Introduction
Leukemia is a multistep process involving the
alteration of different pathways, which ultimately affect cell
proliferation and maturation (1).
However, the hematopoietic microenvironment of the bone marrow is
also important in the development of leukemia. Bone marrow
mesenchymal stem cells (bMSCs) are important elements of the
hematopoietic microenvironment that frequently influence the
development of leukemia via the secretion of hematopoietic growth
factors (2). The communication
between bMSCs and hematopoietic cells alters leukemia progression
(3). A large number of proteins are
involved in these regulatory pathways between bMSCs and
hematopoietic cells, including Wnt5a, based on extracellular
receptor-ligand interactions. Wnt5a is one of the most extensively
studied proteins of the Wnt family and has a number of important
functions in different types of cancer by antagonizing the
canonical and inducing the non-canonical Wnt signaling pathways
(4–6). Wnt5a also promotes the expansion and
self-renewal of apoptosis-resistant transgenic hematopoietic stem
cells (HSCs), as well as the self-renewal of leukemia cells in
vitro (7,8). Our previous study showed that
Wnt5a-overexpressing bMSCs regulate the maturation and
proliferation of HL60 cells when the bMSCs are cocultured with HL60
cells (9). However, the molecular
mechanisms of this process remain unclear.
Wnt signaling is important in embryonic development,
adult homeostasis and tumor progression via the canonical and
non-canonical β-catenin pathways. In addition, Wnt signaling is
also responsible for primary acute myeloid and chronic lymphocytic
leukemia (10). Receptor tyrosine
kinase-like orphan receptor 2 (Ror2) is the coreceptor of Wnt5a and
belongs to the Ror subfamily of cell surface receptors. Ror2
induces the activation of the non-canonical signaling pathway by
binding to Wnt5a, which triggers the downstream signaling cascades,
including Ca2+/calmodulin-dependent protein kinase II
(CaMKII) (11). Therefore, we
hypothesized that bMSC-derived Wnt5a influences the proliferation
and maturation of HL60 cells via activation of the non-canonical
Wnt signaling pathway.
The present study investigates whether bMSCs-induced
Wnt5a was capable of regulating the matuation and proliferation of
HL60 cells through stimulation with with culture supernatants
containing Wnt5a protein obtained from bMSCs infected with
adeno-Wnt5a, adeno-vector or normal bMSCs, and examines which Wnt
signaling pathways are responsible for regulation.
Materials and methods
Cell culture
The HL60 leukemia and HEK293 cell lines was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and cultured with RPMI-1640 medium (Hyclone, Thermo
Scientific, Logan City, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco, Carlsbad, CA, USA) at 37°C in a humidified
atmosphere of 5% CO2. The human bone marrow cells were
harvested from the hips of three consenting healthy patients, two
males and one female (age range, 56–68 years) following
institutional review board approval from the Children’s Hospital of
Chongqing Medical University (Chongqing, China). The bMSCs were
isolated from the bone marrow cells via Ficoll-Paque (Amersham
Pharmacia Biotech, Amersham, UK) density gradient separation
medium, and the separated mononuclear cells were washed twice with
phosphate-buffered saline (Beyotime Institute of Biotechnology,
Shanghai, China).
RNA extraction and quantitative
polymerase chain reaction (qPCR)
Total RNAs were extracted from the bMSCs and HL60
cells via TRIzol reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions, and finally
resuspended in 35 μl of preheated (68°C) nuclease-free water.
For the qPCR analysis of Wnt5a, ROR2, frizzled
family receptor 5 (FZD5), β-catenin, CaMKII, cyclin D1 and β-actin,
the total RNAs were transcribed to cDNAs using the PrimeScript™ RT
reagent kit (Takara Bio, Inc., Shiga, Japan). The qPCR was then
performed using the SYBR® Green real-time PCR master mix
(QPK-201; Toyobo Corporation, Osaka, Japan) and C1000™ Thermal
Cycler (CXF96; Bio-Rad, Hercules, CA, USA). The Ct value of β-actin
was used to normalize the mRNA levels and the primer sequences of
the above genes are shown in Table
I. The qPCR was performed using the following parameters:
Initial hold at 95°C for 30 sec, followed by 40 cycles of 95°C for
10 sec, 60°C for 10 sec and 72°C for 20 sec.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primers | Product, bp |
|---|
| Wnt5a | F:
5′-TGTGGTTTAATGGTGCCTGA-3′
R: 5′-TTCGTCGTGCTCAAGGTATG-3′ | 253 |
| ROR2 | F:
5′-ATGGAACTGTGTGACGTACCC-3′
R: 5′-GCGAGGCCATCAGCTG-3′ | 186 |
| FZD5 | F:
5′-TGTCTGCTCTTCTCGGC-3′
R: 5′-CCGTCCAAAGATAAACTGCT-3′ | 142 |
| β-catenin | F:
5′-TGGTTGCCTTGCTCAACA-3′
R: 5′-AGCTTGGGGTCCACCACT-3′ | 125 |
| CaMKII | F:
5′-AAGATGTGCGACCCTGGAATG-3′
R: 5′-TGTAGGCGATGCAGGCTGAC-3′ | 784 |
| Cyclin D1 | F:
5′-CCCTCGGTGTCCTACTTCAAA-3′
R: 5′-CACCTCCTCCTCCTCCTCTTC-3′ | 726 |
| β-actin | F:
5′-TTCCTTCCTGGGCATGGAGTCC-3′
R: 5′-TGGCGTACAGGTCTTTGCGG-3′ | 191 |
Construction and purification of
adenovirus-mediated vectors
The adenoviruses expressing green fluorescent
protein (AdGFP) and Wnt5a protein (adeno-Wnt5a) were provided by Dr
T.C. He (Molecular Oncology Laboratory, Department of Surgery, The
University of Chicago Medical Center, Chicago, IL, USA). The
adenoviruses were propagated and purified as described prevoiously
(12). Briefly, the adenoviruses
were propagated into the HEK293 cells, and subsequently the
successful viral infection was confirmed by GFP expression. Cell
pellets were resuspended in 1× PBS and lysed using four
freeze-thaw-vortex cycles. The vectors were dialyzed in a storage
buffer (Promega, Madison, WI, USA) following purification using a
cesium chloride gradient (Sigma-Aldrich, Shanghai, China), followed
by transfection into HEK293 cells via lipofectamine 2000
(Invitrogen Life Technologies) for 24 h. Subsequently the titers of
the adenovirus were determined.
Cell proliferation assay
The cells were seeded at a density of
1×104 cells per well in 96-well plates. Next, 100 μl of
the culture supernatants of bMSCs, bMSCs infected with
Wnt5a-encoding adenovirus (adeno-Wnt5a bMSCs) or bMSCs infected
with empty adenoviral vector (adeno-vector bMSCs) (provided by T.C.
He) and 100 μl of RPMI 1640 medium supplemented with 10% FBS
(Sigma-Aldrich, St. Louis, MO, USA) were mixed and added to the
each experimental well of the 96-well plates (12,13).
Following culture for 24 or 48 h, the cell proliferation was
detected using the Cell Counting Kit-8 assay (CCK-8; Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer’s instructions.
ELISA
The protein levels of Wnt5a were measured using a
human Wnt5a ELISA kit (Groundwork Biotechnology Diagnosticate Ltd.,
San Diego, CA, USA) according to the manufacturer’s instructions,
and the absorbance was read at 450 nm using a microplate reader
(SpectraMax 190, Molecular Devices LLC, Sunnyvale, CA, USA).
Immunohistochemical (IHC) staining
For the IHC staining, the HL60 cells were seeded on
microscope glass plates and non-specific staining was blocked using
Dako Protein Block (Dako, Carpinteria, CA, USA) according to the
manufacturer’s instructions. Next, the anti-CD13, -CD14 and -CD68
(all at a dilution of 1:200) primary antibodies were diluted in
Dako antibody diluent and incubated with the cell sections for 1 h
at room temperature. Finally, staining was visualized using the
Dako Envision kit and developed using a DAB chromogen substrate
(Dako).
Western blot analysis
The protein expression of Ror2 (105 kDa), FZD5 (65
kDa), CaMKII (50 kDa) β-catenin (86 kDa), cyclin D1 (34 kDa), Wnt5a
(45 kDa), GAPDH (37 kDa) and β-actin (43 kDa) were analyzed by
western blot analysis using anti-Ror2 rabbit monoclonal (ab92379;
Abcam, Cambridge, UK), anti-Frizzled 5 rabbit polyclonal (ab75234;
Abcam), anti-CaMKII rabbit polyclonal (sc-13082; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), anti-β-catenin rabbit
monoclonal (ab32572; Abcam), anti-cyclin D1 rabbit polyclonal
(ab95281; Abcam), anti-Wnt5a rabbit polyclonal (2392; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-GAPDH rabbit
monoclonal (5174; Cell Signaling Technology, Inc.) and anti-β-actin
rabbit monoclonal (4970; Cell Signaling Technology, Inc.)
antibodies according to the manufacturer’s instructions.
Statistical analysis
Data are presented as the mean ± standard deviation.
Student’s t-test was used for comparisons between the two groups.
P<0.05 was considered to indicate a statistically significant
difference. All experiments were conducted in duplicate and the
statistical analyses were performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA).
Results
Wnt5a overexpression in bMSCs induced by
an adenoviral vector
To identify the role of Wnt5a derived from bMSCs in
influencing the growth of HL60 cells, an adenoviral vector,
adeno-Wnt5a-GFP, was constructed which expresses the Wnt5a protein
in bMSCs. The bMSCs expressing GFP were sorted from non-expressing
bMSCs and bMSCs infected with the adenoviruses containing
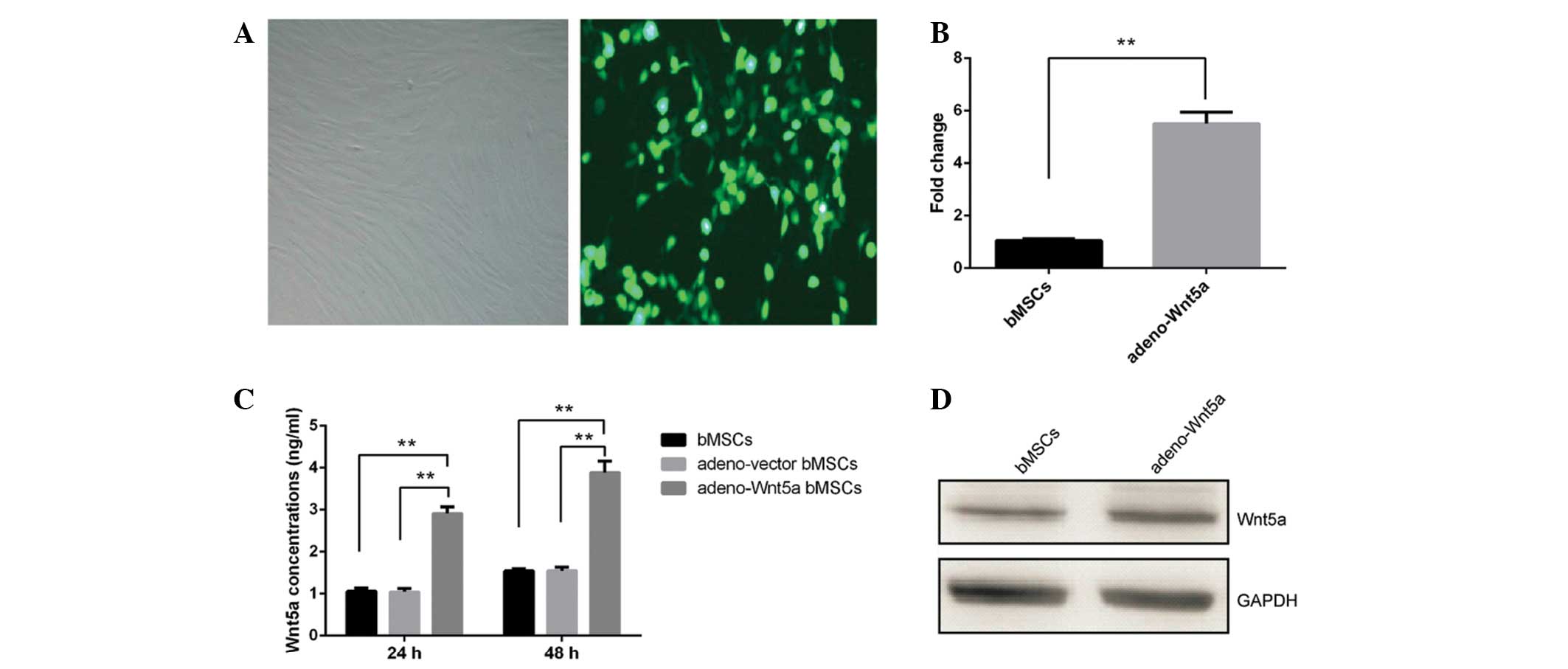
adeno-Wnt5a-GFP or adeno-vector-GFP. As shown in Fig. 1A, approximately all of the bMSCs
expressed GFP. The mRNA and protein levels of Wnt5a were
significantly increased in the adeno-Wnt5a bMSCs compared with
those in the adeno-vector bMSCs (P<0.01; Figs. 1B and C). The protein levels of
Wnt5a in the culture supernatant of adeno-Wnt5a bMSCs were also
significantly elevated when compared with those in the culture
supernatant of adeno-vector bMSCs, following culture for 24 or 48 h
(P<0.01; Fig. 1D). These results
indicated that Wnt5a expression can be induced in bMSCs and
secreted in the culture supernatant of the bMSCs.
bMSC-derived Wnt5a inhibits HL60 cell
proliferation
To analyze whether bMSC-derived Wnt5a influences
HL60 cell proliferation, the HL60 cells were stimulated with the
culture supernatants of bMSCs, adeno-Wnt5a bMSCs and adeno-vector
bMSCs for 24 or 48 h. The culture supernatants were collected
following cell culture for 24 h and the proliferation of the HL60
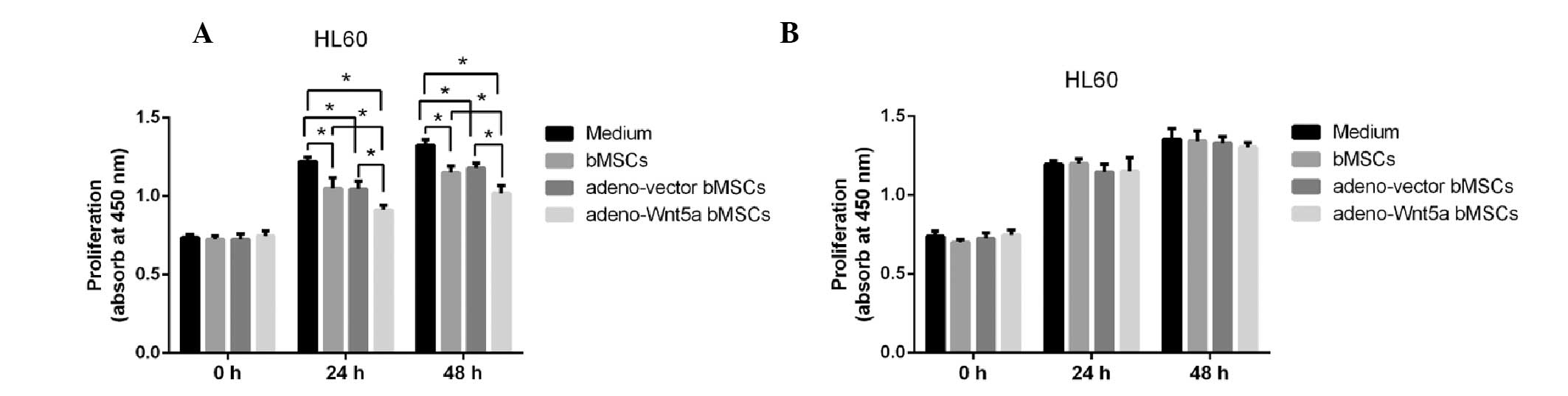
cells was detected using the CCK-8 assay. As shown in Fig. 2A, the proliferation of HL60 cells
was significantly decreased following stimulation with the culture
supernatant of adeno-Wnt5a bMSCs when compared with that following
stimulation with the culture supernatants of bMSCs and adeno-vector
bMSCs (P<0.05). Although, the proliferation of HL60 cells was
significantly decreased in the bMSC and adeno-vector bMSC groups
when compared with that in the conditional medium of HL60 cells
(P<0.05), no significant difference was identified in the
proliferation of HL60 cells between the bMSC and adeno-vector bMSC
groups.
To identify the role of bMSC-derived Wnt5a in the
proliferation of leukemia HL60 cells, a neutralization antibody
against Wnt5a was added to the culture supernatant prior to
stimulation for 1 h. The proliferation of HL60 cells following
stimulation with the Wnt5a-blocked culture supernatant was then
detected, and no significant difference was identified in the
proliferation of HL60 cells among all of the groups (Fig. 2B). These results suggested that
bMSC-derived Wnt5a inhibits the proliferation of leukemia HL60
cells.
bMSC-derived Wnt5a promotes HL60 cell
maturation
To analyze the effect of bMSC-derived Wnt5a on the
maturation of HL60 cells, the culture supernatants of bMSCs,
adeno-Wnt5a bMSCs and adeno-vector bMSCs were assembled following
cell culture for 24 h and used to stimulate HL60 cells for 24 or 48
h. The maturation of HL60 cells was then analyzed by detecting the
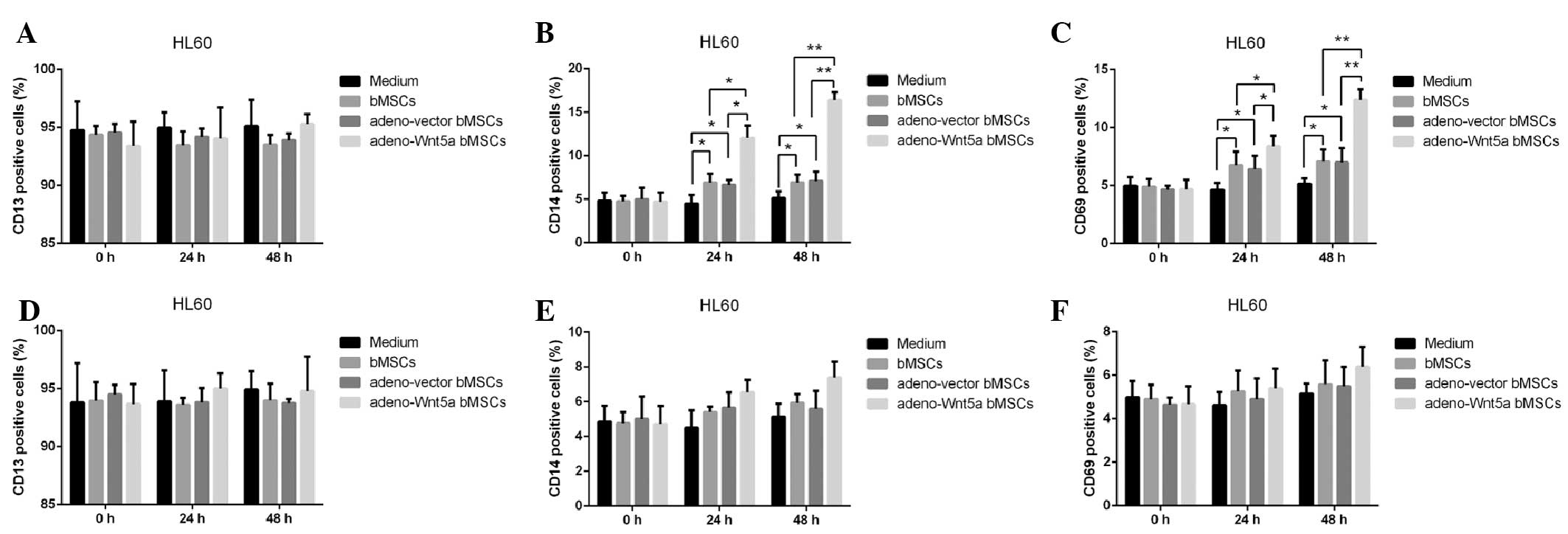
number of cells expressing CD13, CD14 or CD68. As shown in Fig. 3A–C, no significant difference was
identified in the number of CD13-positive cells among the
conditional medium, bMSC, adeno-vector bMSC and adeno-Wnt5a bMSC
groups. However, a significantly increased number of CD14- and
CD68-positive cells were observed in the adeno-Wnt5a bMSC group
than that in the bMSC and adeno-vector bMSC groups at 24 h
(P<0.05) or 48 h (P<0.01). Furthermore, a significantly
increased number of CD14- and CD68-positive cells were identified
in the bMSC and adeno-vector bMSC groups than that in the
conditional medium group (P<0.05). However, no significant
difference in the number of CD14- and CD68-positive cells was
identified between the bMSC and adeno-vector bMSC groups.
To investigate the effect of bMSC-derived Wnt5a on
HL60 cell maturation, the neutralization antibody against Wnt5a was
added to the culture supernatants of the bMSCs, adeno-Wnt5a bMSCs
or adeno-vector bMSCs for 1 h prior to stimulation. Subsequently,
the number of CD13-, CD14- and CD68-positive cells was calculated
and, as shown in Fig. 3D–F, no
significant difference was identified in the number of positive
cells among the four groups. These results indicated that
bMSC-derived Wnt5a promotes the maturation of leukemia HL60
cells.
bMSC-derived Wnt5a performs its functions
by enhancing the non-canonical and inhibiting the canonical Wnt
signaling pathways in HL60 cells
It is well known that Wnt5a affects the canonical
and non-canonical Wnt signaling pathways (7,14–16).
However, it remains unknown whether bMSC-induced Wnt5a influences
the canonical, non-canonical or the two Wnt signaling pathways.
Thus, the expression of Wnt5a receptors, FZD5 and ROR2, were
detected, as well as the downstream proteins, β-catenin and cyclin
D1, the alteration of which is hypothesized to activate or inhibit
the canonical Wnt signaling (17).
In addition, the expression of CaMKII was investigated, as it is
considered to alter the non-canonical Wnt signaling pathway
(18). The expression of the
abovementioned proteins was analyzed in HL60 cells stimulated for
48 h with the culture supernatants of adeno-Wnt5a bMSCs or
adeno-vector bMSCs, or with the culture supernatant with blocked
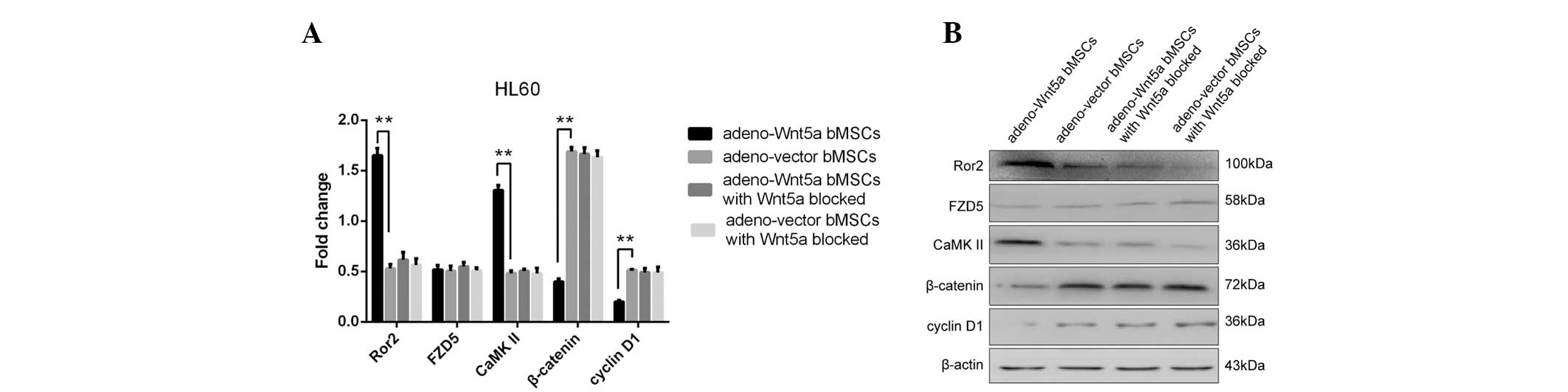
Wnt5a expression. As shown in Fig.
4, the significantly increased expression of Ror2 and CaMKII
and decreased expression of β-catenin and cyclin D1 were observed
in the group of cells stimulated with the culture supernatant of
adeno-Wnt5a bMSCs when compared with the group stimulated with the
supernatant of adeno-vector bMSCs. However, no significant
difference was identified in the expression of the four proteins
between the two groups stimulated with the culture supernatants of
adeno-Wnt5a bMSCs or adeno-vector bMSCs containing a neutralization
antibody against Wnt5a. Furthermore, no significant difference was
identified in the expression of FZD5 among the four groups. These
results indicated that bMSC-derived Wnt5a activates the
non-canonical and inhibits the canonical Wnt signaling
pathways.
Discussion
The present study confirmed that bMSC-derived Wnt5a
inhibits the proliferation of HL60 cells and promotes their
maturation. Furthermore, it was identified that bMSC-derived Wnt5a
activates the non-canonical and inhibits the canonical Wnt
signaling pathways in HL60 cells.
Although Wnts are secreted proteins, it is difficult
to purify active Wnt molecules, including Wnt5a. Therefore, the
present study constructed an adeno-Wnt5a-GFP vector which expresses
the Wnt5a protein in bMSCs to simulate the active Wnt5a molecule.
Accumulating evidence has indicated that the Wnt5a/Ror2 pathway is
associated with the differentiation fate of bMSCs (19), and that the canonical and
non-canonical Wnt signaling pathways differentially affect the
developmental potential of bMSCs (20). An additional study has demonstrated
that bMSCs control HSC migration and proliferation via secreted
molecules, including chemokine stromal derived factor-1 and Wnt5a
(21). The results of our previous
study indicated that Wnt5a-overexpressing bMSCs modify the
proliferation and maturation of HL60 cells when the two cells are
cocultured for three, five or seven days (9). Therefore, the present study focused on
bMSC-derived Wnt5a to investigate its effect on the growth of
leukemia HL60 cells and to determine which factor or factors alter
the proliferation and maturation of HL60 cells, and whether Wnt5a
or other factors are altered as a result of the overexpression of
Wnt5a in bMSCs. As predicted, the culture supernatants of bMSCs,
adeno-Wnt5a bMSCs and adeno-vector bMSCs were also found to
suppress the proliferation of HL60 cells and promote HL60 cell
maturation. In particular, the culture supernatant of adeno-Wnt5a
bMSCs exhibited the most significant effect. In addition, the
phenotype of the Wnt5a protein was lost when the cells were
stimulated with the culture supernatant containing a neutralization
antibody against Wnt5a. These results provide strong evidence that
bMSC-derived Wnt5a modifies the proliferation and maturation of
leukemia HL60 cells.
It is well known that Wnt5a functions in mammalian
cells via binding to its receptors, including FZD5 (a common
receptor) and Ror2 (a coreceptor). FZD5 belongs to the mammalian
frizzled family, which mediates Wnt5a signaling via FZD3, FZD4,
FZD5 and FZD8, but not FZD6, according to the assessment of the
phosphorylation of disheveled protein (Dvl1 human homolog) in
Drosophila Schneider 2 cells (22).
In addition, mutation in the KTxxxW motif or the first or third
loop of the human FZD5 has been found to prevent the binding of Dvl
and the resultant signaling (23);
therefore, FZD5 is crucial in Wnt5a signaling. Ror-2, however, is a
single-pass transmembrane receptor with a tyrosine kinase domain,
which may be more closely involved in the specific activation of
Wnt5a signaling and activation of the non-canonical Wnt signaling
pathway. Unlike Wnt1 and Wnt3a which activate the β-catenin
pathway, Wnt5a has been frequently shown to activate the
β-catenin-independent and non-canonical Wnt signaling pathways via
binding to its receptors, frizzled and Ror2 (24), as well as inhibiting the β-catenin
pathway (25). In contrast to the
inhibitory effects of Wnt5a on the β-catenin pathway, several
studies have reported that Wnt5a stimulates the β-catenin pathway
(26). To investigate whether
bMSC-derived Wnt5a is involved in the modification of HL60 cells,
the present study detected the effect of Wnt5a on the canonical and
non-canonical Wnt signaling pathways. As predicted, Wnt5a was
observed to activate the non-canonical and inhibit the canonical
Wnt signaling pathways.
In conclusion, the present study confirmed that
bMSC-derived Wnt5a inhibits the proliferation of leukemia HL60
cells and promotes their maturation via activating the
non-canonical and impairing the canonical Wnt signaling
pathways.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 39970768 and
30471985).
References
|
1
|
Gilliland DG, Jordan CT and Felix CA: The
molecular basis of leukemia. Hematology Am Soc Hematol Educ
Program. 1:80–97. 2004.
|
|
2
|
Arai KI, Lee F, Miyajima A, Miyatake S,
Arai N and Yokota T: Cytokines: coordinators of immune and
inflammatory responses. Annu Rev Biochem. 59:783–836. 1990.
|
|
3
|
Ploemacher RE, Mayen AE, De Koning AE, et
al: Hematopoiesis: gap junction intercellular communication is
likely to be involved in regulation of stroma-dependent
proliferation of hemopoietic stem cells. Hematology. 5:133–147.
2000.
|
|
4
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005.
|
|
5
|
Taketo MM: Shutting down Wnt
signal-activated cancer. Nat Genet. 36:320–322. 2004.
|
|
6
|
McDonald SL and Silver A: The opposing
roles of Wnt-5a in cancer. Br J Cancer. 101:209–214. 2009.
|
|
7
|
Clevers H and Nusse R: Wnt/beta-catenin
signaling and disease. Cell. 149:1192–1205. 2012.
|
|
8
|
Kikuchi A, Yamamoto H, Sato A and
Matsumoto S: Wnt5a: its signalling, functions and implication in
diseases. Acta Physiol (Oxf). 204:17–33. 2012.
|
|
9
|
Shen YL, Xu YH, Luo Q, Guo ZH, Zheng GH
and Guo YX: Growth and differentiation effect on HL60 cells by
adenovirus-mediated exogenous wnt5a gene modification on human bone
marrow mesenchymal stem cells. Sichuan Da Xue Xue Bao Yi Xue Ban.
41:931–935. 2010.
|
|
10
|
Liang H, Chen Q, Coles AH, et al: Wnt5a
inhibits B cell proliferation and functions as a tumor suppressor
in hematopoietic tissue. Cancer Cell. 4:349–360. 2003.
|
|
11
|
Shrivastava A, Radziejewski C, Campbell E,
et al: An orphan receptor tyrosine kinase family whose members
serve as nonintegrin collagen receptors. Mol Cell. 1:25–34.
1997.
|
|
12
|
Pan Z, Sun X, Shan H, et al: MicroRNA-101
inhibited postinfarct cardiac fibrosis and improved left
ventricular compliance via the FBJ osteosarcoma
oncogene/transforming growth factor-beta1 pathway. Circulation.
126:840–850. 2012.
|
|
13
|
Luo J, Deng ZL, Luo X, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007.
|
|
14
|
van Amerongen R, Fuerer C, Mizutani M and
Nusse R: Wnt5a can both activate and repress Wnt/beta-catenin
signaling during mouse embryonic development. Dev Biol.
369:101–114. 2012.
|
|
15
|
Nemeth MJ, Topol L, Anderson SM, Yang Y
and Bodine DM: Wnt5a inhibits canonical Wnt signaling in
hematopoietic stem cells and enhances repopulation. Proc Natl Acad
Sci USA. 104:15436–15441. 2007.
|
|
16
|
Cheng CW, Yeh JC, Fan TP, Smith SK and
Charnock-Jones DS: Wnt5a-mediated non-canonical Wnt signalling
regulates human endothelial cell proliferation and migration.
Biochem Biophys Res Commun. 365:285–290. 2008.
|
|
17
|
Koehler A, Schlupf J, Schneider M, Kraft
B, Winter C and Kashef J: Loss of Xenopus cadherin-11 leads to
increased Wnt/beta-catenin signaling and up-regulation of target
genes c-myc and cyclin D1 in neural crest. Dev Biol. 383:132–145.
2013.
|
|
18
|
Kühl M and Pandur P: Measuring CamKII
activity in Xenopus embryos as a read-out for non-canonical Wnt
signaling. Methods Mol Biol. 468:173–86. 2008.
|
|
19
|
Xin H, Xin F, Zhou S and Guan S: The
Wnt5a/Ror2 pathway is associated with determination of the
differentiation fate of bone marrow mesenchymal stem cells in
vascular calcification. Int J Mol Med. 31:583–588. 2013.
|
|
20
|
Baksh D and Tuan RS: Canonical and
non-canonical Wnts differentially affect the development potential
of primary isolate of human bone marrow mesenchymal stem cells. J
Cell Physiol. 212:817–826. 2007.
|
|
21
|
de Barros AP, Takiya CM, Garzoni LR, et
al: Osteoblasts and bone marrow mesenchymal stromal cells control
hematopoietic stem cell migration and proliferation in 3D in vitro
model. PLoS One. 5:e90932010.
|
|
22
|
Takada R, Hijikata H, Kondoh H and Takada
S: Analysis of combinatorial effects of Wnts and Frizzleds on
beta-catenin/armadillo stabilization and Dishevelled
phosphorylation. Genes Cells. 10:919–928. 2005.
|
|
23
|
Cong F, Schweizer L and Varmus H: Wnt
signals across the plasma membrane to activate the beta-catenin
pathway by forming oligomers containing its receptors, frizzled and
LRP. Development. 131:5103–5115. 2004.
|
|
24
|
Yamamoto H, Sakane H, Yamamoto H, Michiue
T and Kikuchi A: Wnt3a and Dkk1 regulate distinct internalization
pathways of LRP6 to tune the activation of beta-catenin signaling.
Dev Cell. 15:37–48. 2008.
|
|
25
|
Torres MA, Yang-Snyder JA, Purcell SM,
DeMarais AA, McGrew LL and Moon RT: Activities of the Wnt-1 class
of secreted signaling factors are antagonized by the Wnt-5A class
and by a dominant negative cadherin in early Xenopus development. J
Cell Biol. 133:1123–1137. 1996.
|
|
26
|
Umbhauer M, Djiane A, Goisset C, et al:
The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled
receptors mediates Wnt/beta-catenin signalling. EMBO J.
19:4944–4954. 2000.
|