Introduction
A blood supply is essential for the growth and
hematogenous metastasis of tumors. Maniotis et al (1) previously reported an
angiogenesis-independent pathway known as vasculogenic mimicry
(VM). This pathway is a novel phenomenon in which highly aggressive
human melanoma cells imitate endothelial cells and form vascular
channel-like structures to convey blood plasma and red blood cells
without the involvement of endothelial cells. Periodic acid-Schiff
(PAS)-positive patterns identify VM channels. Subsequently, VM was
identified in lung cancer, hepatocellular carcinoma, gallbladder
carcinoma, gastric adenocarcinoma (GAC) and other types of cancer
(2–5). A study by Li et al (5) described the expression of VM in GAC,
particularly in poorly differentiated GAC. VM may play an extremely
significant role in the biological behavior of multiple tumors
(2–6). However, establishing the detailed
mechanism of VM formation is required.
It has been reported that positive expression of
hypoxia-inducible factor-1α (HIF-1α) is associated with the
formation of VM in primary gallbladder, non-small cell lung cancer
and hepatocellular carcinoma (2–4). STAT3
modulates the stability and activity of HIF-1α, and activated STAT3
increases the HIF-1α protein level by increasing HIF-1α stability
through blocking HIF-1α degradation and accelerating its de
novo synthesis (7). Pawlus
et al (8) found that STAT3
exhibited specific binding to the promoters of HIF1 or HIF2 target
genes respectively, even when overexpressed, and STAT3 interacted
with HIF-1α to activate HIF1 target gene promoters. Taking into
consideration the aforementioned details, STAT3 activation is
possibly associated with VM formation. Therefore, investigating the
association between STAT3 and VM formation in GAC is worthwhile to
learn more about tumor development, invasion and metastasis.
In the present study, the expression levels of
STAT3, p-STAT3, HIF-1α and VM were explored simultaneously for the
first time. Firstly, the existence of VM in GAC was confirmed by a
cluster of differentiation 31 (CD31)/PAS double-staining method.
Subsequently, combining VM existence with the expression levels of
STAT3, p-STAT3 and HIF-1α, the association was assessed between
them and the possible formation mechanism of VM was investigated.
Additionally, prognosis was assessed by Kaplan-Meier survival
analysis for univariate analysis and by Cox proportional hazards
model for multivariate analysis.
Materials and methods
Subjects
A total of 80 cases of paraffin-embedded specimens
were collected in the Department of Pathology at the Qilu Hospital
of Shandong University (Jinan, China). These cases included 60 GAC
specimens (46 male and 14 female patients; median age, 60.0 years)
and 20 gastritis specimens (11 male and 9 female patients; median
age, 56.2 years). Primary gastric cancer in these patients was
diagnosed and treated at the Qilu Hospital between July 2005 and
December 2006. The patients with GAC had well-documented clinical
histories and follow-up information. None of the patients underwent
preoperative chemotherapy and/or radiation therapy. The follow-up
time ranged between 6 and 72 months until July 2012, although the
follow-up data of one case was lost. Overall survival (OS) time was
defined as the interval between the dates of surgery and mortality.
The gastritis cases were derived from gastritis biopsy specimens.
All the cases were reviewed by two highly qualified pathologists.
The study was approved by the ethics committee of Shandong
University School of Medicine (Jinan, China) and written informed
consent was obtained from the patients or their family.
Construction of the tissue
microarray
A tissue microarray instrument (HT-1 type; Hengtai
Technology Development Co., Ltd., Chaoyang, China) was used to
construct a blank receptor wax block of six rows and seven columns.
Marked and collected tissues from the paraffin-embedded specimens
were inserted into the holes of the receptor wax block. From each
case, two specimens were acquired to overcome the loss of tissue.
The first two holes on the first line were filled with ash, which
served as a ‘blank’ specimen-positioning reference. Each receptor
wax block accommodated 40 specimens, which represented a total of
20 cases. The GAC specimens were built into the three tissue
microarrays. Each was subjected to repeated wax melting at 56°C to
become a whole specimen. The tissue microarrays and gastritis
tissue specimens were sectioned into 4-μm-thick slices that served
as a continuous backup source.
Immunohistochemical staining
The slices were dewaxed in xylene and then
rehydrated through a graded series of alcohols. For antigen
retrieval, the slides were heated in 10 mmol/l EDTA buffer (pH
8.0). Subsequent to washing with phosphate-buffered saline (PBS)
three times, the endogenous peroxidase activity was blocked by 3%
hydrogen peroxidase for 10 min of incubation at room temperature.
Following washing with PBS again, the sections were incubated with
polyclonal rabbit anti-human STAT3 (bs-1141R; Bioss, Inc., Beijing,
China), polyclonal rabbit anti-phospho-STAT3 (bs-3429R; Bioss,
Inc.) and monoclonal rabbit anti-human HIF-1α (ZA-0552; ZSGB-BIO,
Beijing, China) primary antibodies at 4°C overnight separately. The
slides were washed with PBS and incubated with biotinylated
horseradish peroxidase-conjugated secondary antibody, polyclonal
goat anti-rabbit immunoglobulin G (PV-6001; ZSGB-BIO), at room
temperature for 30 min. Subsequent to washing, the slides were
colored with 3,3-diaminobenzidine and counterstained with
hematoxylin (9). VM was obtained by
CD31/PAS double-staining, and monoclonal rabbit anti-human CD31
(ZA-0568; ZSGB-BIO) was colored with 3,3-diaminobenzidine(ZLI-9017;
ZSGB-BIO). Then, the slides were placed in 10 mg/ml periodic acid
buffer (P0430-25G; Sigma-Aldrich, Carlsbad, CA, USA) for 10 mins.
Following washing with water, the slides were colored with Schiff
(3952016; Sigma-Aldrich) for 15 min. Following washing with water,
the slides were stained with hematoxylin (ZLI-9609; ZSGB-BIO).
Immunohistochemical analysis
A positive result of immunohistochemical staining is
characterized by the existence of yellow-to-brown granules. The
positive staining of STAT3 was mainly located in the cytoplasm and
partly in the nuclei, while the positive staining of p-STAT3 and
HIF-1α was mainly located in the nuclei and partly in the
cytoplasm. There were two factors that determined the final
outcomes: The staining intensity observed under microscope (BX53,
OLympus, Tokyo, Japan) and the proportion of positive cells
estimated in an average of 100 cells counted in 10
high-magnification fields. The staining intensity was subjected to
the following numerical scoring: Specimens were colorless, 0
points; pale yellow, 1 point; yellow, 2 points; or brown, 3 points.
The proportion of positive cells was scored as follows: The number
of positive cells was <5%, 0 points; 5–25%, 1 point; 26–50%, 2
points; 51–75%, 3 points; and >75%, 4 points. Immunostaining was
considered positive when the product of the two types of scores was
multiplied and was ≥4 (10). VM was
identified in GAC tissues by CD31/PAS double-staining. VM,
characterized by CD31-negative/PAS-positive vascular-like patterns
and the presence of red blood cells, was formed by GAC cells, while
typical blood vessels showed CD31-positive/PAS-negative in their
vascular wall. All sections were scored blindly by two independent
observers.
Statistical analysis
The statistical analysis was performed with the SPSS
Graduate Park 19.0 software (SPSS, Inc., Chicago, IL, USA). The
count column was analyzed by the χ2 test. For the
correlation analysis of STAT3, p-STAT3, HIF-1α and VM expression,
Spearman's rank correlation test was applied; whereas for the
survival analysis, the Kaplan-Meier method and Cox regression
analysis were applied. P<0.05 was considered to indicate a
statistically significant difference.
Results
STAT3, p-STAT3, HIF-1α and VM expression
in GAC and gastritis tissues
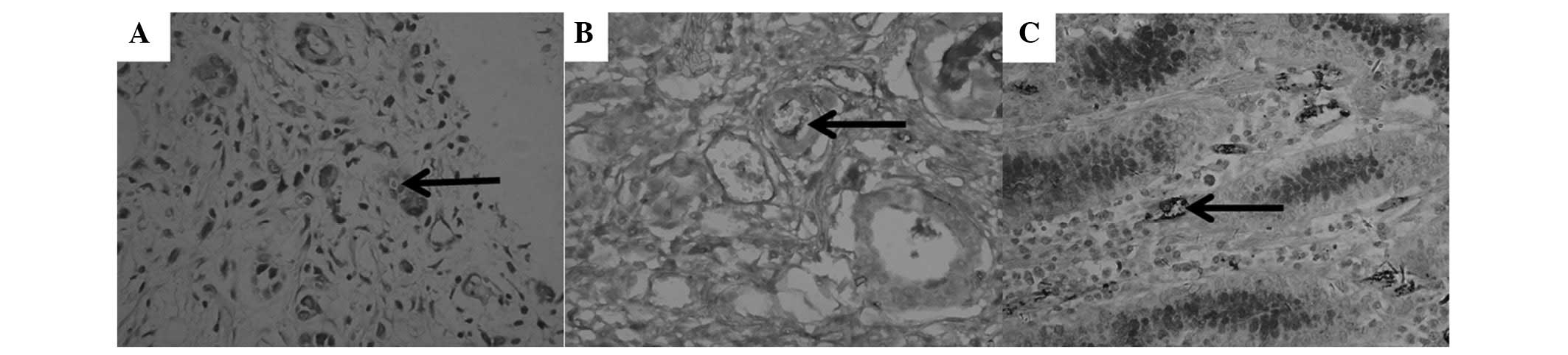
VM (Fig. 1A and B,
arrow), characterized by CD31-negative/PAS-positive channels, and
containing red blood cells, was only found in GAC specimens (31.7%;
P<0.05). In the vascular wall of typical blood vessels from
gastritis specimens, only CD31-positive/PAS-negative staining
(Fig. 1C, arrow) was found instead
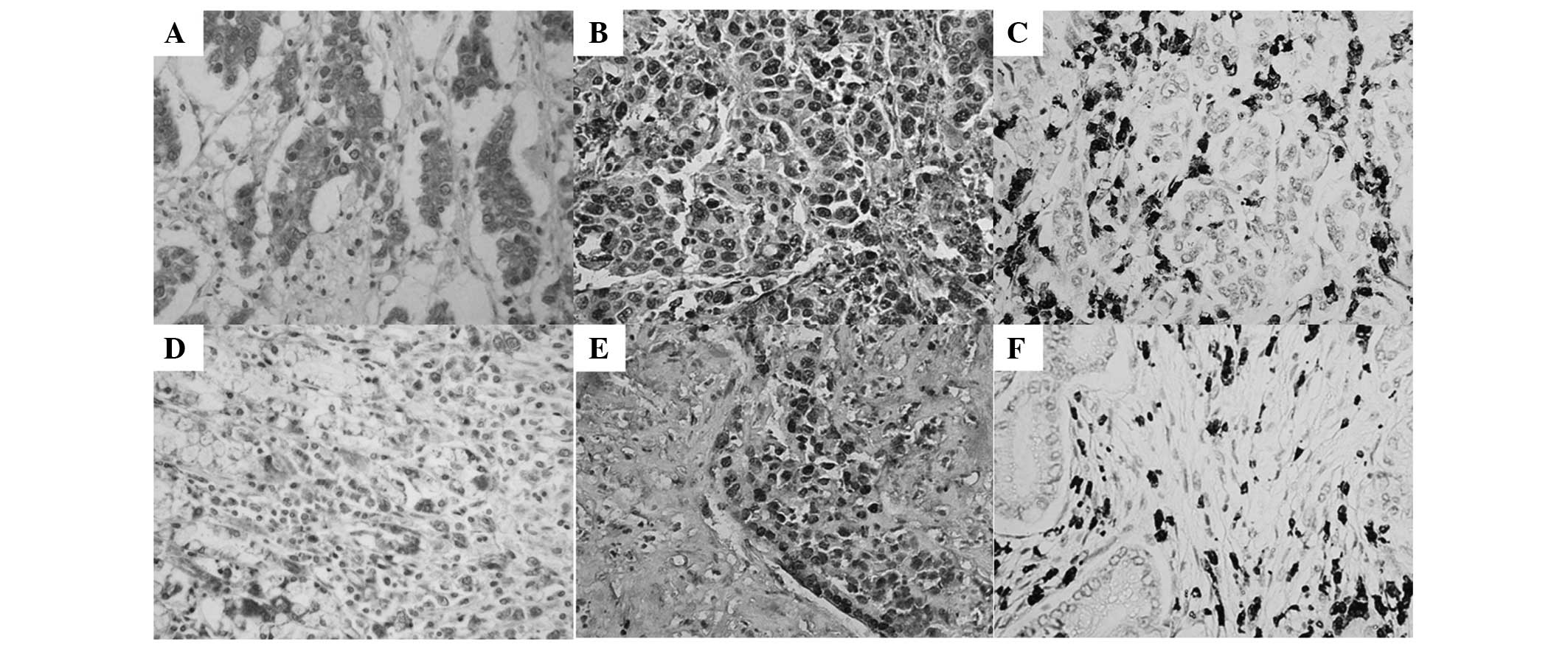
of VM formation. STAT3-positive expression (Fig. 2A and D) was detected mainly in the
cytoplasm and partly in the nuclei of GAC tissue cells. p-STAT3-
(Fig. 2B and E) and HIF-1α-positive
expression (Fig. 2Cand F) was
detected mainly in the nuclei and partly in the cytoplasm of GAC
tissue cells.
Positive expression levels of STAT3, p-STAT3 and
HIF-1α were significantly increased in the GAC specimens compared
with the gastritis specimens, respectively (81.7 vs. 15.0, 58.3 vs.
5.0 and 63.3 vs. 10.0%; P<0.05). Notably, STAT3-, p-STAT3- and
HIF-1α-positive expression and VM formation in tissues from
patients with lymph node metastasis were significantly higher than
those from patients without lymph node metastasis, respectively
(92.7 vs. 57.9, 75.6 vs. 21.1, 78.0 vs. 31.6 and 41.5 vs. 10.5%;
P<0.05). In addition, STAT3- and p-STAT3-positive expression and
VM formation were increased in poorly differentiated GAC tissues
compared with those in well-differentiated GAC tissues, separately
(94.1 vs. 65.4, 78.0 vs. 31.6 and 44.1 vs. 15.4%; P<0.05). The
various expression levels of STAT3, p-STAT3 and HIF-1α were
detected in VM GAC and non-VM GAC tissues, and it was found that
STAT3 (Fig. 2A), p-STAT3 (Fig. 2B) and HIF-1α (Fig. 2C) showed higher expression,
respectively, in VM GAC compared with non-VM GAC tissues (Fig. 2E, F and G) (P=0.012, P=0.013 and
P=0.010, respectively). These results indicated a specific type of
association between STAT3, p-STAT3, HIF-1α and VM formation
(Table I).
 | Table ICorrelation between STAT3, p-STAT3,
HIF-1α, VM and clinicopathological parameters |
Table I
Correlation between STAT3, p-STAT3,
HIF-1α, VM and clinicopathological parameters
| STAT3 | p-STAT3 | HIF-1α |
|---|
|
|
|
|
|---|
| Factors | Positive | Negative | P value | Positive | Negative | P value | Positive | Negative | P value |
|---|
| Group |
| Gastritis | 3 | 17 | <0.001 | 1 | 19 | <0.001 | 2 | 18 | <0.001 |
| GAC | 49 | 11 | | 35 | 25 | | 38 | 22 | |
| Gender |
| Male | 37 | 9 | 0.958a | 25 | 21 | 0.256 | 28 | 18 | 0.473 |
| Female | 12 | 2 | | 10 | 4 | | 10 | 4 | |
| Age at surgery,
years |
| <60 | 19 | 8 | 0.087a | 15 | 12 | 0.693 | 15 | 12 | 0.258 |
| ≥60 | 30 | 3 | | 20 | 13 | | 23 | 10 | |
| Tumor size, cm |
| <5 | 20 | 6 | 0.406 | 14 | 12 | 0.538 | 14 | 12 | 0.182 |
| ≥5 | 29 | 5 | | 21 | 13 | | 24 | 10 | |
| Status of lymph node
metastasis |
| 0 | 11 | 8 | 0.007b | 4 | 15 | <0.001 | 6 | 13 | 0.002 |
| 1–6 | 25 | 2 | | 21 | 6 | | 20 | 7 | |
| >6 | 13 | 1 | | 10 | 14 | | 12 | 2 | |
| Degree of
differentiation |
| Poor | 32 | 2 | 0.012a | 25 | 9 | 0.006 | 24 | 10 | 0.182 |
| Mid to well | 17 | 9 | | 10 | 16 | | 14 | 12 | |
| TNM stage |
| I-II | 21 | 5 | 0.875 | 15 | 11 | 0.930 | 13 | 13 | 0.061 |
| III-IV | 28 | 6 | | 20 | 14 | | 25 | 9 | |
| VM |
| Positive | 19 | 0 | 0.032a | 16 | 3 | 0.006 | 17 | 2 | 0.004 |
| Negative | 30 | 11 | | 19 | 22 | | 21 | 20 | |
Correlation analysis of STAT3, p-STAT3,
HIF-1α and VM in GAC tissues
The results showed that the expression levels of VM
exhibited a positive correlation with those of STAT3 (r=0.480 and
P=0.001), p-STAT3 (r=0.480 and P=0.001) and HIF-1α (r=0.480 and
P=0.001), separately. The expression levels of HIF-1α were also
positively associated with those of STAT3 (r=0.480 and P=0.001) and
p-STAT3 (r=0.480 and P=0.001), separately (Table II).
 | Table IICorrelation between STAT3, p-STAT3,
HIF-1α and VM expression in GAC. |
Table II
Correlation between STAT3, p-STAT3,
HIF-1α and VM expression in GAC.
| STAT3 | p-STAT3 | HIF-1α |
|---|
|
|
|
|
|---|
| Factors | Negative | Positive | P value | r | Negative | Positive | P value | r | Negative | Positive | P value | r |
|---|
| VM |
| Negative | 11 | 30 | 0.012 | 0.323 | 22 | 19 | 0.028 | 0.285 | 20 | 21 | 0.004 | 0.369 |
| Positive | 0 | 19 | | | 3 | 16 | | | 2 | 17 | | |
| HIF-1α |
| Negative | 9 | 13 | <0.001 | 0.444 | 14 | 8 | 0.008 | 0.339 | | | | |
| Positive | 2 | 36 | | | 11 | 27 | | | | | | |
| p-STAT3 |
| Negative | 9 | 16 | 0.002 | 0.386 | | | | | | | | |
| Positive | 2 | 33 | | | | | | | | | | |
Survival analysis of STAT3, p-STAT3,
HIF-1α and VM
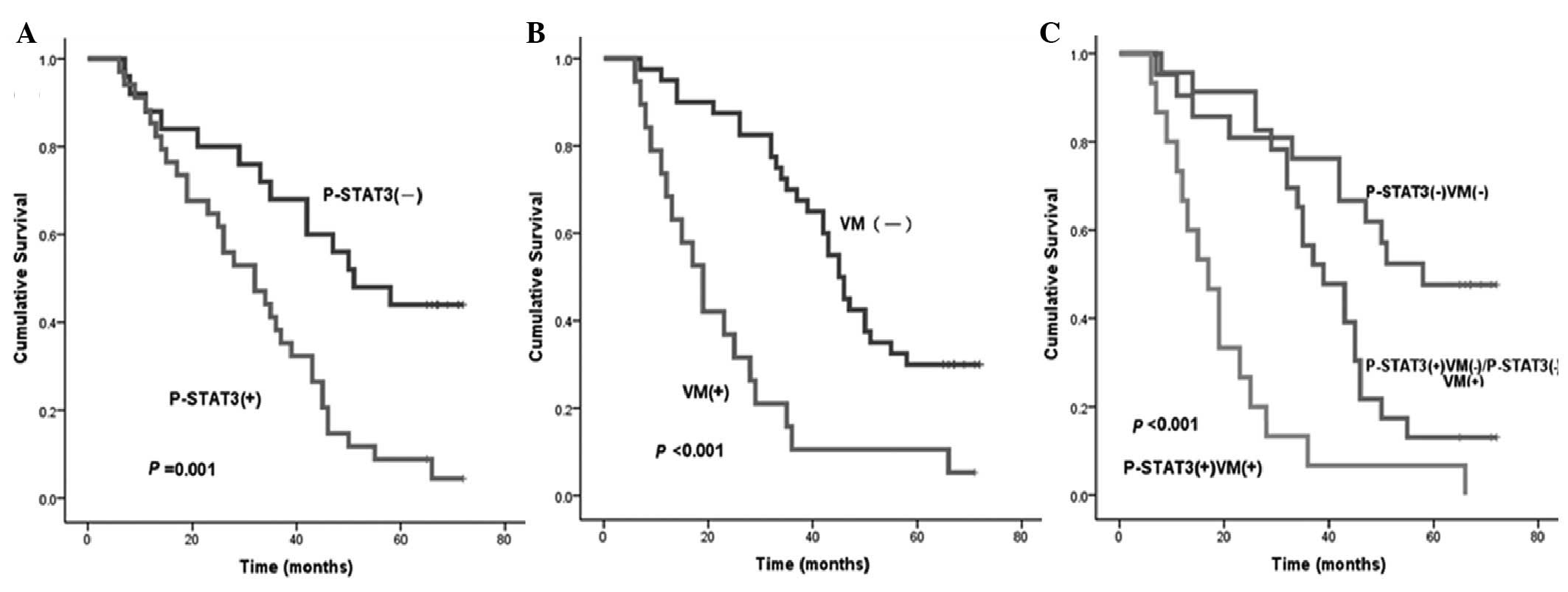
Using Kaplan-Meier univariate analysis, six factors
were found to have statistically significant associations with the
OS time of patients with GAC following curative surgery, including
STAT3, p-STAT3 (Fig. 3A), HIF-1α,
VM (Fig. 3B), status of lymph node
metastasis and degree of differentiation (P<0.05). In addition,
VM combined with STAT3, p-STAT3 or HIF-1α, respectively, was also
found to have statistically significant associations with the OS
time of patients with GAC. Patients with p-STAT3- and VM-negative
expression were more likely to have a longer median OS time
compared with those with p-STAT3- and (or) VM-positive expression
(P<0.05) (Fig. 3C and Table III).
 | Table IIIUnivariate analysis of factors
affecting the overall survival time of 60 patients with GAC by the
Kaplan-Meier method. |
Table III
Univariate analysis of factors
affecting the overall survival time of 60 patients with GAC by the
Kaplan-Meier method.
| Factor | χ2 | P-value |
|---|
| Gender | 0.001 | 0.976 |
| Age | 0.449 | 0.503 |
| Tumor size | 1.664 | 0.197 |
| Depth of primary
tumor invasion | 0.220 | 0.639 |
| Status of lymph
nodes metastasis | 9.312 | 0.002 |
| Degree of
differentiation | 5.506 | 0.019 |
| TNM stage | 1.374 | 0.241 |
| STAT3 | 9.271 | 0.002 |
| p-STAT3 | 11.793 | 0.001 |
| HIF-1α | 8.013 | 0.005 |
| VM | 18.312 | <0.001 |
| VM and STAT3 | 16.301 | <0.001 |
| VM and p-STAT3 | 29.102 | <0.001 |
| VM and HIF-1α | 26.305 | <0.001 |
All the aforementioned six variables were analyzed
by a multivariate Cox proportional hazards model (forward stepwise
procedure). In this model, VM (HR, 3.021 and P=0.001), and p-STAT3
(HR, 2.520 and P=0.006) showed significant correlations with the OS
times of patients with GAC following curative surgery, which
indicated that VM and p-STAT3 were the independent risk factors of
the OS time of patients with GAC (Table IV).
 | Table IVMultivariate analysis of factors
affecting the overall survival time of patients with GAC by Cox
proportional hazards model. |
Table IV
Multivariate analysis of factors
affecting the overall survival time of patients with GAC by Cox
proportional hazards model.
| Factor | P-value | Relative risk
(HR) | 95%CI |
|---|
| Status of lymph
node metastasis | 0.100 | | |
| Degree of
differentiation | 0.364 | | |
| STAT3 | 0.164 | | |
| p-STAT3 | 0.006 | 2.520 | 1.310–4.849 |
| HIF-1α | 0.244 | | |
| VM | 0.001 | 3.021 | 1.613–5.660 |
Discussion
Recently, various factors have been studied to
reveal the mechanism of VM formation. VM is considered to play a
key role in tumor growth, progression and metastasis (5,6). Li
et al (5) found the
existence of VM in GAC, and that hypoxia may participate in VM
formation of GAC, particularly in poorly differentiated GAC. In the
present study, it was found that VM was detected only in GAC
specimens, particularly in the poorly differentiated GAC tissues.
Patients with VM formation had a significantly shorter median OS
time than those without VM formation (P<0.001). By multivariate
survival analysis, VM was found to be an independent risk factor of
the OS time of patients with GAC. Therefore, VM was indicated to be
a detective marker of GAC tissues.
The most significant difference in the
microenvironment between tumor and normal tissues is ischemia of
the tumor due to structural imperfections of the tumor vessels,
which induces to anoxia of tumor tissues (11). As a hypoxia-dependent protein,
HIF-1α can be rapidly degraded when oxygen is normal, but when
oxygen is not sufficient, it can upregulate cell proliferation at
the transcription level, activate the expression of numerous
hypoxia response genes, and be closely associated with energy
metabolism, angiogenesis, infiltration and metastasis of the tumor
by binding with the hypoxia response element of the hypoxia
response (12). As a tumorigenesis
factor, HIF-1α could induce angiogenesis of lung cancer when
activated in hypoxia (13). In the
present study, it was found that HIF-1α-positive expression was
significantly increased in GAC specimens, particularly in VM GAC
specimens, compared with the gastritis specimens (P<0.05).
Similarly, the HIF-1α-positive expression was positively associated
with VM formation (r=0.480 and P=0.001). These demonstrated that
HIF-1α was a positive index of VM formation in GAC tissues.
Patients with HIF-1α- and VM-positive expression were more likely
to have a shorter median of OS compared with those with HIF-1α- and
(or) VM-negative expression by survival analysis (P<0.05).
Therefore, we propose that the phenomenon of HIF-1α-VM
double-positive expression is a more promising index of prognosis
than that of HIF-1α- or VM-positive expression.
As a member of the STAT family, STAT3 plays a
significantly important role in human cancers, and is closely
associated with the proliferation and apoptosis of tumor cells in a
wide variety of tumor types. A study by Yakata et al
(14) showed increased expression
of STAT3 in gastric cancer, and found that STAT3 expression was
significantly associated with invasion depth and lymph node
metastasis of GAC tissues. STAT3 could be transformed into p-STAT3
by activation under hypoxic conditions. In the present study, the
expression levels of STAT3 and p-STAT3 were found to be higher in
GAC than those in gastritis tissues, particularly in poorly
differentiated GAC (P<0.05). This result agreed with the
findings of Yakata et al (14).
Xu et al (15) demonstrated that HIF-1 expression
induced by Src was inhibited when blocking STAT3 signaling in
breast cancer and melanoma cell lines. STAT3 converted to p-STAT3,
and p-STAT3 directly bound HIF-1α and upregulated HIF-1α stability
through delaying protein degradation and accelerating protein
synthesis (7). STAT3 can promote
HIF-1α transcription and increase HIF-1α protein stability by
inhibiting the expression of p53 (8,16,17).
All these results reveal that STAT3 is a positive factor of HIF-1α.
Furthermore, p-STAT3 can upregulate the expression of matrix
metalloproteinase 2 (MMP2) to promote the formation of VM in tumor
tissues (6,18–20).
Hypoxia is a possible mechanism of VM genesis by the induction of
the expression of HIF-1α, MMP-2 and MMP-9 (6,19,20).
Above all, the results of the present study concluded that STAT3
activation could upregulate and stabilize the expression of HIF-1α
by various pathways intending to promote the VM formation under
hypoxic conditions.
The results of the present study showed that STAT3-
and p-STAT3-positive expression was increased in the VM group
(P<0.05). Additionally, STAT3 expression was positively
correlated with p-STAT3, HIF-1α and VM expression, respectively, in
GAC tissues. By univariate and multivariate survival analysis,
patients with both negative expression of p-STAT3 and VM were found
to be more likely to have a longer median OS time compared with
those with p-STAT3- and (or) VM-positive expression (P<0.05),
and p-STAT3 was an independent risk factor of the OS time of
patients with GAC. These indicated a specific type of association
between STAT3, p-STAT3, HIF-1α and VM in GAC tissues.
Combining the aforementioned studies with the
results of the present study, it was deemed that STAT3 may be a
novel positive factor of VM formation in GAC tissues through the
effect of p-STAT3. STAT3 and p-STAT3 were positive factors of
HIF-1α expression and VM formation in GAC tissues. STAT3 was
significantly associated with progression and prognosis of GAC.
Combining the previous studies with the present study results, it
can be concluded that STAT3 may promote the formation of VM to
affect the invasion and metastasis in GAC tissue by a specific type
of mechanism (STAT3-p-STAT3-HIF-1α-VM).
In conclusion, it was found that p-STAT3 and VM
played a significant role in indicating the prognosis of patients
with GAC. STAT3 activation may play a positive role in VM formation
of GAC tissues by the STAT3-p-STAT3-HIF-1α-VM effect axis. These
results provide opportunities to develop potential novel
therapeutic targets for GAC.
Acknowledgments
This study was financially supported by the National
Natural Science Foundation of China (grant no. 81000869), the
Natural Science Foundation of Shandong Province (grant no.
ZR2011HM075) and the Promotive Research Fund for Excellent Young
and Middle-aged Scientists of Shandong Province (grant no.
BS2011YY039).
References
|
1
|
Maniotis AJ, Folberg R, Hess A, et al:
Vascular channel formation by human melanoma cells in vivo and
vitro: vasculogenic mimicry. Am J Pathol. 155:739–752. 1999.
|
|
2
|
Liu WB, Xu GL, Jia WD, et al: Prognostic
significance and mechanisms of patterned matrix vasculogenic
mimicry in hepatocellular carcinoma. Med Oncol. 28(Suppl 1):
S228–S238. 2011.
|
|
3
|
Sun W, Shen ZY, Zhang H, et al:
Overexpression of HIF-1α in primary gallbladder carcinoma and its
relation to vasculogenic mimicry and unfavourable prognosis. Oncol
Rep. 27:1990–2002. 2012.
|
|
4
|
Wu S, Cheng Z, Yu L, Song W and Tao Y:
Expression of CD82/KAI1 and HIF-1α in non-small cell lung cancer
and their relationship to vasculogenic mimicry. Zhongguo Fei Ai Za
Zhi. 14:918–925. 2011.(In Chinese).
|
|
5
|
Li M, Gu Y, Zhang Z, et al: Vasculogenic
mimicry: a new prognostic sign of gastric adenocarcinoma. Pathol
Oncol Res. 16:259–266. 2010.
|
|
6
|
Sun B, Qie S, Zhang S, et al: Role and
mechanism of vasculogenic mimicry in gastrointestinal stromal
tumors. Hum Pathol. 39:444–451. 2008.
|
|
7
|
Jung JE, Lee HG, Cho IH, et al: STAT3 is a
potential modulator of HIF-1-mediated VEGF expression in human
renal carcinoma cells. FASEB J. 19:1296–1298. 2005.
|
|
8
|
Pawlus MR, Wang L, Murakami A, Dai G and
Hu CJ: STAT3 or USF2 contributes to HIF target gene specificity.
PLoS One. 8:e723582013.
|
|
9
|
Chen QR, Guan F, Yan DJ, Lei DS, Fu L, et
al: The dynamic expression of allograft inflammatory factor-1 in
hepatic tissues and splenic cells of BALB/c mice with
Schistosoma japonicum infection. Tissue Antigens. 79:33–41.
2012.
|
|
10
|
Yu HF, Zhao G, Ge ZJ, et al: High RIN1
expression is associated with poor prognosis in patients with
gastric adenocarcinoma. Tumour Biol. 33:1557–1563. 2012.
|
|
11
|
Crowther M, Brown NJ, Bishop ET and Lewis
CE: Microenvironmental influence on macrophage regulation of
angiogenesis in wounds and malignant tumors. J Leukoc Biol.
70:478–490. 2001.
|
|
12
|
Huang GW, Yang LY and Lu WQ: Expression of
hypoxia-inducible factor 1 alpha and vascular endothelial growth
factor in hepatocellular carcinoma: Impact on neovascularization
and survival. World J Gastroenterol. 11:1705–1708. 2005.
|
|
13
|
Noman MZ, Buart S, Van Pelt J, et al: The
cooperative induction of hypoxia-inducible factor-1 alpha and STAT3
during hypoxia induced an impairment of tumor susceptibility to
CTL-mediated cell. J Immunol. 182:3510–3521. 2009.
|
|
14
|
Yakata Y, Nakayama T, Yoshizaki A, et al:
Expression of p-STAT3 in human gastric carcinoma: significant
correlation in tumour invasion and prognosis. Int J Oncol.
30:437–442. 2007.
|
|
15
|
Xu Q, Briggs J, Park S, et al: Targeting
Stat3 blocks both HIF-1 and VEGF expression induced by multiple
oncogenic growth signaling pathways. Oncogene. 24:5552–5560.
2005.
|
|
16
|
Hu CJ, Wang LY, Chodosh LA, Keith B and
Simon MC: Differential roles of hypoxia-inducible factor 1alpha
(HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell
Biol. 23:9361–9374. 2004.
|
|
17
|
Niu G, Wright KL, Ma Y, et al: Role of
Stat3 in regulating p53 expression and function. Mol Cell Biol.
25:7432–7440. 2005.
|
|
18
|
Xie TX, Wei D, Liu M, et al: Stat3
activation regulates the expression of matrix metalloproteinase-2
and tumor invasion and metastasis. Oncogene. 23:3550–3560.
2004.
|
|
19
|
Sun B, Zhang D, Zhang S, Zhang W, Guo H
and Zhao X: Hypoxia influences vasculogenic mimicry channel
formation and tumor invasion-related protein expression in
melanoma. Cancer Lett. 249:188–197. 2007.
|
|
20
|
Xu X, Jia R, Zhou Y, Song X and Fan X:
Investigation of vasculogenic mimicry in sebaceous carcinoma of the
eyelid. Acta Ophthalmol. 88:e160–e164. 2010.
|