Introduction
Cellular senescence and apoptosis are potent tumor
suppression mechanisms for the regulation of cell growth arrest and
limitation of aberrant cell proliferation. It has been reported
that age is the major risk factor for the development of cancer in
mammals, and aging is often associated with the incidence of
cancer, including human primary lung adenocarcinoma (1–3).
Silent information regulator 2 (Sir2) has been reported to extend
the lifespan by ≤70% in budding yeast. Mammals possess seven
homologs of yeast Sir2, which are known as the Sirtuin family
(Sirtuin 1–7). Sirtuin 1 (Sirt1) is the most thoroughly studied and
is similar to yeast Sir2, which is a nicotinamide adenine
dinucleotide-dependent class III histone deacetylase. The foremost
function of Sirt1 is to deacetylate histone proteins, including H1,
H3 and H4, and non-histone proteins. Non-histone proteins comprise
of three major groups: Transcription factors, including p53, p73,
androgen receptor, forkhead box protein O (FOXO), E2F1,
hypermethylated in cancer 1 (HIC1) and nuclear factor-κB; signaling
factors, including mothers against decapentaplegic homolog 7 and
endothelial nitric oxide synthase; and DNA repair proteins, such as
Ku-70. Sirt1, with its wide distribution in the cell nucleus and
cytoplasm, has been found to regulate a series of normal
physiological processes, including cell senescence, DNA repair and
stress response (4–7). There are vast numbers of downstream
molecules of Sirt1, including p53, FOXO1, FOXO3, FOXO4 and E2F1,
which are regulated by Sirt1. Simultaneously, Sirt1 activity is
regulated by its upstream molecules, for instance, p53, HIC1, E2F1,
deleted in breast cancer 1 (DBC1), human antigen R (HuR) and active
regulator of Sirt1 (AROS). Notably, a few downstream molecules of
Sirt1, including E1F2 and p53, are also considered to be upstream
molecules. These findings indicate that the interaction between
Sirt1 and its upstream or downstream molecules is the reason for
the multiple functions of Sirt1, and plays an essential and
complicated role in cells.
Under normal conditions, the tumor suppressor gene
HIC1 can negatively regulate Sirt1 transcription to inhibit Sirt1
expression. Additionally, it has been identified that in cell and
animal models, there is a circular regulator loop among HIC1, Sirt1
and p53, in which HIC1 directly represses Sirt1 transcription,
Sirt1 represses p53 activity by its deacetylation and inactivated
p53 leads to HIC1 inactivation. However, a study by Tseng et
al (8) indicated that this loop
deregulates in patients with lung squamous carcinoma and lung
adenocarcinoma (9). Similarly, DBC1
binds Sirt1 to form a stable complex to suppress the level of
Sirt1, thus inducing cell apoptosis in response to oxidase
(10).
Of course, certain agents can activate Sirt1
activity, including the tumor suppressor HuR and AROS (11). Knockdown of AROS can repress Sirt1
levels and enhance P21WAF1 to increase the G0/G1 population and
cell apoptosis (12) Certain
observations have indicated that the depletion of Sirt1 by small
interfering RNA (siRNA) induces tumor cell death with no toxicity
on normal cells (13). A previous
study has provided strong evidence that Sirt1 is significantly
overexpressed to function as a tumor promoter in mouse and human
prostate cancer (14) and acute
myeloid leukemia (15). However,
previous studies indicate that Sirt1 is an inhibitor in colon
cancer (16,17).
Certain study results indicate that regulated
transcription of Sirt1 levels in cancer cells can affect the
expression level of Sirt1. At least two feedback loops, Sirt1-p53
and Sirt1-E2F1, regulate this Sirt1 transcription. Two binding
sites of p53 interact on the Sirt1 gene promoter and usually
suppress Sirt1 gene transcription (6). In turn, Sirt1 can inhibit the activity
of p53 by deacetylating its C-terminal Lys382 residue, thus further
suppressing p53-mediated cell apoptosis following DNA damage.
Acetylation of p53 is an indispensable process for the suppression
of cell apoptosis (18). Thus,
there is a Sirt1-p53 negative-feedback loop regulating the
transcription of Sirt1. The other negative-feedback loop is located
between Sirt1 and E2F1. Etoposide-mediated DNA damage induces E2F1
expression. E2F1 induces Sirt1 expression and is an apoptosis gene
activator that can induce cell apoptosis dependently or
independently of the p53 mechanism. E2F1 is also a downstream
molecule of Sirt1, which deacetylates E2F1-induced transcription of
target genes, including Sirt1, itself to prevent cell apoptosis
(19).
In addition, it has been reported that the FOXO1,
FOXO3 and FOXO4 members of the Forkhead transcription factor family
interact with Sirt1, leading to increased resistance to stress and
apoptosis, and thus to cancer cell survival (20–23).
According to previous studies into the biological
properties of Sirt1, it was found to play an essential and complex
role in normal physiological functions, and it was demonstrated
that the dual function of Sirt1 caused by its upstream and
downstream molecules was distributed differently in various
tissues. However, it remains unknown whether Sirt1 is expressed in
patients with primary lung carcinoma. In the present study, the
expression of Sirt1 was analyzed immunohistochemically in
surgically resected human primary pulmonary adenocarcinoma tissues
from 125 patients. The effect of Sirt1 expression in tumor tissues
on the outcome of these patients was also investigated.
Materials and methods
Patients
The data was analyzed for 125 patients (71 males and
54 females) who underwent surgery for primary lung adenocarcinoma
subsequent to being diagnosed and treated at Kobe University
Hospital, Japan, between 2001 and 2004. The study was approved by
the Regional Ethics Committee for Clinical Research of Kobe
University and conducted according to the principles of the
Declaration of Helsinki. Dated and written informed consent was
obtained from all patients. Primary tumors and adjacent
non-neoplastic lung tissues were obtained at the time of surgery.
Peripheral parts of the resected lung carcinomas were sectioned,
evaluated by a pathologist and used for immunohistochemistry (IHC).
All patients were enrolled consecutively. Detailed clinical and
demographical information, prognostic factors and disease
progression were collected retrospectively.
IHC
Formalin-fixed paraffin-embedded specimens were
sectioned at the maximal area of tumor mass into 5-μm-thick slices
and sections were deparaffinized in xylene and rehydrated in
ethanol, heat-treated for 20 min in Dako REAL™ Target Retrieval
solution (no. S1699; Dako, Glostrup, Denmark) for antigen retrieval
and 10 min in Dako Protein Block Serum-Free solution (code no.
X909; Dako, Carpinteria, CA, USA). Rabbit anti-human Sirt1
monoclonal antibody ab32441 (concentration 1:200; Abcam, Cambridge,
UK) was used as the primary antibody for detection of Sirt1. The
Dako LSAB2 System-horseradish peroxidase (HRP) (DAB) kit was used
for endogenous peroxidase blocking, treatment with a secondary
antibody against anti-rabbit antibody and the visualization of HRP.
Hematoxylin staining was used as the counterstain. Images of
immunohistochemically stained sections were captured by a camera
mounted on a Keyence BZ-8000 digital microscope (Keyence, Osaka,
Japan).
Classification of immunohistochemically
stained patterns
Immunohistochemically stained sections were
classified by means of light microscopy. For the assessment of the
protein expression of Sirt1, samples were classified as
Sirt1-positive when the ratio of stained cells in all epithelial
cancer cells of a tumor tissue was >50%, and as Sirt1-negative
if it was <50%. The cut-off value was set at 50%, as this value
was statistically useful for this study (24).
The Ki67 index (Ki67 expression ratio),
hypoxia-inducible factor 1 (HIF1) expression and tumor protein p53
expression were determined by the Division of Diagnostic Pathology,
Kobe Medical University (Kobe, Japan). IHC was previously performed
for 10 cancer-related proteins (including CDC45, HIF1, psf3,
E-cadhelin and necl5) with the same paraffin-embedded specimens of
the 125 cases investigated in the present study. The correlation
between their expression in cancer cells compared with Sirt1
expression was also examined, however, only HIF1 showed a
statistically significant different (P<0.05) (24–26).
Statistical analysis
All statistical analyses were carried out with PASW
Advanced Statistics 18 software (SPSS, Inc., Chicago, IL, USA).
Baseline characteristics were expressed as percentages for
categorical variables and as means ± standard deviation for
continuous variables. Cross tabulation and χ2 tests were
used to examine the association between Sirt1 expression and
various clinicopathological parameters.
Results
Sirt1 expression in cancer cells of human
lung adenocarcinoma
The expression status of Sirt1 was determined in 125
lung adenocarcinoma and adjacent normal lung tissues by IHC, with
the use of rabbit anti-human monoclonal antibody. In normal lung
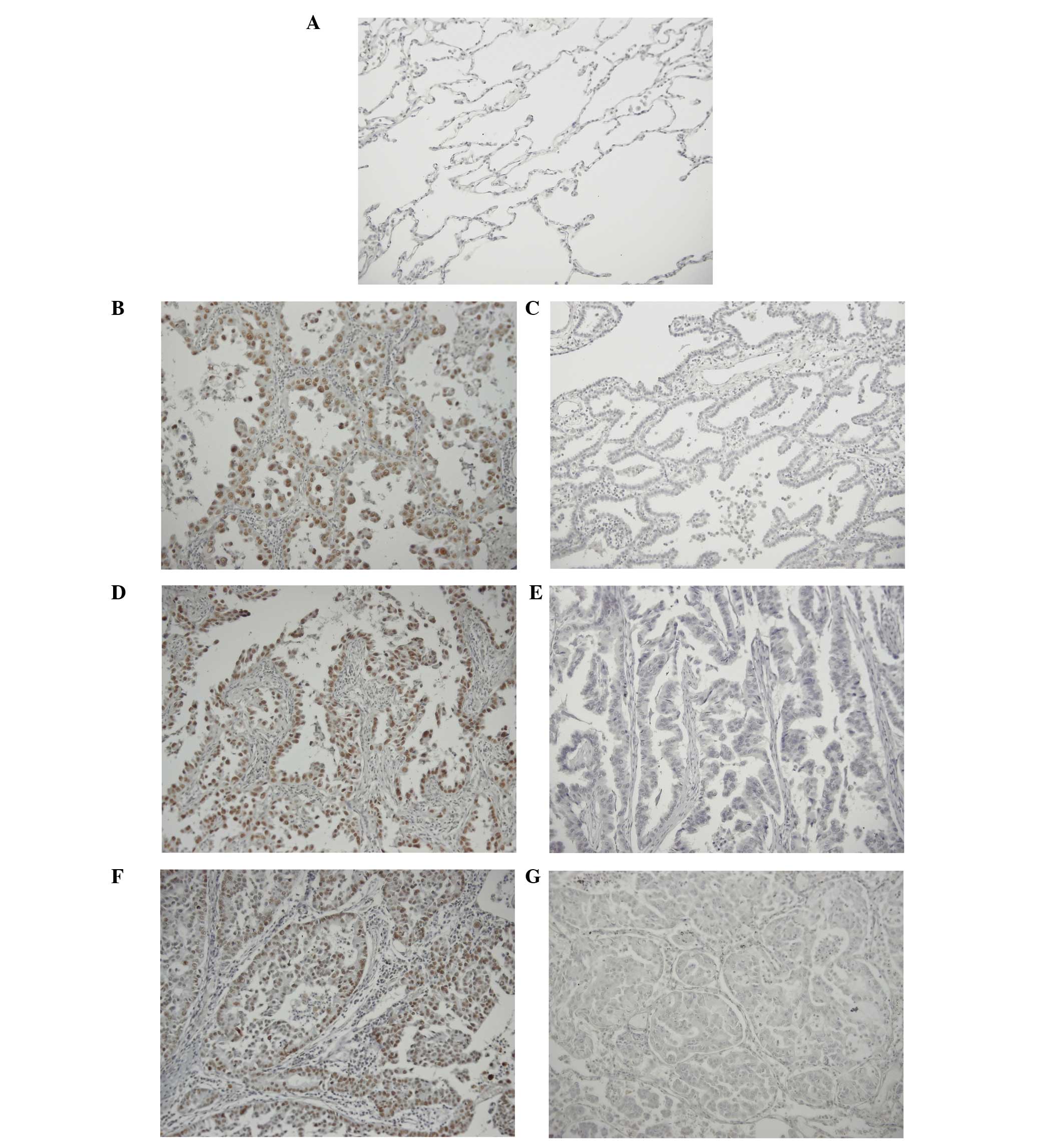
tissue, Sirt1 expression was not detected (Fig. 1A). In certain tumor tissues, the
cancer cells were stained in a scattered pattern, and the ratio of
the Sirt1-positive cells in such tissue was <10%. By contrast,
some tumor tissues showed Sirt1-positive stained cells clustered in
certain areas of the tissue, and the ratio of stained cells in such
tissue samples was >80%. These tissue samples showing clustered
staining were classified as Sirt1-positive (Fig. 1B, D and F; negative controls
Fig. 1C, E and G). Thus, the status
of Sirt1 expression was determined as follow: If >50% of cancer
cells in any microscopic field (magnification, ×200) of tumor
tissue showed staining, the tissue was considered Sirt1-positive;
if the ratio of positive staining was <50% for all the examined
microscopic field, the tissue was deemed Sirt1-negative. Of the
specimens examined, 26 (20.8%) were positive for Sirt1 and 99
(79.2%) were negative for Sirt1 expression.
Association between Sirt1 expression and
clinicopathological characteristics of patients
In order to evaluate the role of Sirt1 in lung
adenocarcinoma, Sirt1 expression was investigated in association
with any of the clinicopathological variables in the 125 enrolled
cases of primary lung adenocarcinoma (Table I). The results of the analysis
revealed that Sirt1 expression was significantly associated with
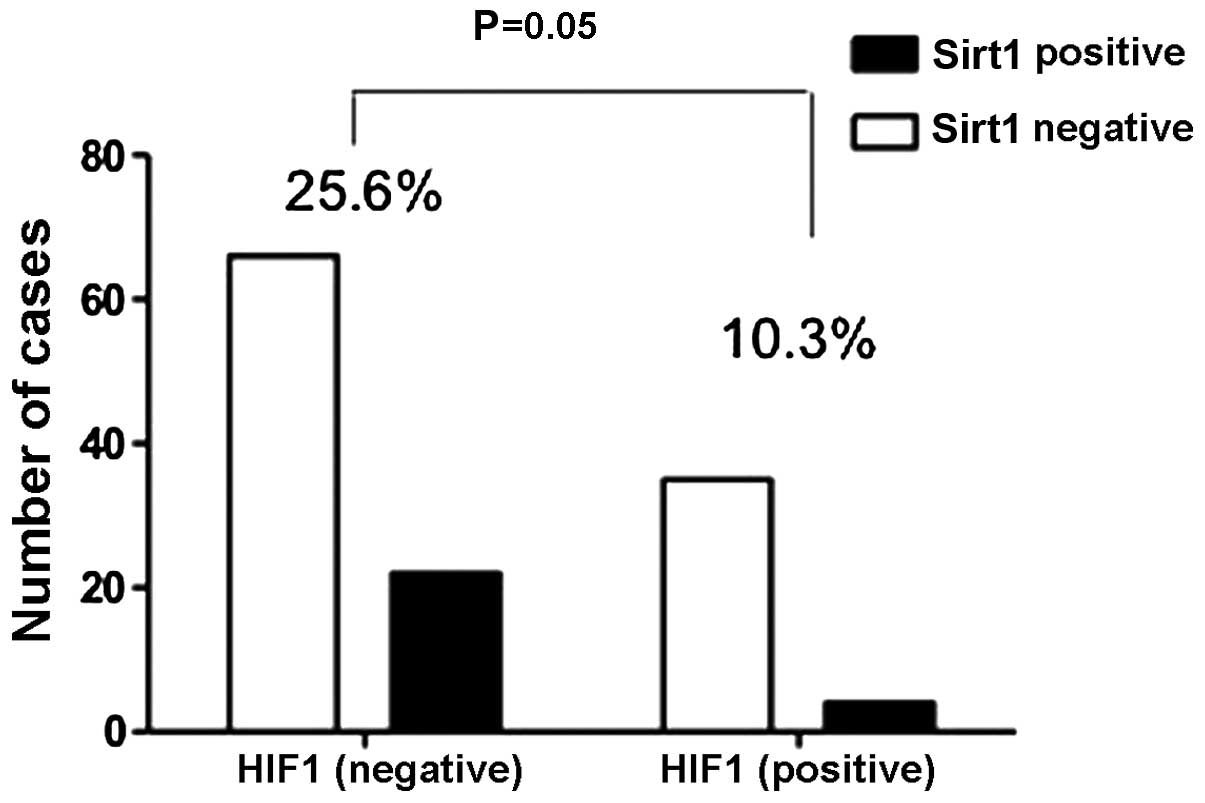
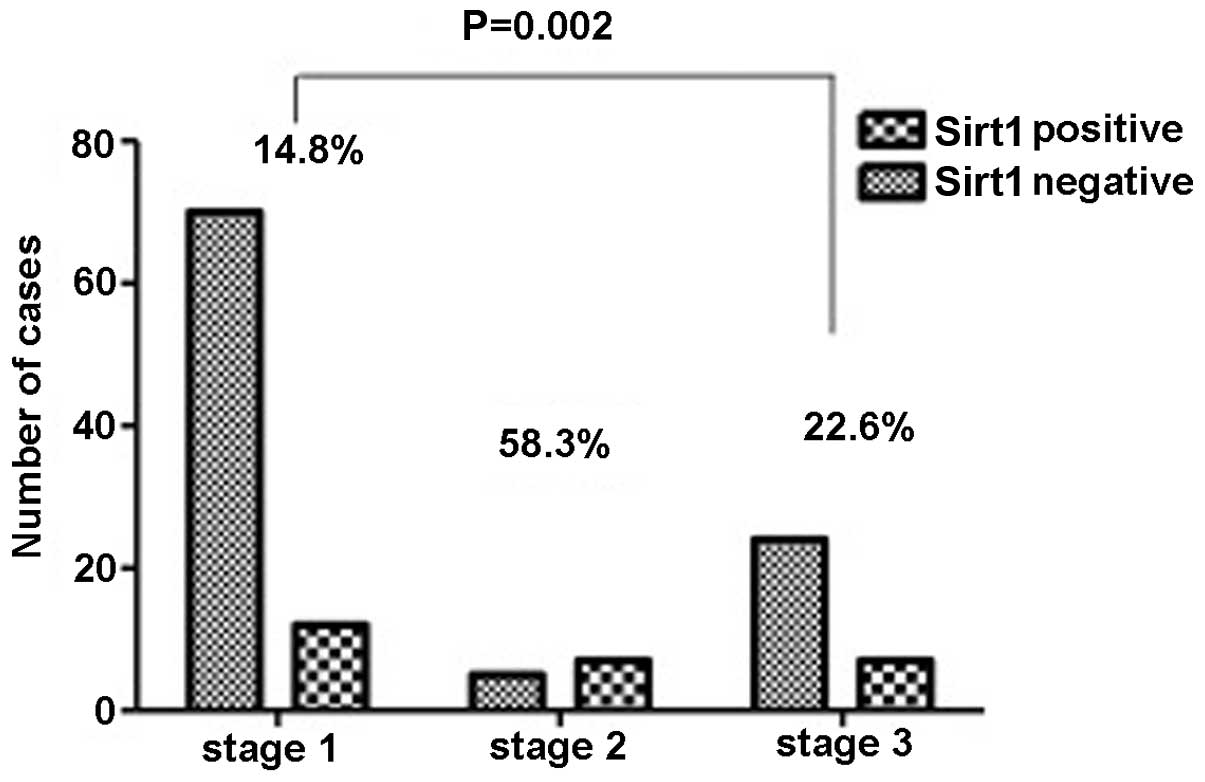
the Ki67 index (P=0.002), HIF1 expression (P=0.05) (Fig. 2), tumour-node-metastasis (TNM) stage
(P=0.002) (Fig. 3), particularly in
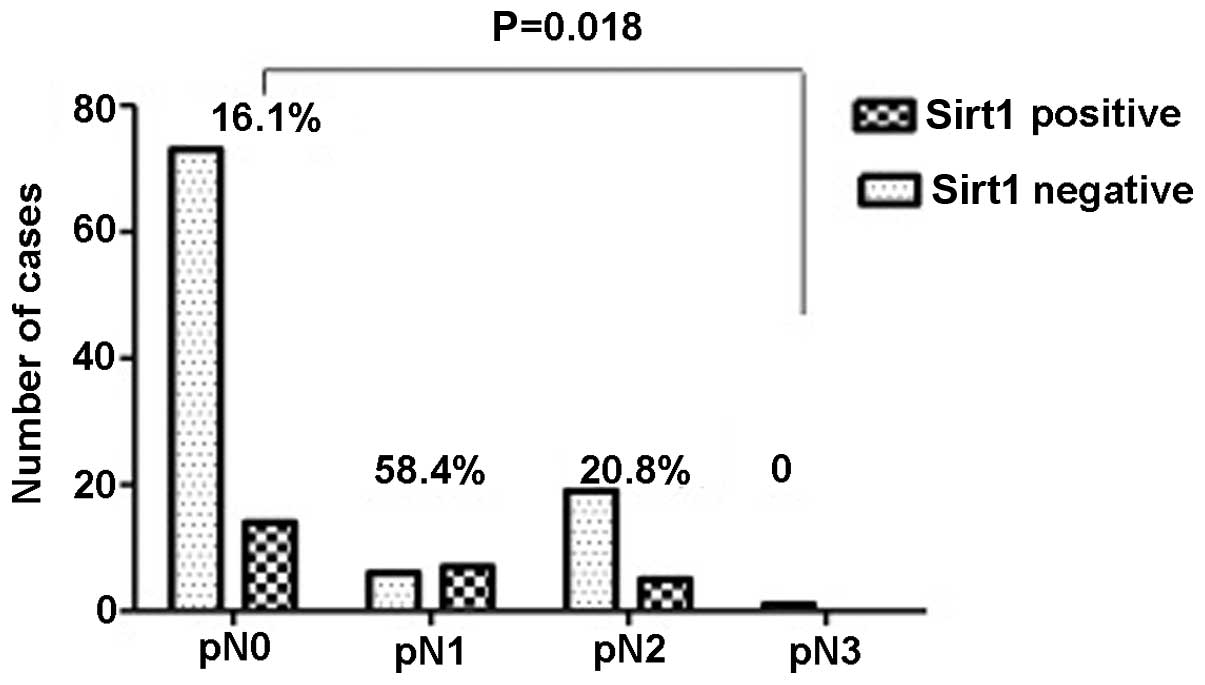
lymph node invasion (Fig. 4) and
metastasis (Fig. 5), and with a
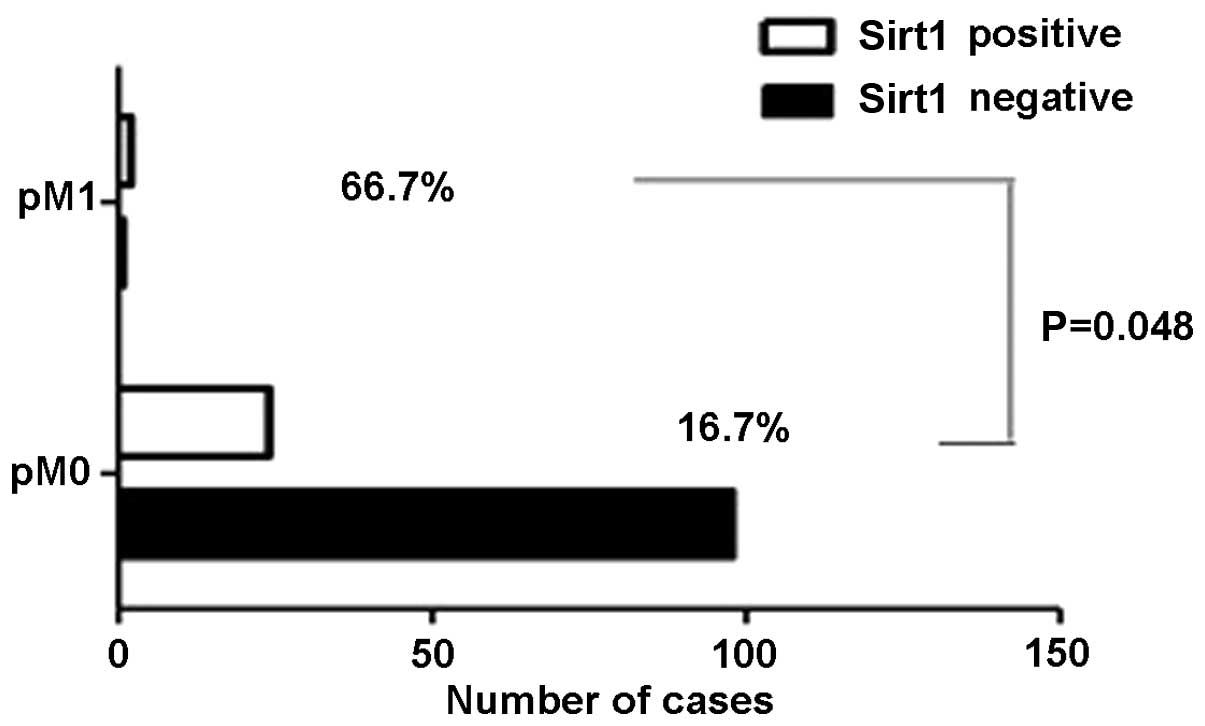
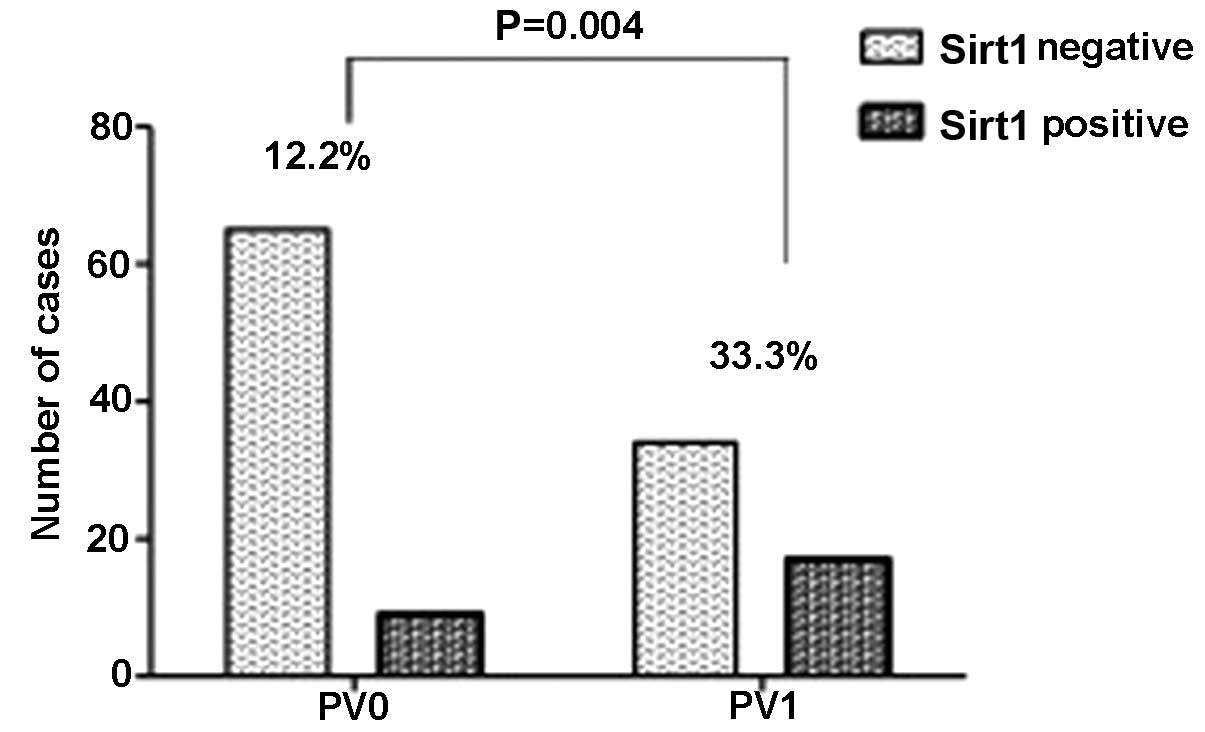
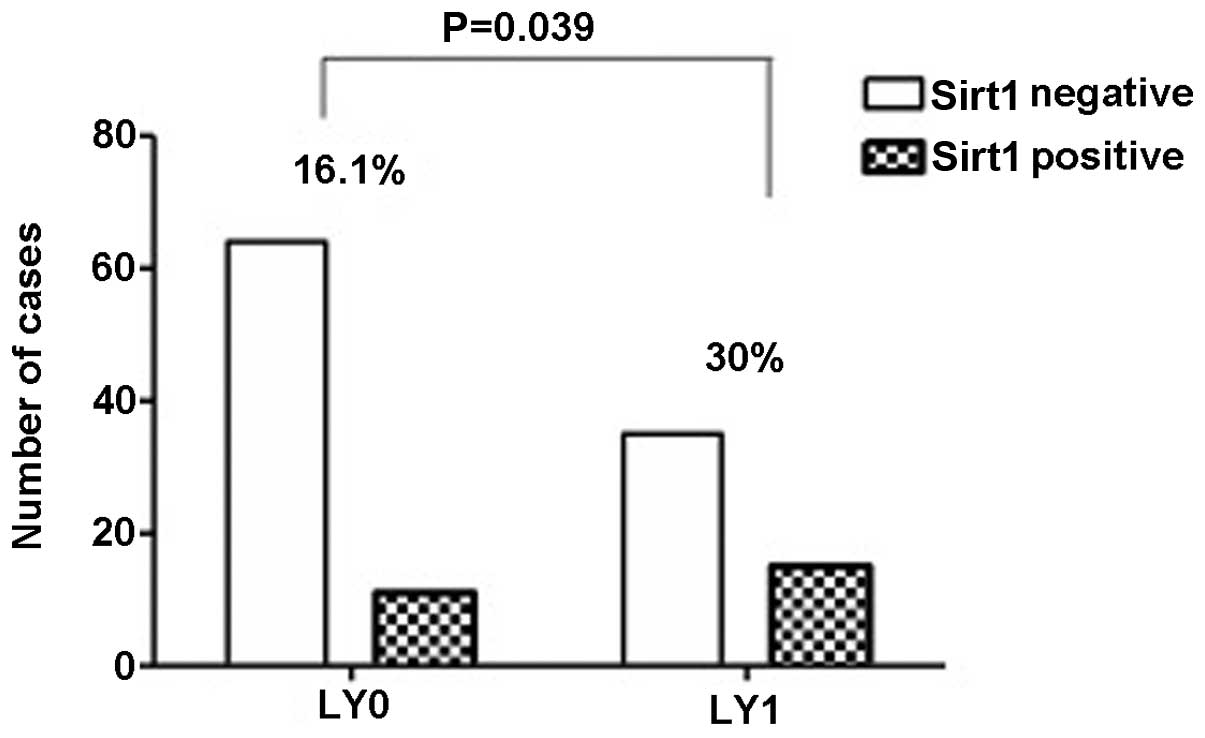
higher number of pulmonary vein invasion (P=0.039) (Fig. 6) and lymphatic duct invasion
(P=0.004) (Fig. 7). Sirt1
overexpression was not significantly correlated with age (P=0.617),
gender (P=0.60), T factor (P=0.442), cancer invasion to the
pulmonary artery (P=0.261) or p53 expression (P=0.577).
 | Table IAssociations between the increased
expression of Sirt1 and clinicopathological characteristics of 125
patients with lung adenocarcinoma. |
Table I
Associations between the increased
expression of Sirt1 and clinicopathological characteristics of 125
patients with lung adenocarcinoma.
| Variable | Total | Sirt1-positive | Sirt1-negative | P-value |
|---|
| No. of patients, n
(%) | 125 (100) | 26 (20.8) | 99 (79.2) | NA |
| Age, years | 85±8.685 | 70.46±4.623 | 66.83±9.307 | 0.617 |
| Mean ± SD
(range) | (42–84) | (60–79) | (42–84) | NA |
| Gender, n (M/F) | 71/54 | 19/7 | 52/47 | 0.600 |
| Succumbed/remained,
n | 30/95 | 8/18 | 22/77 | 0.364 |
| T factor, n |
| TI/T2/T3/T4 | 68/43/4/10 | 12/9/1/4 | 56/34/3/6 | 0.442 |
| N factor, n |
| N0/N1/N2/N3 | 87/13/24/1 | 14/7/5/0 | 73/6/19/1 | 0.018a |
| M factor, n |
| M0/M1 | 122/3 | 24/2 | 98/1 | 0.048a |
| TNM stage, n |
| I/II/III/IV | 82/12/31/0 | 12/7/7/0 | 70/5/24/0 | 0.002a |
| PA invasion, n |
|
Positive/negative | 24/101 | 7/19 | 17/82 | 0.261 |
| PV invasion, n |
|
Positive/negative | 51/74 | 17/9 | 34/65 | 0.004a |
| LY invasion, n |
|
Positive/negative | 50/75 | 15/11 | 35/64 | 0.039a |
| p53 expression,
n |
|
Positive/negative | 67/58 | 14/12 | 53/46 | 0.977 |
| HIFI expression,
n |
|
Positive/negative | 39/86 | 4/22 | 35/64 | 0.050a |
| Ki67 index, n |
|
Positive/negative | 111/14 | 26/0 | 85/14 | 0.002 |
Association between HIF1 and Sirt1
expression
HIF1 is a member of the HIF family that function as
regulators and increase when cells become hypoxic due to oxidative
stress (27). A significant
association was found between HIF1 expression levels and a
Sirt1-positive signal in patients with primary lung adenocarcinoma
(P=0.05) (Fig. 2), and there was a
negative regulation between them. A high level of expression of
HIF1 indicates that cancer cells are in a hypoxic state. However,
Sirt1 can regulate oxidative stress through indirect deactylation
of FOXO3, eventually reducing the oxidative stress burden and thus
leading to cell survival. Therefore, when cancer cells are suited
to the surrounding environment through Sirt1 regulation, the
molecular HIF1 level will decrease, resulting in a negative
correlation between Sirt1 and HIF1 expression.
Association of Ki67 index, TNM
classification and tumor invasion with Sirt1 expression
In the present study, an extremely close association
was found between the Ki67 index (determined by pathologists in the
Division of Diagnostic Pathology of Kobe University Hospital) and
Sirt1-positive expression (P=0.002). The Ki67 index frequently
indicates cancer proliferation and has a strong correlation with
clinical outcomes. This finding is consistent with the function of
Sirt1, which is associated with cell survival and proliferation. In
addition, it was found that the overexpression of Sirt1 is
associated with a high TNM classification (P=0.002) (Fig. 3), particularly in lymph node
invasion (P=0.018) (Fig. 4) and
metastasis (P=0.048) (Fig. 5), but
not primary tumor (P=0.442) and tumor size (P=0.151).
Overexpression of Sirt1 also showed a significant association with
pulmonary vein invasion (P=0.004) and lymphatic duct invasion
(P=0.039) (Figs. 6 and 7), but without the pulmonary arteries
(P=0.261). From these data, it was concluded that overexpression of
Sirt1 is closely associated with invasion and metastasis.
Discussion
Previous studies have indicated that Sirt1 is
considered to play the part of a tumor promoter and tumor
suppressor in tumorigenesis. These seemingly contradictory roles
show that Sirt1 has a complicated function in tumorigenesis. The
function of Sirt1 depends on the temporal and special distribution
of its various upstream regulators and downstream targets in
different tissue contexts (7).
While it is recognized that tumor promoters and suppressors are
significant in tumor development, no analyses of Sirt1
overexpression in a large number of surgically resected human
cancer tissues have been reported, nor has the clinical
significance of Sirt1 been properly ascertained.
In total, 125 surgically resected lung
adenocarcinoma specimens were examined to determine the Sirt1
status in cancer cells and tissue clinically by IHC staining. The
findings presented in the current study show for the first time
that Sirt1 overexpression in lung adenocarcinoma tissue specimens
is associated with HIF1 expression, Ki67 index, TNM stage,
pulmonary vein invasion and lymphatic duct invasion.
A significant correlation was not identified between
p53 and Sirt1-positive expression in the present study. Sirt1 was
originally considered to be a tumor promoter, as it directly
represses p53-mediated cell apoptosis and, as there is a
negative-feedback loop between Sirt1 and p53, they regulate and
interact with each other (5).
However, in the current study, no significant association was found
between Sirt1 and p53 (P=0.977). Similarly, a study by Jung-Hynes
and Ahmad (4) demonstrated that
Sirt1 overexpression occurs in both PC3 cells (which
lack p53) and PC3-p53 cells (with wild-type p53),
regardless of p53 in prostate cancer cells. In addition, a study by
Kim et al (10) indicated
that repression of Sirt1 activity by its specific inhibitor
sirtinol or siRNA in MCF-7 cell leads to cell senescent-like growth
arrest, indicating that the function of Sirt1 is disrupted by its
inhibitor. Notably, Tseng et al (8) reported that the p53 mutation and p53
overexpression do not frequently occur in lung adenocarcinoma.
Therefore, it is indicated that Sirt1 expression does not correlate
with p53 in patients with primary lung adenocarcinoma.
However, in the present study, a direct significant
association was not found between Sirt1-positive expression and the
prognosis for patients with lung adenocarcinoma (P=0.238). Of the
125 cases enrolled in the present study, 32 were diagnosed with
stage 3 of the TNM classification, but only three cases showed
metastasis and succumbed, and of these cases, two showed
Sirt1-positive expression (66.7%). It can therefore be considered
that as the number of patients with stage 3 or metastasis of lung
adenocarcinoma increases, overexpression of Sirt1 and the survival
rate will be inevitably linked. It has been reported that the high
level of HIF1 expression is associated with a good prognosis for
lung cancer. HIF1 levels frequently increase in the extremely early
stages of tumor progression, and are expressed in situ
carcinomas and premalignant tissues. The function of HIF1 may be to
decrease cell hypoxia and induce cell apoptosis (28,29).
By contrast, one of the functions of Sirt1 is to enhance the chance
of cell survival, with excellent growth and proliferation under
hypoxic conditions. In the present study, a negative regulation
between HIF1 expression and Sirt1-positive expression was found.
Additionally, cells located in oxygen-poor conditions are more
frequently observed in medium-term and advanced stages of cancer.
These adaptable cells adjust to hypoxia and survive through Sirt1
regulation and possibly have a more aggressive phenotype and
reduced sensitivity to anticancer treatment (29). Therefore, we suggest that Sirt1
participates in the initiation of tumors and furthermore its
expression is more closely associated with medium-term and advanced
stages of cancer and tumor development. Sirt1 may thus be
associated with a poor prognosis for lung adenocarcinoma. In
addition, hypoxia and oxidative stress can cause DNA damage. Sirt1
also plays a positive role in repairing double-strand DNA breaks.
Erroneous DNA replication or repair causes unceasing proliferation
of aberrant cells as a function of Sirt1 and the probability is
high for these cells to become tumorous (30). DNA damage also accumulates with age
and DNA repair defects can cause phenotypes resembling premature
aging, thus prolonging the presence of senescent cells in a
neoplastic microenvironment, which in turn may promote malignant
progression of adjacent epithelia cells. In young organisms,
cellular senescence can be considered an advantageous mechanism for
reducing aberrant mutations or impeding exposure to oxidative
stress, but it may be harmful in that it may promote phenotypes
associated with old age and thus potentially contribute to
tumorigenesis (31). With an
increase in age, specific inhibitors of Sirt1 function, including
HIC1 and DBC1, become weaker. They repress Sirt1 expression in
normal cells, but with aging of the cells they may gradually lose
their function to promote tumorigenesis (31).
Ki67 index and TNM classification are also
significant indicators for clinical tumor development. The high
Ki67 index and TNM classification indicate that cancer cells have a
faster growth and differentiation in tumorigenesis. Furthermore,
there is a strong possibility for invasion of the surrounding
tissue and metastasis to other areas. These usually occur in
malignant tumors and are associated with a poor prognosis for
patients with lung adenocarcinoma. In the present study, it was
found that a high Ki67 index and TNM classification are closely
correlated with Sirt1-positive expression. Particularly, in TNM
classification, Sirt1-positive expression is more closely
associated with lymph node invasion and metastasis, but not with
tumor size. Thus it can be observed that overexpression of Sirt1 is
closely associated with invasion and metastasis. It is indicated
that overexpression of Sirt1 may be correlated with a poor
prognosis for lung adenocarcinoma again. These indicate that
identifying an inhibitor based on the biological features of Sirt1
may make Sirt1 an ideal target for the development of potent
anticancer drugs.
References
|
1
|
Jeyapalan JC and Sedivy JM: Cellular
senescence and organismal aging. Mech Ageing Dev. 129:467–474.
2008.
|
|
2
|
Krtolica A and Campisi J: Cancer and
aging: a model for the cancer promoting effects of the aging
stroma. Int J Biochem Cell Biol. 34:1401–1414. 2002.
|
|
3
|
Lombard DB, Chua KF, Mostoslavsky R, et
al: DNA repair, genome stability, and aging. Cell. 120:497–512.
2005.
|
|
4
|
Jung-Hynes B and Ahmad N: Role of p53 in
the anti-proliferative effects of Sirt1 inhibition in prostate
cancer cells. Cell Cycle. 8:1478–1483. 2009.
|
|
5
|
Yi J and Luo J: SIRT1 and p53, effect on
cancer, senescence and beyond. Biochim Biophys Acta.
1804:1684–1689. 2010.
|
|
6
|
Liu T, Liu PY and Marshall GM: The
critical role of the class III histone deacetylase SIRT1 in cancer.
Cancer Res. 69:1702–1705. 2009.
|
|
7
|
Fang Y and Nicholl MB: Sirtuin 1 in
malignant transformation: friend or foe? Cancer Lett. 306:10–14.
2011.
|
|
8
|
Tseng RC, Lee CC, Hsu HS, et al: Distinct
HIC1-SIRT1-p53 loop deregulation in lung squamous carcinoma and
adenocarcinoma patients. Neoplasia. 11:763–770. 2009.
|
|
9
|
Deng CX: SIRT1, is it a tumor promoter or
tumor suppressor? Int J Biol Sci. 5:147–152. 2009.
|
|
10
|
Kim JE, Chen J and Lou Z: DBC1 is a
negative regulator of SIRT1. Nature. 451:583–586. 2008.
|
|
11
|
Abdelmohsen K, Pullmann R Jr, Lal A, et
al: Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol
Cell. 25:543–557. 2007.
|
|
12
|
Kim EJ, Kho JH, Kang MR and Um SJ: Active
regulator of SIRT1 cooperates with SIRT1 and facilitates
suppression of p53 activity. Mol Cell. 28:277–290. 2007.
|
|
13
|
Ford J, Jiang M and Milner J:
Cancer-specific functions of SIRT1 enable human epithelial cancer
cell growth and survival. Cancer Res. 65:10457–10463. 2005.
|
|
14
|
Huffman DM, Grizzle WE, Bamman MM, et al:
SIRT1 is significantly elevated in mouse and human prostate cancer.
Cancer Res. 67:6612–6618. 2007.
|
|
15
|
Bradbury C, Khanim FL, Hayden R, et al:
Histone deacetylases in acute myeloid leukaemia show a distinctive
pattern of expression that changes selectively in response to
deacetylase inhibitors. Leukemia. 19:1751–1759. 2005.
|
|
16
|
Kabra N, Li Z, Chen L, et al: SirT1 is an
inhibitor of proliferation and tumor formation in colon cancer. J
Biol Chem. 284:18210–18217. 2009.
|
|
17
|
Yeung F, Hoberg JE, Ramsey CS, et al:
Modulation of NF-kappaB-dependent transcription and cell survival
by the SIRT1 deacetylase. EMBO J. 23:2369–2380. 2004.
|
|
18
|
Tang Y, Zhao W, Chen Y, Zhao Y and Gu W:
Acetylation is indispensable for p53 activation. Cell. 133:612–626.
2008.
|
|
19
|
Wang C, Chen L, Hou X, et al: Interactions
between E2F1 and SirT1 regulate apoptotic response to DNA damage.
Nat Cell Biol. 8:1025–1031. 2006.
|
|
20
|
Stünkel W, Peh BK, Tan YC, et al: Function
of the SIRT1 protein deacetylase in cancer. Biotechnol J.
2:1360–1368. 2007.
|
|
21
|
Brunet A, Sweeney LB, Sturgill JF, et al:
Stress-dependent regulation of FOXO transcription factors by the
SIRT1 deacetylase. Science. 303:2011–2015. 2004.
|
|
22
|
Kobayashi Y, Furukawa-Hibi Y, Chen C, et
al: SIRT1 is critical regulator of FOXO-mediated transcription in
response to oxidative stress. Int J Mol Med. 16:237–243. 2005.
|
|
23
|
An BS, Tavera-Mendoza LE, Dimitrov V, et
al: Stimulation of Sirt1-regulated FoxO protein function by the
ligand-bound vitamin D receptor. Mol Cell Biol. 30:4890–4900.
2010.
|
|
24
|
Hokka D, Maniwa Y, Tane S, et al: Psf3 is
a prognostic biomarker in lung adenocarcinoma. Lung Cancer.
79:77–82. 2013.
|
|
25
|
Satoh N, Maniwa Y, Bermudez VP, Nishimura
K, Nishio W, Yoshimura M, et al: Oncogenic hosphatase Wip1 is a
novel prognostic marker for lung adenocarcinoma patient survival.
Cancer Sci. 102:1101–1106. 2011.
|
|
26
|
Nakai R, Maniwa Y, Tanaka Y, Nishio W,
Yoshimura M, Okita Y, et al: Overexpression of Necl-5 correlates
with unfavorable prognosis in patients with lung adenocarcinoma.
Cancer Sci. 101:1326–1330. 2010.
|
|
27
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006.
|
|
28
|
Volm M and Koomägi R: Hypoxia-inducible
factor (HIF-1) and its relationship to apoptosis and proliferation
in lung cancer. Anticancer Res. 20:1527–1533. 2000.
|
|
29
|
Greijer AE and van der Wall E: The role of
hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J
Clin Pathol. 57:1009–1014. 2004.
|
|
30
|
Halliwell B: Oxidative stress and cancer:
have we moved forward? Biochem J. 401:1–11. 2007.
|
|
31
|
Brooks CL and Gu W: How does SIRT1 affect
metabolism, senescence and cancer? Nat Rev Cancer. 9:123–128.
2009.
|