Introduction
Squamous cell carcinoma (SCC) is the second most
common type of non-melanoma cancer of the skin. Although there are
a number of associated risk factors, the etiology of this cancer
has not yet been determined. microRNA-21 (miRNA/miR-21) is
overexpressed in several types of solid tumors, including
esophageal (1), stomach (2), colorectal (3), prostate (4), pancreatic (5), lung (6) and head and neck (7) cancers. It has been reported that the
downregulation of miR-21 suppresses tumor growth and invasion in
breast, glioma and colon cancer cells. Furthermore, the inhibition
of miR-21 can regulate the expression of phosphatase tensin
homologue (PTEN) and programmed cell death 4 (PDCD4) in cancer
cells. However, the biological roles of miR-21 in SCC of the skin
remain poorly understood and require further study. To the best of
our knowledge, no previous studies have investigated the role of
miR-21 in the A431 cell line. The present study was the first to
examine the expression of PDCD4, PTEN and cell apoptosis in A431
cells transfected with anti-miR-21.
The findings of the current study demonstrate that
miR-21 plays an oncogenic role in the process of SCC and may serve
as a target for effective therapy of SCC of skin.
Material and methods
Cell culture
The A431 cells of human cutaneous SCC were kindly
provided by the Cell Resource Center of the Institute of Basic
Sciences, Chinese Academy of Medical Sciences (Tianjin, China). The
cells were cultured in Dulbecco’s modified Eagle’s medium
containing 10% fetal bovine serum (Gibco-BRL, Carlsbad, CA, USA)
and incubated in a humidified atmosphere of 5% CO2 at
37°C. The cells were divided into three groups as follows: i)
untreated A431 cells; ii) A431 cells transfected with an unrelated
fragment (negative control); and iii) A431 cells transfected with
antisense oligonucleotide (ASO)-miR-21.
Quantitative polymerase chain reaction
(qPCR)
For the A431 cells, total RNA was isolated using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. Total RNA (~200 ng)
was reverse transcribed using gene-specific reverse transcription
primers from the TaqMan microRNA assays (Applied Biosystems, Foster
City, CA, USA) and the TaqMan microRNA Reverse Transcription kit
(Takara Bio, Inc., Shiga, Japan) according to the manufacturer’s
instructions. qPCR was performed on the iQ5 Multicolor Real-time
Detection System (Bio-Rad, Hercules, CA, USA) under the following
conditions: initial denaturation for 3 min at 95°C, followed by 40
cycles for denaturation for 30 sec at 95°C, combined annealing for
30 sec at 56°C and primer extension for 30 sec at 72°C. To estimate
the expression of miR-21, the Ct values were normalized using 18S
rRNA as an internal control. The relative miRNA expression level
was calculated using the 2−ΔΔCt method. The primer for
miR-21 detection was 5′-TGCGGTAGCTTATCAGACTGATG-3′, and the heat
shock RNA-U6 primer was 5′-TGCGGGTGCTCGCTTCGG CAGC-3′.
Transfection of ASO-miR-21
ASOs of human miR-21 were transfected by
Lipofectamine™ 2000 reagent (Invitrogen Life Technologies). The
primer sequence of ASO was 5′-TCAACA TCAGTCTGATAAGCTA-3′.
Immunocytochemistry
PCDC4 and PTEN protein expression was detected by
immunocytochemistry of streptavidin-perosidase. Following culture,
the cells were grown on microscope slides and sections were
deparaffinized, heated in a microwave in 0.01 M sodium citrate
buffer for antigen retrieval, treated with 3%
H2O2 for 10 min and rinsed in H2O
and phosphate-buffered saline (PBS). The sections were blocked in
5% goat serum in PBS, followed by incubation with the anti-PDCD4
and anti-PTEN antibodies (both Tianjin Saier Biotechology Co.,
Ltd., Tianjin, China). Signals were detected with
3,3′-diaminobenzidine substrate (ZSGB-Bio, Inc., Beijing, China).
PDCD4 and PTEN protein expression was evaluated by integrated
optical density.
Western blot analysis
The expression levels of downstream targets of human
miR-21 were determined by western blot analysis. Protein from the
A431 cells was extracted using radioimmunoprecipitation assay lysis
buffer (Tianjin Saier Biotechology Co., Ltd.). Samples were
resolved using SDS-PAGE on a 10% Tris-HCl gel and transferred to a
nitrocellulose filter membrane (Millipore, Billerica, MA, USA). The
membrane was probed with specific antibodies and target proteins,
including GAPDH, rabbit anti-PDCD4 and rabbit anti-PTEN.
Horseradish peroxidase (HRP)-conjugated secondary antibodies (goat
anti-rabbit HRP) and luminal reagent were used to detect
chemiluminescence. Blots were subsequently exposed to X-ray film
and developed. Bands were digitally scanned and analyzed with
Labworks 4.0 system (UVP, LLC, Upland, CA, USA). Western blotting
of GAPDH on the same membrane was used as a loading control. The
average pixel densities and band sizes in the control bands were
used to normalize band density and the size of the target proteins.
Target bands from the cells were directly compared.
Apoptosis assay
The three groups of cells were washed twice with 10
mM cold PBS and resuspended in 1× binding buffer at a concentration
of 1×106 cells/ml. The cells were stained with
4′,6-diamidino-2-phenylindole (DAPI) and terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL), using the
FragEL™ DNA Fragmentation Detection kit (Merck KGaA, Darmstadt,
Germany). The experiments were repeated at least three times. DAPI
stained the nucleus of all the cells, while fluorescein stained the
nucleus of the apoptotic cells. The ratio of apoptotic cell to all
the cells was used to evaluate the level of cell apoptosis.
Statistical analysis
Data are expressed as the mean ± standard deviation
of three independent cell groups. The differences between groups
were assessed by an unpaired two-tailed Student’s t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
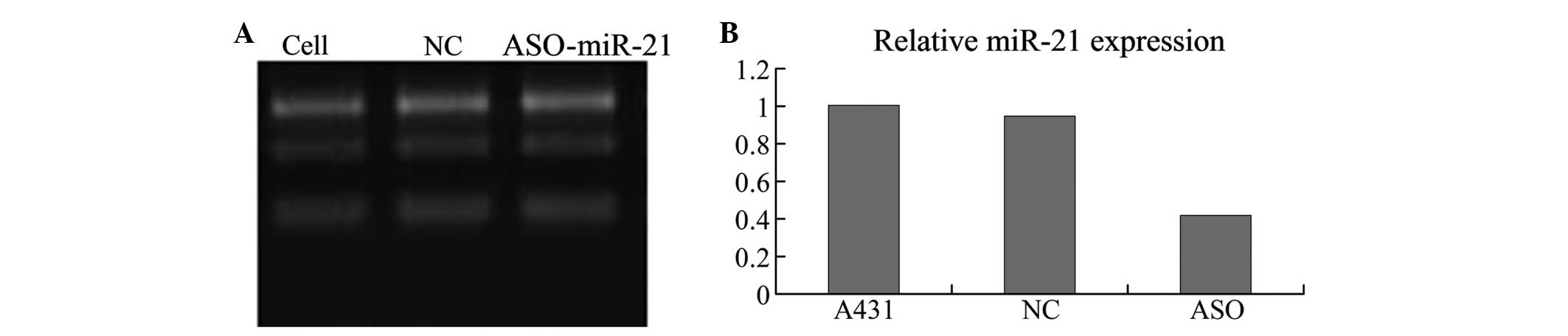
ASO-miR-21 downregulates miR-21 in A431
cells
qPCR analysis demonstrated that the three groups of
cells had different expression levels of miR-21 (F=107.24,
P<0.05). miR-21 was expressed at a significantly higher level in
the A431 cells and cells transfected with an unrelated fragment of
control compared with the cells transfected with ASO-miR-21
(P<0.05). However, similar levels of miR-21 expression were
found between the control A431 cells and the cells without
treatment (P>0.05). Thus demonstrating that ASO-miR-21
downregulates miR-21 in A431 cells (Fig. 1).
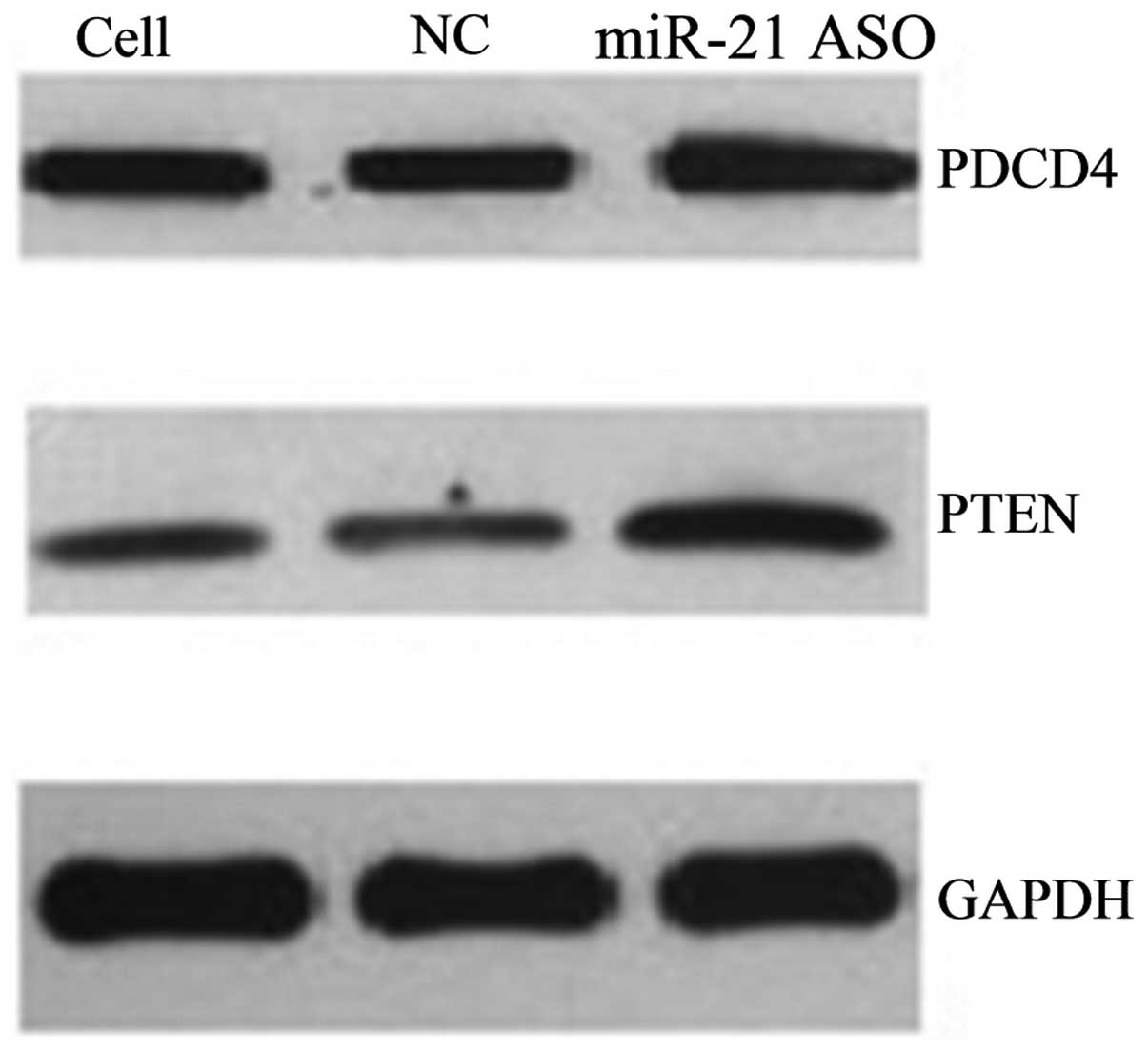
ASO-miR-21 upregulates the expression of
PDCD4 and PTEN in A431 cells
Western blot analysis showed that the three groups
of cells had different expression levels of PDCD4 (F=11,941.13,
P<0.05) and PTEN (F=83,249.64, P<0.05). Following ASO-miR-21
transfection, the expression of PDCD4 and PTEN in the A431 cells
was evidently increased compared with the control group (Figs. 2 and 3), while similar levels of PDCD4 and PTEN
expression were found between the control group and the cells
without any treatment. In addition, to determine the effects of
miR-21 on PDCD4 and PTEN expression, the protein levels of PDCD4
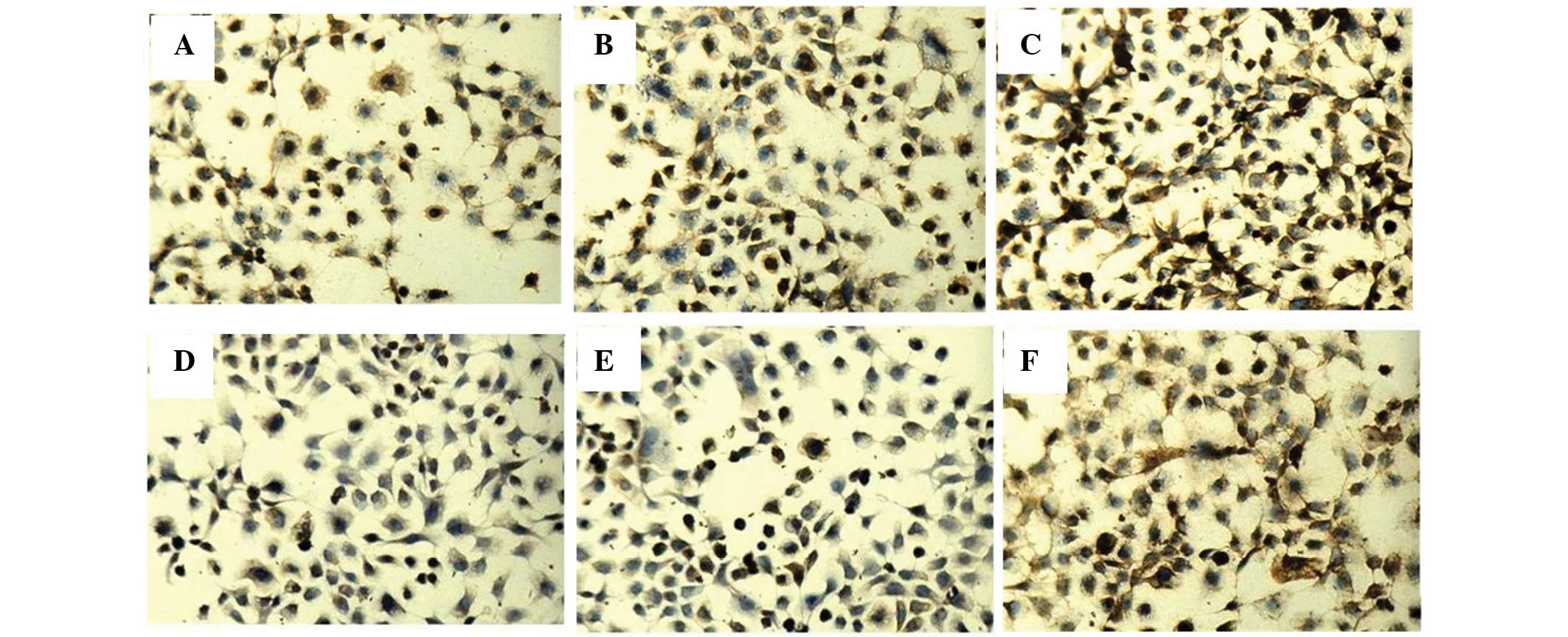
and PTEN were detected by immunocytochemistry and western blotting.
The positive staining of PDCD4 and PTEN was localized in the
cytoplasm and the nucleus (Fig. 3).
Immunocytochemistry revealed that the three groups of cells had
different expression levels of PDCD4 (F=50.12, P<0.05) and PTEN
(F=576.54, P<0.05).
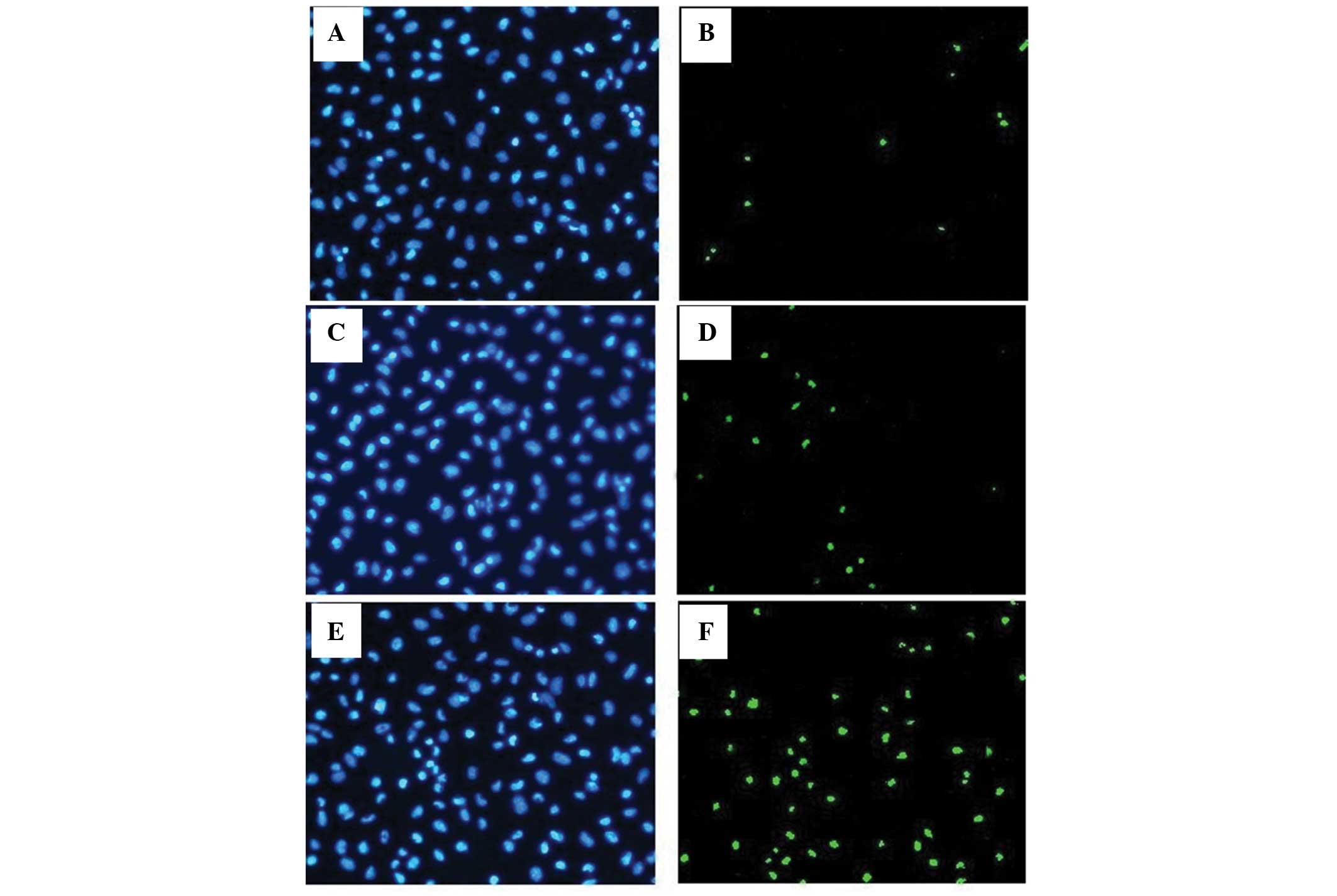
Downregulation of miR-21 induces
apoptosis of A431 cells
To determine the effects of miR-21 on apoptosis,
cell apoptosis was detected by TUNEL assay and the apoptotic ratio
was used to evaluate the level of apoptosis in the cells. The three
groups of cells had different apoptotic ratios (F=201.79,
P<0.05). Following ASO-miR-21 transfection, the apoptotic ratio
in the A431 cells was evidently increased compared with the control
group (Fig. 4), while similar
apoptotic ratios were found between the control A431 cells and the
cells without any treatment.
Discussion
SCC is one of the most common types of skin cancer
in dermatology, and there are numerous risk factors associated with
SCC of the skin, including ultraviolet-B radiation, radiation
therapy, previous burns, exposure to arsenic and coal tar, human
papilloma virus infection, inflammatory lesions and ulcers of long
standing (8). miR-21 is
overexpressed in several types of cancers and induces the invasion,
intravasion and metastasis of cancer (9). PDCD4 and PTEN are the target genes of
miR-21. In the present study, we hypothesized that miR-21
downregulates the expression of PDCD4 and PTEN in A431 cells, and
that the inhibition of miR-21 subsequently increases PDCD4 and PTEN
expression and suppresses tumor cell growth. The results showed
that miR-21 affected the expression of PDCD4 and PTEN and the
apoptosis of the A431 cells.
PDCD4 suppresses several targets that regulate
translation and cell proliferation, and has been indicated to be
involved in tissue invasion and proliferation. In a mouse cancer
model, PDCD4 suppressed benign and malignant skin tumor formation
and progression (10). In a study
of colorectal cancer, PDCD4 mRNA levels were negatively regulated
by miR-21 at each stage of cancer (11). PTEN is a phosphatase that maintains
low levels of phosphatidylinositol 3,4,5-triphosphate (PIP-3) by
conversion to PIP-2. When PTEN fails to maintain this homeostasis,
PIP-3 levels increase and activate the protein kinase B (Akt)
pathway. Activation of the Akt pathway has several effects,
including the promotion of cell growth and proliferation and the
inhibition of apoptosis (12,13).
Meng et al reported that the aberrant expression of miR-21
contributed to hepatocellular carcinoma growth and spread by
modulating PTEN expression and PTEN-dependent pathways involved in
mediating the cell growth, migration and invasion of cancer cells
(14). Ming and He reported that
PTEN negatively regulates the oncogenic phosphatidylinositol
3-kinase/Akt signaling pathway and showed that PTEN is a critical
tumor suppressor for skin cancer in humans and in mice (15). A previous study of gastric cancer
indicated that miR-21 inhibition may upregulate the PTEN expression
level, and that the downregulation of miR-21 exhibits a stronger
inhibitory effect on the biological behavior of cancer cells
(16).
In the present study, ASO-miR-21 was efficiently
transfected into the A431 cells resulting in a marked
downregulation of miR-21 in vitro. Previous studies have
shown that transfection of anti-miR-21 can downregulate miR-21
expression of oral SCC (17) and
laryngeal SCC (18). In the present
study, immunocytochemistry and western blot analysis revealed a
significant increase in the expression of PDCD4 and PTEN in the
A431 cell line transfected with anti-miR-21. Previous studies have
shown that the inhibition of miR-21 in cancer cells increases PTEN
and PDCD4 protein levels in HeLa and MCF-7/ADR cells (19,20).
Furthermore, in the present study, the TUNEL assay showed a
significant increase in the apoptotic ratio in the A431 cell line
transfected with anti-miR-21. miR-21 has also been found to be
upregulated in laryngeal carcinoma tissues, and the knockdown of
miR-21 by a specific ASO inhibited the proliferation potential of
Hep-2 cells (21).
In conclusion, the present study demonstrated that
miR-21 downregulates the expression of PDCD4 and PTEN, and that the
inhibition of miR-21 suppresses tumor growth and invasion.
Considering that PDCD4 and PTEN function as tumor suppressor genes
and are the target genes of miR-21, ASO-miR-21 may have potential
applications as a therapeutic target. Understanding the complex
regulation of miR-21 with regard to its target gene expression in
SCC may be valuable for exploring potential therapeutic methods for
SCC, and gene therapy targeting miR-21 should be further
investigated as a potential alternative strategy for SCC
therapy.
References
|
1
|
Zhu L, Yan W, Rodriguez-Canales J, et al:
MicroRNA analysis of microdissected normal squamous esophageal
epithelium and tumor cells. Am J Cancer Res. 1:574–584. 2011.
|
|
2
|
Motoyama K, Inoue H, Mimori K, et al:
Clinicopathological and prognostic significance of PDCD4 and
microRNA-21 in human gastric cancer. Int J Oncol. 36:1089–1095.
2010.
|
|
3
|
Chang KH, Miller N, Kheirelseid EA, et al:
MicroRNA-21 and PDCD4 expression in colorectal cancer. Eur J Surg
Oncol. 37:597–603. 2011.
|
|
4
|
Ribas J and Lupold SE: The transcriptional
regulation of miR-21, its multiple transcripts, and their
implication in prostate cancer. Cell Cycle. 9:923–929. 2010.
|
|
5
|
Giovannetti E, Funel N, Peters GJ, et al:
MicroRNA-21 in pancreatic cancer: correlation with clinical outcome
and pharmacologic aspects underlying its role in the modulation of
gemcitabine activity. Cancer Res. 70:4528–4538. 2010.
|
|
6
|
Wei J, Gao W, Zhu CJ, et al:
Identification of plasma microRNA-21 as a biomarker for early
detection and chemosensitivity of non-small cell lung cancer. Chin
J Cancer. 30:407–414. 2011.
|
|
7
|
Tran N, Mclean T, Zhang X, et al: MicroRNA
expression profiles in head and neck cancer cell lines. Biochem
Biophys Res Commun. 358:12–17. 2007.
|
|
8
|
LeBiot PE, Burg G, Weedon D and Sarasin A:
World Health Organization Classification of Tumours. Pathology and
Genetics of Skin Tumours. IARC Press; Lyon: 2006
|
|
9
|
Asangani IA, Rasheed SA, Nikolova DA, et
al: MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor
suppressor Pdcd4 and stimulates invasion, intravasation and
metastasis in colorectal cancer. Oncogene. 27:2128–2136. 2008.
|
|
10
|
Jansen AP, Camalier CE and Colburn NH:
Epidermal expression of the translation inhibitor programmed cell
death 4 suppresses tumorigenesis. Cancer Res. 65:6034–6041.
2005.
|
|
11
|
Horiuchi A, Linuma H, Akahane T, et al:
Prognostic significance of PDCD4 expression and association with
microRNA-21 in each Dukes’ stage of colorectal cancer patients.
Oncol Rep. 27:1384–1392. 2012.
|
|
12
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005.
|
|
13
|
Wen YG, Wang Q, Zhou CZ, et al: Mutation
analysis of tumor suppressor gene PTEN in patients with gastric
carcinomas and its impact on PI3K/AKT pathway. Oncol Rep. 24:89–95.
2010.
|
|
14
|
Meng F, Henson R, Wehbe-Janek H, et al:
MicroRNA-21 regulates expression of the PTEN tumor suppressor gene
in human hepatocellular cancer. Gastroenterology. 133:647–658.
2007.
|
|
15
|
Ming M and He YY: PTEN: new insights into
its regulation and function in skin. J Invest Dermatol.
129:2109–2112. 2009.
|
|
16
|
Zhang BG, Li JF, Yu BQ, et al: MicroRNA-21
promotes tumor proliferation and invasion in gastric cancer by
targeting PTEN. Oncol Rep. 27:1019–1026. 2012.
|
|
17
|
Reis PP, Tomenson M, Cerciqne NK, et al:
Programmed cell death 4 loss increased tumor cell invasion and is
regulated by miR-21 in oral squamous cell carcinoma. Mol Cancer.
9:2382010.
|
|
18
|
Ren J, Zhu D, Liu M, et al: Downregulation
of miR-21 modulates Ras expression to promote apoptosis and
suppression invasion of laryngeal squamous cell carcinoma. Eur J
Cancer. 46:3409–3416. 2010.
|
|
19
|
Yao Q, Xu H, Zhang QQ, et al: MicroRNA-21
promotes cell proliferation and down-regulates the expression of
programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells.
Biochem Biophys Res Commun. 388:539–542. 2009.
|
|
20
|
Wang ZX, Lu BB, Wang H, et al: MicroRNA-21
modulates chemosensitivity of breast cancer cells to doxorubicin by
targeting PTEN. Arch Med Res. 42:281–290. 2011.
|
|
21
|
Liu M, Wu H, Liu T, et al: Regulation of
the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma.
Cell Res. 19:828–837. 2009.
|