Introduction
Voltage-gated sodium channels (VGSCs) are
responsible for the rising phase of the action potential in the
majority of electrically excitable cells and, thus, are important
in impulse generation and propagation (1). VGSCs are composed of a pore-forming α
subunit and one or more auxiliary subunits (β1–β4). Nine sodium
channel α subunits (Nav1.1–Nav1.9), encoded by the SCN1A-SCN5A and
SCN8A-SCN11A genes, have been found in vertebrates (2). Different VGSC α subunits are
abundantly expressed in the majority of excitable tissues. For
example, in the heart, Nav1.5 is the main subtype of sodium
channel. The functions of VGSCs in such excitable tissues are well
understood. VGSCs contribute to impulse generation, conduction,
axonal migration and synaptic connectivity (1). Therefore, dysfunction of VGSCs leads
to several diseases in excitable tissues, including Brugada
syndrome (3,4), long QT syndrome (5), chronic pain syndromes (6) and epilepsy (7). Recently, however, VGSCs have been
found to have relatively high expression levels in a range of cell
types that are considered ‘non-excitable’, including immune cells,
fibroblasts and cancer cells (8).
Prostate cancer is the most common type of cancer in
males worldwide (9). It has been
reported that the expression of certain VGSC α subtypes, such as
Nav1.7 (encoded by the SCN9A gene), are upregulated in human and
murine prostate cancer cells (10,11).
In vitro experiments have shown that tetrodotoxin (TTX), a
specific VGSC blocker, inhibits cancer invasion, proliferation and
migration in PC-3 prostate cancer cells (12,13).
Therefore, identifying the aberrant expression of a major ion
channel subtype in cancer cells will aid in the targeted diagnosis
and treatment of cancer. However, whether the expression of other
sodium channel subtypes, other than Nav1.7, is also altered in
prostate cancer in humans remains controversial. In addition,
whether the expression of sodium channels is altered in individuals
with BPH, another common disease of the prostate, compared with
that in individuals with a normal prostate or in prostate cancer
patients remains unknown. Therefore, the present study set out to
determine the mRNA levels of VGSC α subunits in prostate samples
from healthy males and BPH patients, and in human prostate cancer
cells. By using quantitative polymerase chain reaction (qPCR), we
systematically determined the mRNA expression levels of all types
of VGSC α subunits in normal human prostate, BPH and prostate
cancer cells. By using a patch-clamp technique, it was investigated
whether the highly expressed VGSC α subunits in prostate cancer
cells were functional. The current study provides a basis for the
correlation of VGSC α subunits with prostate cancer and aids in the
clinical diagnosis and drug targets for the treatment of prostate
cancer.
Materials and methods
Tissue samples and cell lines
Three normal human prostate samples and three BPH
patient samples were collected from the First Hospital of
Shijiazhuang City, China. The age range of the subjects was 50–60
years. Informed consent was obtained from all patients and control
subjects who participated in this study. Ethical approval for the
study was obtained from the ethics committee of the First Hospital
of Shijiazhuang (Shijiazhuang, China). PC-3 and LNCaP cells were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China).
Cell culture
PC-3 and LNCaP cells were cultured and harvested as
described previously (12,13). In brief, cells were grown in a
humidified atmosphere of 5% CO2 at 37°C and were
maintained in F-12K medium (for PC-3 cells) or in RPMI-1640 medium
(for LNCaP cells), with 10% fetal bovine serum (all Gibco-BRL,
Carlsbad, CA, USA).
RNA extraction and purification
Tissue samples were collected, weighed, homogenized
and processed for total RNA isolation at 4°C using RNeasy Plus mini
kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s
instructions. The concentration of total RNA for each sample was
determined by the Nanodrop ND-1000 spectrophotometer (Thermo Fisher
Scientific, Waltham, MA, USA). The integrity of the extracted RNA
was confirmed by electrophoresis under denaturing conditions.
cDNA synthesis and qPCR
Reverse transcription (RT) was performed using an
iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) for the synthesis of single-stranded cDNA library
according to the manufacturer’s instructions. qPCR was performed
using the iCycler iQ Real-Time PCR detection system (Bio-Rad
Laboratories, Inc.), and each sample was run in triplicate. Three
controls aimed at detecting DNA contamination in the RNA samples or
during the RT or qPCR reactions were always included: i) an RT
mixture without reverse transcriptase; ii) an RT mixture including
the reverse transcriptase enzyme, but no RNA; and iii) a water only
control (reaction mixture with water instead of the cDNA template).
Table I lists the primer pairs used
for the amplification of each VGSC subtypes and
β2-microglobulin. PCR products were visualized on a 1.5%
agarose gel. The data were collected and analyzed using iCycler
software (Bio-Rad Laboratories, Inc.). β2-microglobulin
was used as internal control. Relative quantification was performed
using the comparative threshold (CT) method (ΔΔCT) after
determining the CT values for the reference
(β2-microglobulin) and target (Nav1.1–Nav1.9) genes in
each sample set.
 | Table IqPCR primer pairs used for detecting
VGSC α subunits mRNA levels in human normal prostate, in BPH
samples and in human prostate cancer cells. |
Table I
qPCR primer pairs used for detecting
VGSC α subunits mRNA levels in human normal prostate, in BPH
samples and in human prostate cancer cells.
| Gene symbol
(human) | Channel name | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Product length
(bp) |
|---|
| SCN1A | Nav1.1 |
CAGTGCAGCAGGCAGGC |
TCAATCGGTTCCCTTCAATGGAG | 212 |
| SCN2A | Nav1.2 |
AGACTTCAGTGGTGCTGGTG |
CTCTTCTTCTCCAGACTGTTC | 139 |
| SCN3A | Nav1.3 |
GGGTTAGGAGAGCTGTTGG |
CAAGGTGCTCTCTCTGTCTTC | 109 |
| SCN4A | Nav1.4 |
CTCGAGCTGGACCACCTTAA |
TCTCCTCTGCCTGCTCCTC | 232 |
| SCN5A | Nav1.5 |
CAACAGCTGGAATATCTTCG |
CCAAAGATGGAGTAGATGAAC | 260 |
| SCN8A | Nav1.6 |
TCAGCATCCCAGGCTCGC |
CTGGCTGTAGCCGCTGTA | 223 |
| SCN9A | Nav1.7 |
TATGACCATGAATAACCC |
TCAGGTTTCCCATGAACAGC | 389 (297a) |
| SCN10A | Nav1.8 |
GTTGGCACAGCAATAGATCTCC |
GACAGCCATGTCATTCTTGAC | 246 |
| SCN11A | Nav1.9 |
CCATCCTTGACCATCTCAACTG |
GGAAAGGAATGTGCTCCTGA | 186 |
| β2-microglobulin | |
TGCTGTCTCCATGTTTGATGTATCT |
TCTCTGCTCCCCACCTCTAAGT | 80 |
Electrophysiology
Na+ currents (INa) were
recorded using the whole-cell patch-clamp technique as previously
described (14–17). In brief, patch pipettes were
fabricated from borosilicate glass (Warner Instruments LLC, Hamden,
CT, USA) by a P-97 Flaming/Brown micropipette puller (Sutter
Instrument, Novato, CA, USA) and fire-polished using a microforge
(MF 830; Narishige Scientific Instrument Lab., Tokyo, Japan).
Pipette resistance was between 1.0 and 2.0 MΩ, and voltage-clamp
experiments were performed with an Axopatch 200B amplifier
(Molecular Devices, LLC, Sunnyvale, CA, USA). All recordings were
performed at room temperature (20–22°C). INa was
recorded in bath solution containing 140 mmol/l NaCl, 1 mmol/l
MgCl2, 1 mmol/l CaCl2, 10 mmol/l HEPES, 3
mmol/l KCl and 10 mmol/l glucose (pH 7.35), adjusted with CsOH. The
pipette solution contained the following: 10 mmol/l NaCl, 140
mmol/l CsF, 10 mmol/l EGTA, 5 mmol/l MgATP and 10 mmol/l HEPES (pH
7.35), adjusted with CsOH. Osmolarity was adjusted to 310 mOsm with
sucrose for all solutions. Recordings were filtered at 5 kHz and
digitally sampled at 40 kHz. To determine the voltage-dependence of
steady-state activation, currents were elicited by a 40-msec pulse
from a holding potential of −100 mV to test potentials between −80
and +40 mV in 5-mV increments. TTX (Enzo Life Sciences, New York,
NY, USA), a VGSC blocker, was used to identify sodium currents.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Results are presented as the means
± SEM. Statistical significance of differences between groups was
assessed using one-way analysis of variance. P<0.05 was
considered to indicate a statistical difference.
Results
mRNA expression profile of VGSC α
subunits in normal and hyperplastic prostate samples
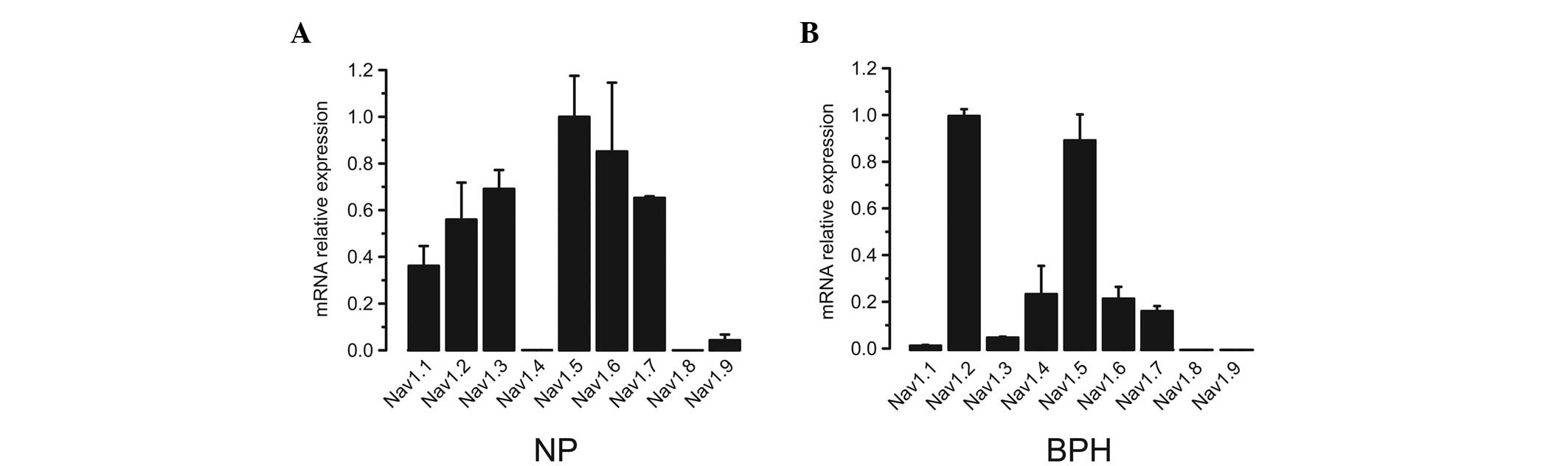
Initially, all nine VGSC α subunit expression
profiles in normal and hyperplastic prostates were analyzed. Three
human normal prostate (NP) and three human BPH biopsy samples were
analyzed using qPCR. Isoform-specific primers were used to amplify
different subtypes (Table I). In NP
samples, with the exception of Nav1.8, all subtypes of VGSC α
subunits were detected by qPCR. Among the expressed subtypes,
Nav1.2, Nav1.3, Nav1.6, Nav1.7, and particularly Nav1.5, had
relatively higher expression levels compared with those of the
other subtypes (Fig. 1A). Similar
to NP samples, with the exception of Nav1.8 and Nav1.9, all VGSC
subtypes were identified in BPH samples (Fig. 1B). Among those subtypes, Nav1.2 and
Nav1.5 were the predominant types.
mRNA expression profile of VGSC α
subunits in prostate cancer cells
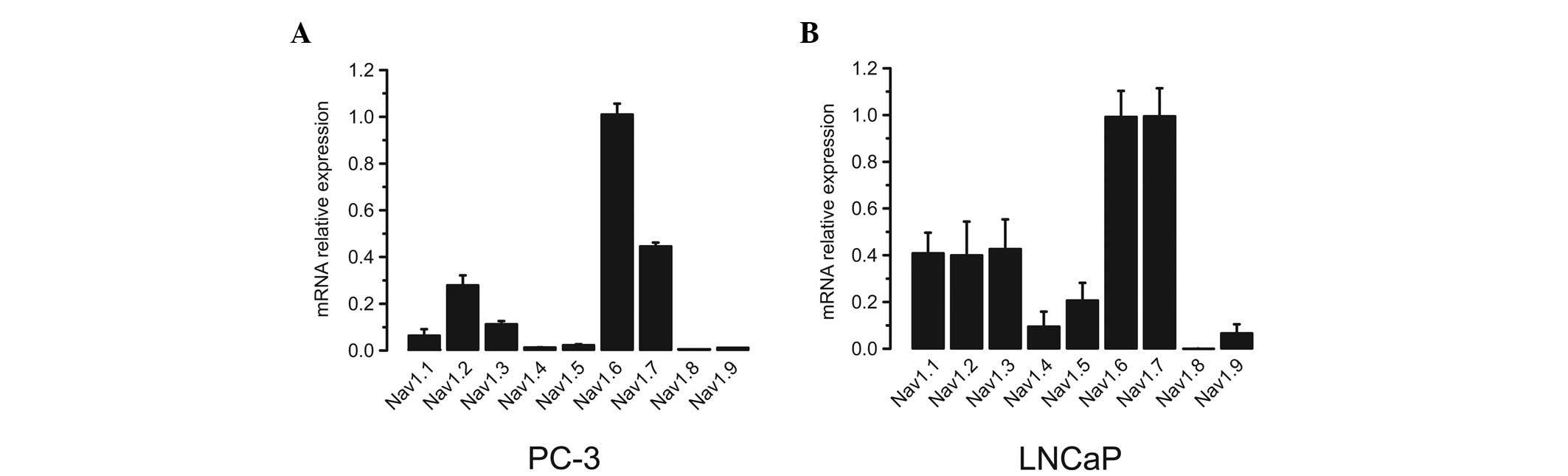
The VGSC α subunit mRNA expression profiles were
subsequently determined in human prostate cancer cells. Two typical
human prostate cancer cell lines, PC-3 and LNCaP, were utilized.
Using the same method as that for normal and hyperplastic prostate
samples, it was identified that the expression levels of Nav1.6
were ≥2.5-fold higher than those of any other subtype in PC-3 cells
(Fig. 2A). In LNCaP cells, however,
Nav1.6 and Nav1.7 were the predominate isoforms, which showed 2- to
3-fold higher expression levels compared with the remaining
subtypes (Fig. 2B).
Nav1.6 and Nav1.7 were up-regulated in
prostate cancer cells
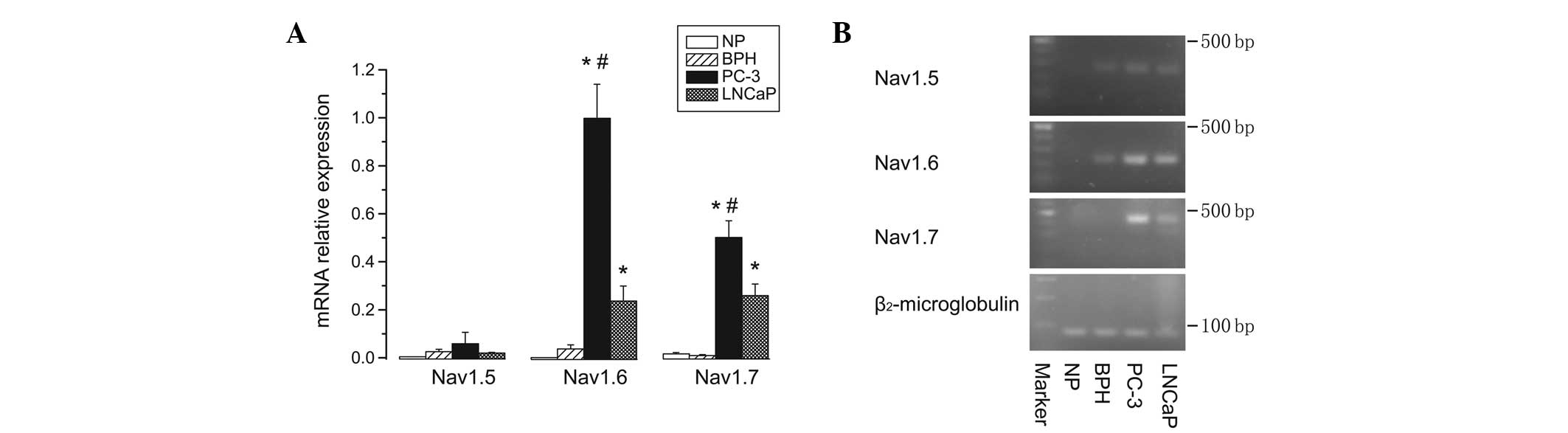
The expression profiles of all VGSC α subunit mRNA
levels had previously been determined in each of the three prostate
sample types (NP, BPH and prostate cancer cells). To compare the
relative expression levels of each VGSC α subunit among all types
of prostate cells, Nav1.5, Nav1.6 and Nav1.7 were selected, as
these subtypes exhibited the highest expression levels either in NP
and BPH samples, or in PC-3 and LNCaP cells, respectively. qPCR
data showed that Nav1.5 had almost equally low mRNA expression
levels in normal, BPH and prostate cancer cells. Notably, the
expression levels of Nav1.6 and Nav1.7, particularly Nav1.6, were
significantly upregulated (6- to 27-fold higher) in either PC-3 or
LNCaP cancer cells compared with those in NP and BPH samples
(P<0.05). Furthermore, the mRNA levels of Nav1.6 and Nav1.7 in
PC-3 cells were significantly higher than those in LNCaP cells
(P<0.05) (Fig. 3).
Sodium channels are functional in PC-3
prostate cancer cells
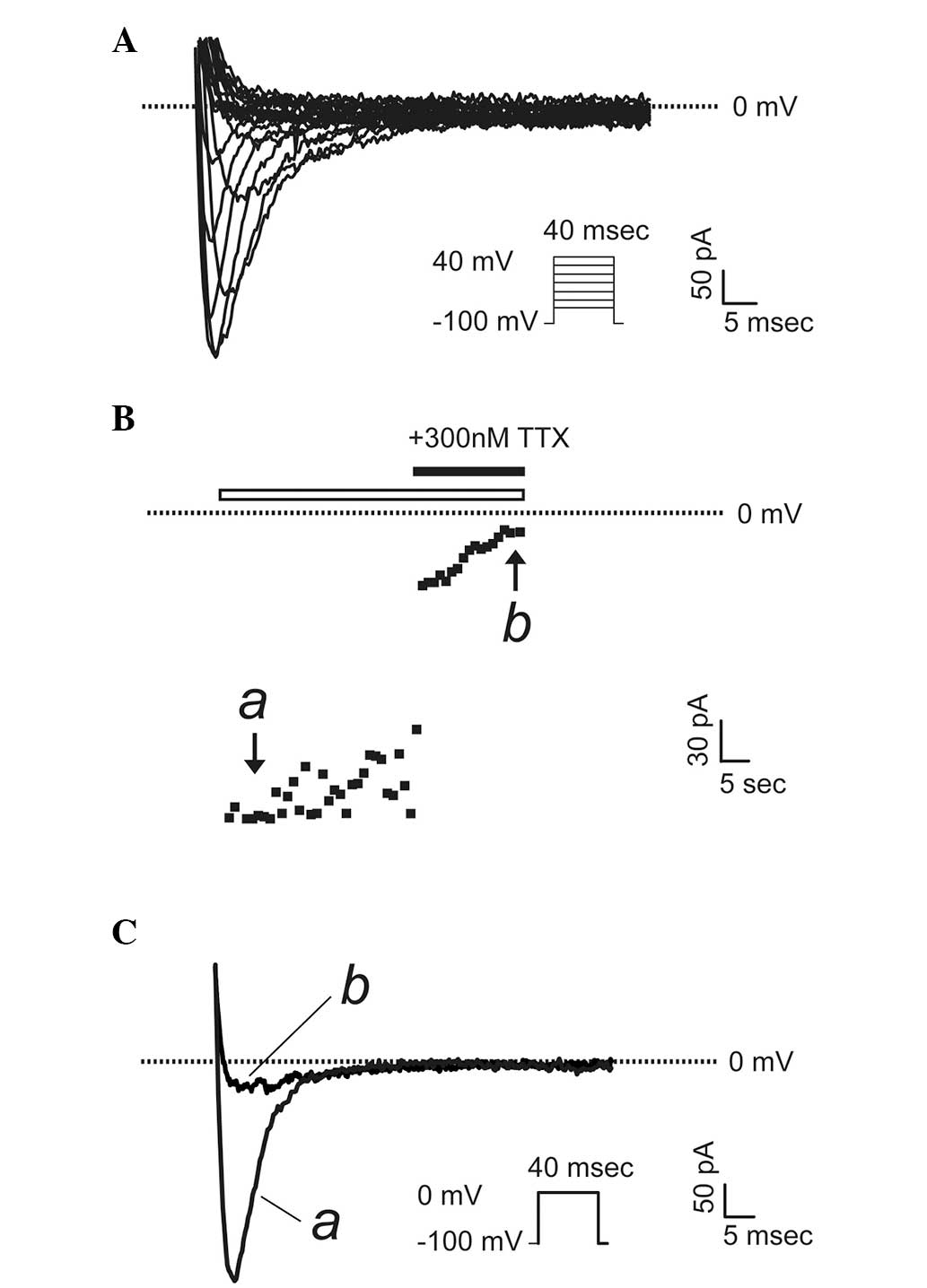
To determine whether the VGSC α subunits that were
detected to be upregulated in prostate cancer cells were
functionally expressed, a patch-clamp technique were used to record
whole-cell sodium currents in PC-3 and LNCaP cells, which possess
markedly different metastatic potentials. Fig. 4A shows a typical voltage-gated
sodium current recorded in PC-3 cells, the more highly metastatic
cancer cells, upon a stimulation from a holding potential of -100
mV to a series of test pluses between −80 and +40 mV in 5-mV
increments. To confirm the currents were sodium currents, a
specific VGSC blocker, TTX (300 nM) was used. The maximum activated
currents were completely abolished upon application of TTX
(Fig. 4B and C). Notably, in all
LNCaP cells (the cells with lower metastatic potential)
investigated, there were no sodium currents present (n=15, data not
shown).
Discussion
BPH, a common disease in adult males, has an
increasing incidence with age (9).
Certain types of prostate cancer are aggressive and have a poor
prognosis and high mortality rate in males (18). Previously, it has been reported that
certain types of VGSC α subunits were expressed in human and rodent
prostate cancer cells (10–13). However, to date, the detailed
expression information of VGSC α subunits in the prostate remain
unclear. To the best of our knowledge, the current study is the
first to use qPCR to explore all VGSC subtype mRNA expression
profiles in NP, BPH and prostate cancer cells. The main findings of
present study are as follows: i) the majority of VGSC α subunits
can be detected in NP and BPH samples; however, Nav1.6/Nav1.7 in NP
and BPH samples exhibit very low expression levels compared with
those in prostate cancer cells; ii) in prostate cancer (PC-3 and
LNCaP) cells, the expression of Nav1.6 and Nav1.7 is dramatically
upregulated; and iii) the upregulated Nav1.6 and Nav1.7 α subunits
in PC-3 cancer cells are functional.
Ion channels are a class of important functional
membrane-spanning proteins responsible for ion permission and ion
carrying. VGSCs are key ion channels to generate and conduct action
potential in excitable tissues (such as nerve and muscle) (2). VGSCs are composed of a pore-forming α
subunit and are associated with one or more auxiliary subunits
(β1–β4). Nine sodium channel α subunits (Nav1.1-Nav1.9), encoded by
the SCN1A-SCN5A and SCN8A-SCN11A genes, have been found in
vertebrates. The α subunits consist of four homologous domains, and
each domain contains six transmembrane segments (S1–S6), wherein a
positively charged S4 acts as a voltage sensor. β subunits are
regulatory subunits responsible for channel gating and trafficking
(2).
Increasing evidence shows that VGSCs also exist in
non-excitatory tissues, such as glial cells, osteoblasts,
lymphocytes, endothelial cells and fibroblasts (19–22). A
series of studies have found VGSCs expressed in prostate cancer
cells, and the expression of VGSCs were positively correlated with
cancer proliferation (12,13,23–25).
VGSCs were identified to be specifically expressed in the MAT-LyLu
highly metastatic murine prostate cancer cell line and, using TTX
blocking, VGSCs can significantly reduce the invasiveness of the
cell line in vitro (13),
suggesting that VGSC expression is important in the invasion and
metastasis of cancer cells. Further study found that the expression
levels of Nav1.7 are 3-fold higher in highly metastatic prostate
cancer cell lines (MAT-LyLu and PC-3) than in weakly metastatic
cell lines (AT-2 and LNCaP) (23).
The present study demonstrated that not only Nav1.7 but also Nav1.6
mRNA levels were significantly increased in prostate cancer cell
lines. Notably, Nav1.6 and Nav1.7 mRNA are expressed at higher
levels in PC-3 cells than in LNCaP cells. PC-3 and LNCaP cells are
two different metastatic prostate cancer cell lines, and PC-3 cells
have a greater invasive capacity than LNCaP cells. The functional
testing in the current study also showed typical sodium currents
are only present in PC-3 cells. These findings suggest that
inhibition of VGSC α subunits (Nav1.6 and Nav1.7) may be a useful
treatment strategy to reduce the metastatic spread of prostate
cancer. To clarify whether the upregulation of VGSC α subunits is
disease-associated, we also detected and compared the VGSC α
subunit expression profiles in prostate biopsy samples from normal
subjects and from BPH patients. The majority of VGSC subtypes were
expressed basically at very low levels in those samples.
As for the mechanisms accounting for sodium channels
affecting cancer cell migration and metabolism, there are several
different interpretations. Brisson et al propose that in
MDA-MB-231 breast cancer cells, Nav1.5 increases Na+
influx, which activates the Na+/H+ exchanger
type 1, which is an important regulator of H+ efflux.
Increased H+ release alters the pH of the local pH,
leading to pH-dependent extracellular matrix degradation and cell
invasiveness (26). However,
Carrithers et al suggests that Nav1.6 regulates cellular
invasion through its effects on podosome and invadopodia formation
in macrophages and melanoma cells (27). Overall, the mechanisms by which
sodium channel mRNA is upregulated and sodium channels regulate
cell invasion in cancer cells require further study.
Clinical pathological staging, biopsy Gleason score
and prostate-specific antigen have been widely used in the
diagnosis and monitoring of disease progression of prostate cancer
(28,29). However, due to the low specificity
and sensitivity, the application is limited. With the progress of
high-throughput genomics and proteomics technology, increasing
prostate cancer-related tumor markers, such as MIB-1/Ki67 labeling
indices, DD3, EGR-1 and Bcl-2, are constantly being discovered and
used in the diagnosis and prediction of prognosis of prostate
cancer (30,31). However, thus far, there remains a
lack of specific and sensitive tumor markers that can accurately
diagnose early prostate cancer and determine its invasiveness.
Therefore, it has become important to study new molecular biomarker
of genotype and phenotype in prostate cancer cells. Previous
studies have demonstrated that sodium channels influence the
metabolism of cancer cells through affecting cancer cell adhesion,
proliferation, invasion, migration and apoptosis (8,10–13),
suggesting that sodium channels may be an important diagnosis
indicator for prostate cancer. The present study showed that mRNA
expression levels of Nav1.6 and Nav1.7 were significantly
upregulated in prostate cancer cells compared with those in NP or
BPH samples, suggesting Nav1.6 and Nav1.7 may be potential
diagnostic markers for prostate cancer. It has been reported that
prostate surgery using classical sodium channel blockers/anesthetic
drugs could inhibit prostate cancer spread and recurrence (32). Therefore, it may be speculated that
sodium channel blockers, particularly Nav1.6 and Nav1.7 α
subunit-specific blockers, have the potential to participate in the
prevention and treatment of prostate cancer.
Overall, VGSCs have been demonstrated to be
upregulated in numerous types of metastatic prostate cancer cells.
The upregulated sodium channels play important roles in regulating
cellular migration, invasion and proliferation. Notably, the
altered VGSC expression has a potential utility as a diagnostic and
therapeutic target. In the present study, the mRNA expression
levels of all nine types of VGSCs α subunit were analyzed in human
NP, BPH and prostate cancer (PC-3 and LNCaP) cells by qPCR assay.
Compared with those in NP and BPH samples, the mRNA expression
levels of Nav1.6 and Nav1.7 were dramatically upregulated in
prostate cancer cells, suggesting these subtypes may be potential
diagnostic markers for certain types of prostate cancer in
humans.
Acknowledgements
This study was supported by a grant (no. 31171097)
from the National Natural Science Foundation of China (to CW) and
by a grant (no. C2012003031) from Hebei Province Overseas Returnees
Start-up Fund (to CW). Dr Chuan Wang was supported by the Backbone
of Scientific Research Training Program of Hebei Medical
University.
References
|
1
|
Catterall WA: The molecular basis of
neuronal excitability. Science. 223:653–661. 1984.
|
|
2
|
Catterall WA: From ionic currents to
molecular mechanisms: the structure and function of voltage-gated
sodium channels. Neuron. 26:13–25. 2000.
|
|
3
|
Antzelevitch C, Brugada P, Borggrefe M, et
al: Brugada syndrome: report of the second consensus conference:
endorsed by the Heart Rhythm Society and the European Heart Rhythm
Association. Circulation. 111:659–670. 2005.
|
|
4
|
Veltmann C, Schimpf R, Echternach C, et
al: A prospective study on spontaneous fluctuations between
diagnostic and non-diagnostic ECGs in Brugada syndrome:
implications for correct phenotyping and risk stratification. Eur
Heart J. 27:2544–2552. 2006.
|
|
5
|
Bennett PB, Yazawa K, Makita N and George
AL Jr: Molecular mechanism for an inherited cardiac arrhythmia.
Nature. 376:683–685. 1995.
|
|
6
|
Waxman SG: Painful Na-channelopathies: an
expanding universe. Trends Mol Med. 19:406–409
|
|
7
|
Oliva M, Berkovic SF and Petrou S: Sodium
channels and the neurobiology of epilepsy. Epilepsia. 53:1849–1859.
2012.
|
|
8
|
Brackenbury WJ: Voltage-gated sodium
channels and metastatic disease. Channels (Austin). 6:352–361.
2012.
|
|
9
|
Baade PD, Youlden DR and Krnjacki LJ:
International epidemiology of prostate cancer: geographical
distribution and secular trends. Mol Nutr Food Res. 53:171–184.
2009.
|
|
10
|
Diss JK, Stewart D, Pani F, et al: A
potential novel marker for human prostate cancer: voltage-gated
sodium channel expression in vivo. Prostate Cancer Prostatic Dis.
8:266–273. 2005.
|
|
11
|
Diss JK, Fraser SP, Walker MM, Patel A,
Latchman DS and Djamgoz MB: Beta-subunits of voltage-gated sodium
channels in human prostate cancer: quantitative in vitro and in
vivo analyses of mRNA expression. Prostate Cancer Prostatic Dis.
11:325–333. 2008.
|
|
12
|
Laniado ME, Lalani EN, Fraser SP, et al:
Expression and functional analysis of voltage-activated
Na+ channels in human prostate cancer cell lines and
their contribution to invasion in vitro. Am J Pathol.
150:1213–1221. 1997.
|
|
13
|
Grimes JA, Fraser SP, Stephens GJ, et al:
Differential expression of voltage-activated Na+
currents in two prostatic tumour cell lines: contribution to
invasiveness in vitro. FEBS Lett. 369:290–294. 1995.
|
|
14
|
Nguyen TP, Wang DW, Rhodes TH and George
AL Jr: Divergent biophysical defects caused by mutant sodium
channels in dilated cardiomyopathy with arrhythmia. Circ Res.
102:364–371. 2008.
|
|
15
|
Benson DW, Wang DW, Dyment M, et al:
Congenital sick sinus syndrome caused by recessive mutations in the
cardiac sodium channel gene (SCN5A). J Clin Invest. 112:1019–1028.
2003.
|
|
16
|
Sato PY, Musa H, Coombs W, et al: Loss of
plakophilin-2 expression leads to decreased sodium current and
slower conduction velocity in cultured cardiac myocytes. Circ Res.
105:523–526. 2009.
|
|
17
|
Wang C, Hennessey JA, Kirkton RD, et al:
Fibroblast growth factor homologous factor 13 regulates
Na+ channels and conduction velocity in murine hearts.
Circ Res. 109:775–782. 2011.
|
|
18
|
Nomiya T, Tsuji H, Toyama S, et al:
Management of high-risk prostate cancer: radiation therapy and
hormonal therapy. Cancer Treat Rev. 39:872–878. 2013.
|
|
19
|
Diaz D, Delgadillo DM, Hernández-Gallegos
E, et al: Functional expression of voltage-gated sodium channels in
primary cultures of human cervical cancer. J Cell Physiol.
210:469–478. 2007.
|
|
20
|
Fraser SP, Diss JK, Chioni AM, et al:
Voltage-gated sodium channel expression and potentiation of human
breast cancer metastasis. Clin Cancer Res. 11:5381–5389. 2005.
|
|
21
|
Hernandez-Plata E, Ortiz CS,
Marquina-Castillo B, et al: Overexpression of NaV 1.6 channels is
associated with the invasion capacity of human cervical cancer. Int
J Cancer. 130:2013–2023. 2012.
|
|
22
|
Roger S, Besson P and Le Guennec JY:
Involvement of a novel fast inward sodium current in the invasion
capacity of a breast cancer cell line. Biochim Biophys Acta.
1616:107–111. 2003.
|
|
23
|
Fraser SP, Grimes JA and Djamgoz MB:
Effects of voltage-gated ion channel modulators on rat prostatic
cancer cell proliferation: comparison of strongly and weakly
metastatic cell lines. Prostate. 44:61–76. 2000.
|
|
24
|
Abdul M and Hoosein N: Voltage-gated
sodium ion channels in prostate cancer: expression and activity.
Anticancer Res. 22:1727–1730. 2002.
|
|
25
|
Smith P, Rhodes NP, Shortland AP, et al:
Sodium channel protein expression enhances the invasiveness of rat
and human prostate cancer cells. FEBS Lett. 423:19–24. 1998.
|
|
26
|
Brisson L, Gillet L, Calaghan S, et al:
Na(V)1.5 enhances breast cancer cell invasiveness by increasing
NHE1-dependent H(+) efflux in caveolae. Oncogene. 30:2070–2076.
2011.
|
|
27
|
Carrithers MD, Chatterjee G, Carrithers
LM, et al: Regulation of podosome formation in macrophages by a
splice variant of the sodium channel SCN8A. J Biol Chem.
284:8114–8126. 2009.
|
|
28
|
Diamandis EP and Yu H: Nonprostatic
sources of prostate-specific antigen. Urol Clin North Am.
24:275–282. 1997.
|
|
29
|
Foster CS, Cornford P, Forsyth L, Djamgoz
MB and Ke Y: The cellular and molecular basis of prostate cancer.
BJU Int. 83:171–194. 1999.
|
|
30
|
de Kok JB, Verhaegh GW, Roelofs RW, et al:
DD3 (PCA3), a very sensitive and specific marker to detect prostate
tumors. Cancer Res. 62:2695–2698. 2002.
|
|
31
|
Chakravarti A and Zhai GG: Molecular and
genetic prognostic factors of prostate cancer. World J Urol.
21:265–274. 2003.
|
|
32
|
Biki B, Mascha E, Moriarty DC, Fitzpatrick
JM, Sessler DI and Buggy DJ: Anesthetic technique for radical
prostatectomy surgery affects cancer recurrence: a retrospective
analysis. Anesthesiology. 109:180–187. 2008.
|