expression in normal
blood cells and leukemia cells. Exp Ther Med. 4:397–400.
2012.PubMed/NCBI
|
|
22
|
Mansour MR, Sulis ML, Duke V, et al:
Prognostic implications of NOTCH1 and FBXW7 mutations in adults
with T-cell acute lymphoblastic leukemia treated on the MRC
UKALLXII/ECOG E2993 protocol. J Clin Oncol. 27:4352–4356. 2009.
View Article : Google Scholar
|
|
23
|
Zhang H, Zhang YW, Chen Y, et al:
Appoptosin is a novel pro-apoptotic protein and mediates cell death
in neurodegeneration. J Neurosci. 32:15565–15576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bennett JM, Catovsky D, Daniel MT, et al:
Proposals for the classification of the acute leukaemias.
French-American-British (FAB) co-operative group. Br J Haematol.
33:451–458. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vardiman JW, Thiele J, Arber DA, et al:
The 2008 revision of the World Health Organization (WHO)
classification of myeloid neoplasms and acute leukemia: rationale
and important changes. Blood. 114:937–951. 2009. View Article : Google Scholar
|
|
26
|
Ferrando AA and Look AT: Clinical
implications of recurring chromosomal and associated molecular
abnormalities in acute lymphoblastic leukemia. Semin Hematol.
37:381–395. 2000. View Article : Google Scholar
|
|
27
|
Ferrando AA, Neuberg DS, Staunton J, et
al: Gene expression signatures define novel oncogenic pathways in T
cell acute lymphoblastic leukemia. Cancer Cell. 1:75–87. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soulier J, Clappier E, Cayuela JM, et al:
HOXA genes are included in genetic and biologic networks defining
human acute T-cell leukemia (T-ALL). Blood. 106:274–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferrando AA, Armstrong SA, Neuberg DS, et
al: Gene expression signatures in MLL-rearranged T-lineage and
B-precursor acute leukemias: dominance of HOX dysregulation. Blood.
102:262–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aifantis I, Raetz E and Buonamici S:
Molecular pathogenesis of T-cell leukaemia and lymphoma. Nat Rev
Immunol. 8:380–390. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Budhu A, Ji J and Wang XW: The clinical
potential of microRNAs. J Hematol Oncol. 3:372010. View Article : Google Scholar
|
|
32
|
Fernando TR, Rodriguez-Malave NI and Rao
DS: MicroRNAs in B cell development and malignancy. J Hematol
Oncol. 5:72012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Whitehead KA, Langer R and Anderson DG:
Knocking down barriers: advances in siRNA delivery. Nat Rev Drug
Discov. 8:129–138. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin L, Velazquez OC and Liu ZJ: Notch
signaling: emerging molecular targets for cancer therapy. Biochem
Pharmacol. 80:690–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Breit S, Stanulla M, Flohr T, et al:
Activating NOTCH1 mutations predict favorable early treatment
response and long-term outcome in childhood precursor T-cell
lymphoblastic leukemia. Blood. 108:1151–1157. 2006. View Article : Google Scholar
|
|
36
|
Weng AP, Ferrando AA, Lee W, et al:
Activating mutations of NOTCH1 in human T cell acute lymphoblastic
leukemia. Science. 306:269–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aster JC, Pear WS and Blacklow SC: Notch
signaling in leukemia. Annu Rev Pathol. 3:587–613. 2008. View Article : Google Scholar
|
|
38
|
Zou J, Li P, Lu F, et al: Notch1 is
required for hypoxia-induced proliferation, invasion and
chemoresistance of T-cell acute lymphoblastic leukemia cells. J
Hematol Oncol. 6:32013. View Article : Google Scholar : PubMed/NCBI
|