Introduction
Pancreatic cancer is a highly lethal disease and
tumors are often late stage at diagnosis, at which point there are
few effective treatment options. In 2012, pancreatic cancer
resulted in ~85.13% of cancer-related mortality within the United
States, the five-year survival rate was ≤5% and the median survival
was less than six months (1).
Notable improvements have been made in the five-year survival rates
for a number of cancers over the past 30 years, with the exception
of pancreatic cancer (1,2). Therefore, it is imperative to identify
novel treatment strategies to prolong patient survival time for
pancreatic cancer. Qingyihuaji formula (QYHJ) has been used in
pancreatic cancer treatment for a number of years at the Fudan
University Cancer Center (Shanghai, China). Our previous
retrospective studies have shown that QYHJ treatment prolongs the
survival time of pancreatic cancer patients, with multivariate
analysis demonstrating that QYHJ acted as an independent protective
factor for pancreatic cancer with liver metastases (3–5).
However, QYHJ appeared to prolong the survival time for one
subgroup of patients, however, was ineffective for a different
group of patients. Our previous study showed that different
pancreatic cancer cell lines with different levels of
erythropoietin-producing hepatoma cell line-B2 (EphB2) expression
exhibited different responses to QYHJ treatment (6). It has previously been confirmed that
EphB2 is a prognostic factor for several types of cancer and acts
preferentially as a tumor suppressor in various cancers (7–15).
Furthermore, our previous study revealed that inhibition of EphB2
expression in pancreatic cancer CFPAC-1 cells resulted in the
promotion of cancer growth by stimulating cell proliferation and
decreasing apoptosis. Therefore, EphB2 acts as a tumor suppressor
in pancreatic cancer (16).
Traditional Chinese medicine (TCM) is regarded as a multi-target
therapy, which is similar to target therapy in modern medicine
(17,18). Previous studies revealed that not
all patients benefit from target therapies as patients may have
their own effective population according to their distinctive
predictors. Thus, the aim of the present study was to investigate
the correlation between the different levels of EphB2 expression
and the response to QYHJ treatment. In addition, to elucidate
whether EphB2 acts as a predictive factor for QYHJ treatment.
Materials and methods
Cell cultures
In 1990, the human pancreatic cancer CFPAC-1 cell
line was established from a patient with cystic fibrosis by
Schoumacher et al (19).
CFPAC-1 EphB2 RNAi and CFPAC-1 control RNAi cells were transfected
by lentivirus-based RNAi to inhibit EphB2 expression, and served as
a control RNAi in our previous study (16). Cells were cultured in RPMI-1640
medium (Gibco-BRL, Carlsbad, CA, USA) with 10% heat-inactivated
fetal bovine serum (Hyclone, Logan, UT, USA) under a 5%
CO2 atmosphere at 37°C. The medium was changed at 24 h
intervals when the culture had almost reached confluence.
Drug preparation and intervention
QYHJ is composed of Hedyotidis herba,
Amorphophallus konjac, Herba scutelleriae barbatae,
coix seed, akebia stem, Gynostemma pentaphyllum and java
amomum fruit. The herb powder was produced by Jiangyin Tianjiang
Pharmaceutical Co., Ltd., (Jiangyin, China). QYHJ was prepared by
dissolving the herb powder into distilled water to the required
concentration. The daily dosage of QYHJ for the nude mice was
calculated according to the following human-mouse transfer formula:
Db = Da × (Rb/Ra) × 2/3
(Wb/Wa) where D, R, and W represent dosage,
weight coefficient and body weight, respectively, and a and b
represent human and mouse, respectively. The QYHJ group received a
total of 200 μl liquid QYHJ twice a day by oral gavage as well as a
36 g/kg daily dosage, the gemcitabine group received an
intraperitoneal injection of 120 mg/kg gemcitabine on days one,
eight and 15 and the control group received an oral gavage of a
total of 200 μl normal saline twice a day. All of the animal
studies were reviewed and approved by the Animal Care and Use
Committee of Fudan University (Shanghai, China) and were in
accordance with the guidelines of the Department of Health and
Human Services.
Assessment of tumorigenicity in vivo
In total, 1×106 CFPAC-1, CFPAC-1 control
RNAi and CFPAC-1 EphB2 RNAi cells (200 μl) with different levels of
EphB2 expression were injected subcutaneously into the right flank
of eight-week-old female BALB/c nude mice. Tumor volume was
measured twice per week and calculated using the following formula:
Tumor volume = 0.52 × A × B2, where A is the length
(long diameter) and B is the width (short diameter) of the tumor.
Following four weeks of intervention with drugs, the mice were
sacrificed, the tumors were dissected and the tumor weight was
measured. The tumor weight inhibitory rate was calculated according
to the following formula: Tumor weight inhibitory rate = 100 ×
(tumor weight of control group - tumor weight of QYHJ group) /
tumor weight of control group.
Cell cycle and apoptosis analyses
The tumors were dissected, ground, centrifuged and
washed with phosphate-buffered saline (PBS). Next, 1×106
cells were fixed in 1 ml ethanol at 4°C for 1 h. Following
centrifugation at 1,500 × g for 10 min (L-550, Changsha Xiangyi
Centrifuge Instrument Co., Ltd., Changsha, China) and washing with
PBS, the cells were resuspended in 250 μl PBS containing 12.5 μg
RNase and incubated for 30 min at 37°C. Cellular DNA was stained
with 250 μl propidium iodide (PI) for 30 min at room temperature in
the dark. The stained cells were analyzed by flow cytometry (FCM)
for cell cycle analysis, and 1×106 cells were
centrifuged at 400 × g for 10 min and resuspended in 250 μl PBS;
these were incubated for 10 min at 37°C with 1 μg/ml Heochst 33342.
Following centrifugation and washing with PBS, the cells were
resuspended in 1 ml PI and incubated for an additional 15 min at
room temperature in the dark. The stained cells were analyzed by
FCM to assess the cell apoptosis.
Reverse transcription-polymerase chain
reaction analysis (RT-PCR)
Total RNA from the tumor tissue was extracted using
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA), according
to the manufacturer’s instructions. RT was performed using the
RevertAid H Minus First Strand cDNA Synthesis kit (Fermentas,
Waltham, MA, USA), according to the manufacturer’s instructions.
The primer sequences used for the targeted genes were as follows:
Sense, 5′-TGTAAACAAACCCAGATGCAGGA-3′ and antisense,
5′-CAGGTACATATCACGCGCACAG-3′ for EphB2; sense,
5′-CGCCGTTGGCCAAGAACCTGG-3′ and antisense,
5′-CAGCTTGTCTCCAATCTTCGG-3′ for Eph receptor-interacting B1
(EphrinB1); sense, 5′-GAAGATCGTCGCCACCTG-3′ and antisense,
5′-GACCTCCTCCTCGCACTTCT-3′ for cyclin D1; sense,
5′-CAGTACGAATGCGTGGCG-3′ and antisense, 5′-CTCCTCGCCGGTCTGCAC-3′
for cyclin-dependent kinase 6 (CDK6); sense,
5′-TTGCTTTACGTGGCCTGTTTC-3′ and antisense,
5′-GAAGACCCTGAAGGACAGCCAT-3′ for Bcl-2; and sense,
5′-GGGAGCCAAAAGGGTCATCATCTC-3′ and antisense,
5′-CCATGCCAGTGAGCTTCCCGTTC-3′ for GAPDH. PCR was performed with 30
cycles of 30 sec at 94°C, 30 sec at 58°C and 45 sec at 72°C. The
PCR products were electrophoresed and the bands were visualized
under ultraviolet radiation following staining with ethidium
bromide. The bands were determined and semi-quantified using
Labworks 4.6 software (UVP Products, Upland, CA, USA).
Western blot analysis
Antibodies against EphB2 and EphrinB1 were obtained
from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA), and
antibodies against cyclin D1, CDK6 and Bcl-2 were obtained from
Cell Signaling Technology, Inc. (Beverly, MA, USA). Proteins were
extracted from the tumor tissue and the concentration was
determined via a bicinchoninic acid assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). In total, 50 μg protein
from each sample was electrophoresed at 180 V for 1.5 h in
Tris-glycine running buffer. The proteins were transferred onto a
nitrocellulose membrane at room temperature overnight and incubated
with EphB2, EphrinB1, cyclin D1, CDK6 or Bcl-2 primary antibodies
for 2 h, followed by washing with the second antibody for 1 h. Goat
anti-β-actin polyclonal antibody served as an internal control and
rabbit anti-goat secondary antibody was subsequently used. The
protein expression was detected using an Enhanced Chemiluminescence
Plus kit (Amersham Pharmacia Biotech, Amersham, UK), exposure to
X-ray film or under ultraviolet radiation. The bands were
semi-quantified using Labworks 4.6 software.
Statistical analysis
Data are presented as the mean ± standard error.
Statistical analyses were performed by one-way analysis of
variance, followed by the Student-Newman-Keuls test for multiple
comparisons to compare the results of the in vivo
experiments, FCM, and the mRNA and protein levels. P<0.05 was
considered to indicate a statistically significant difference.
Results
High expression of EphB2 indicates
advantageous tumor growth suppression with QYHJ treatment in
CFPAC-1 cells
CFPAC-1, CFPAC-1 control RNAi and CFPAC-1 EphB2 RNAi
cells with different levels of EphB2 expression were injected
subcutaneously into female BALB/c nude mice to establish a
tumor-bearing mouse model. The mice were divided into QYHJ,
gemcitabine and control groups. The QYHJ group received a total of
200 μl liquid QYHJ twice a day by oral gavage, the gemcitabine
group received an intraperitoneal injection of 120 mg/kg
gemcitabine on days one, eight and 15, and the control group
received an oral gavage of a total of 200 μl normal saline twice a
day. Tumor volume was measured twice a week for four weeks, and
following four weeks of intervention, the tumor weight was measured
and the tumor weight inhibitory rate was calculated. The results
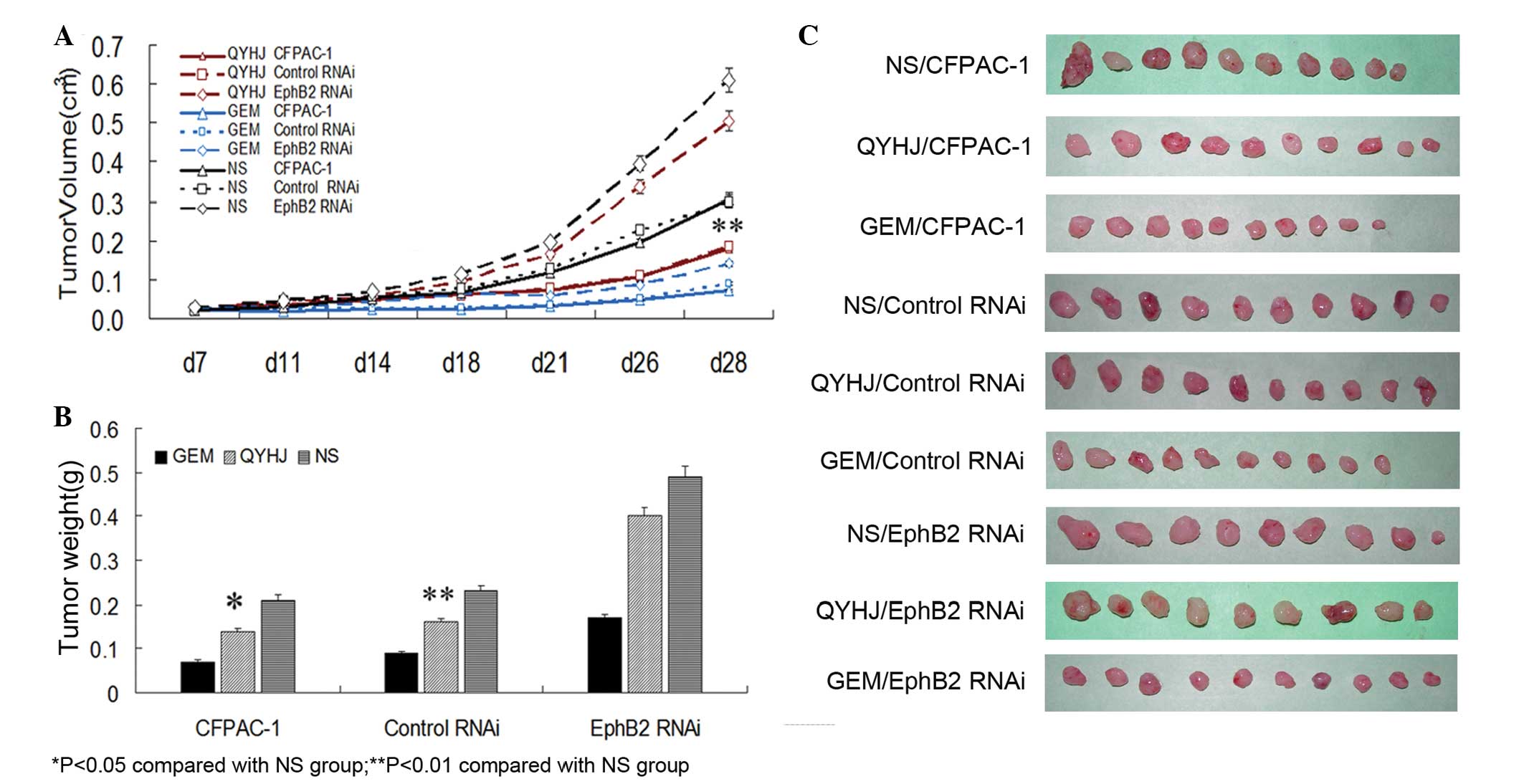
showed that there was no significant difference in tumor volume
between the QYHJ and control groups following 28 days of measuring
in the CFPAC-1 EphB2 RNAi cells, however, a significant inhibition
was observed in the CFPAC-1 and CFPAC-1 control RNAi cells
(P<0.05; Fig. 1A and C). In the
QYHJ group, tumor weight was 0.14±0.04, 0.16±0.04 and 0.40±0.10 g
for the CFPAC-1, CFPAC-1 control RNAi and CFPAC-1 EphB2 RNAi cells,
respectively. In addition, the tumor weight inhibitory rate was
31.40 and 31.33% for CFPAC-1 and CFPAC-1 control RNAi cells,
respectively, with a statistically significant decrease in the
corresponding QYHJ group compared with the control group
(P<0.05, P<0.01); the tumor weight inhibitory rate was only
18.36% in the CFPAC-1 EphB2 RNAi cells, with no statistically
significant difference identified between the QYHJ and control
groups (Fig. 1B and C). Different
levels of EphB2 expression reflected the different responses to the
QYHJ treatment. The cells that were expressing higher levels of
EphB2 exhibited more effective tumor growth inhibition as a result
of the QYHJ treatment, therefore, EphB2 may function as an
effective predictive factor for QYHJ treatment.
QYHJ suppresses tumor growth by retarding
the cell cycle process, but not cell apoptosis in CFPAC-1
cells
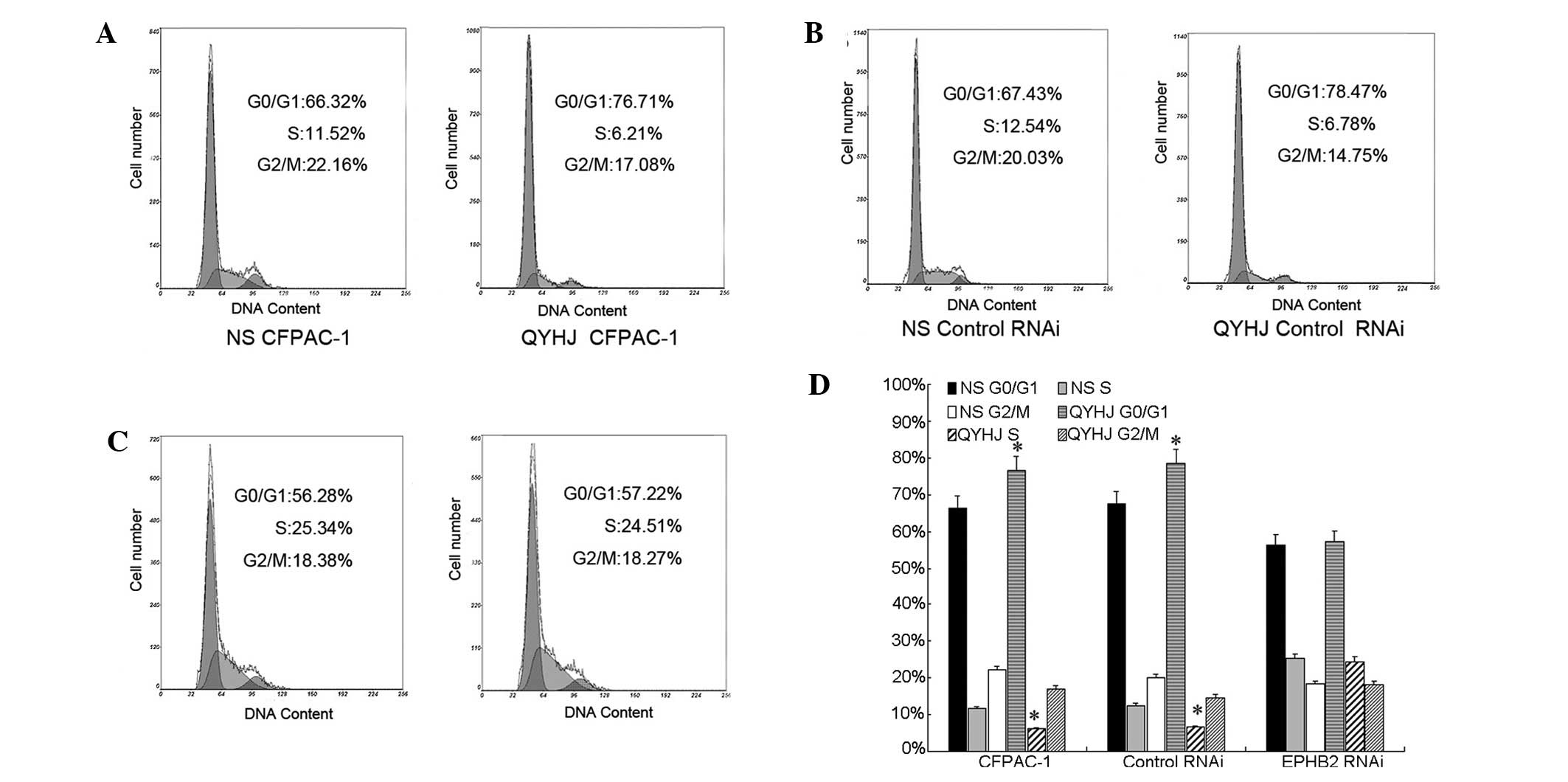
Our previous study showed that the overexpression of
EphB2 suppressed tumor growth by inhibiting the cell cycle process
and inducing cell apoptosis in CFPAC-1 cells (16). Accordingly, in the present study,
the cell cycle and apoptosis were analyzed by FCM to reveal the
mechanisms of the different responses to QYHJ treatment in CFPAC-1
cells, which were expressing different levels of EphB2. In the
CFPAC-1 EphB2 RNAi cells, the proportion of cells in the G0/G1
phase was 25.34 and 24.51% and in the S phase was 56.28 and 57.22%
for the QYHJ and control groups, respectively (Fig. 2C and D). No significant change was
identified in the cell cycle distribution following QYHJ treatment,
however, QYHJ treatment blocked the cell cycle in the G0/G1 phase
and reduced the proportion of cells in the S phase in CFPAC-1 and
CFPAC-1 control RNAi cells. In the CFPAC-1 and CFPAC-1 control RNAi
cells, the proportion of cells in the G0/G1 phase was 76.71 and
66.32% in the QYHJ group, respectively, and 78.47 and 67.43% in the
control group, respectively. The QYHJ treatment resulted in a
statistically significant increase in the G0/G1 phase population in
CFPAC-1 and CFPAC-1 control RNAi cells (P<0.05; Fig. 2A,B and D). An ~50% decrease in the S
phase proportion was observed following QYHJ treatment in the
CFPAC-1 and CFPAC-1 control RNAi cells; the proportion was
decreased from 11.52 to 6.21% in the CFPAC-1 cells and from 12.54
to 6.78% in the CFPAC-1 control RNAi cells. In addition, a
statistically significant decrease was identified in the S phase
population in the CFPAC-1 and CFPAC-1 control RNAi cells following
QYHJ treatment (P<0.05; Fig. 2A,B
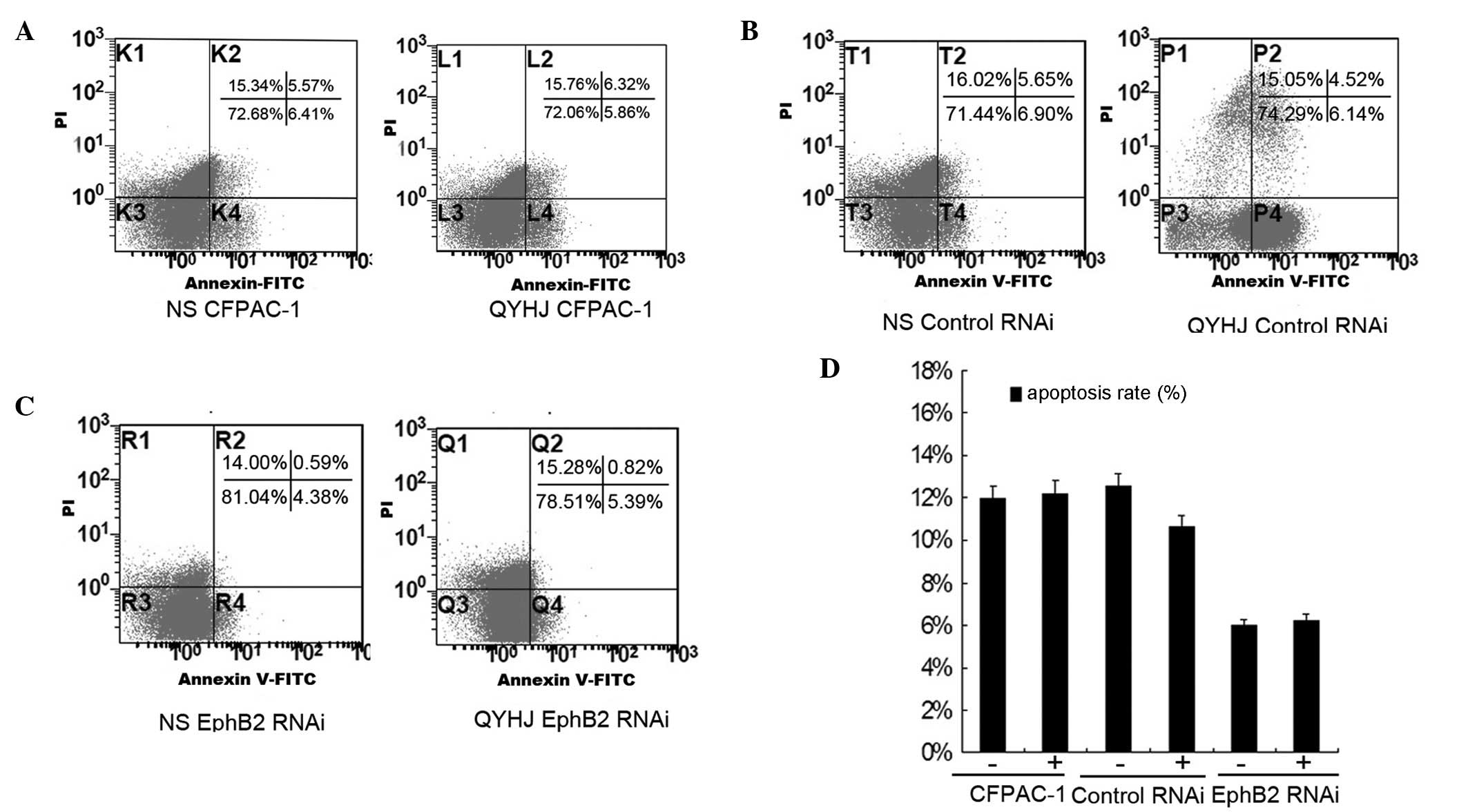
and D). The apoptosis rate was 11.98, 12.55 and 4.97% for the
control group in the CFPAC-1, CFPAC-1 control RNAi and CFAC-1 EphB2
RNAi cells, respectively, and was 12.18, 10.68 and 6.21% following
QYHJ treatment in the corresponding cell lines (Fig. 3A–C). QYHJ treatment did not result
in a statistically significant increase in the proportion of dead
cells in CFPAC-1 cells that were expressing different levels of
EphB2 (Fig. 3D).
High expression of EphB2 predicts the
superior response to QYHJ treatment via the EphrinB1-EphB2-CDK6
pathway in CFPAC-1 cells
Our previous study found that EphB2 induced the
G0/G1 phase by blocking the downregulation of cyclin D1 and CDK6 in
the CFPAC-1 cells (16). Previous
studies have revealed that EphB2 emanates the intracellular signal
by binding the transmembrane EphrinB1 ligands to form the
Eph-EphrinB1 complex, which is required for EphB2 kinase activity
transmission in the receptor-expressing cells (20,21).
In addition, decreased cell growth has been observed in
EphB2-expressing tumor cells in the presence of the EphrinB1/Fc
ligand (22). An additional
experiment was performed in the present study to verify the
mechanism of the superior response to QYHJ treatment in cells,
which were expressing higher levels of EphB2. The results showed
that the mRNA and protein levels of EphB2 changed indistinctly,
following QYHJ treatment, in the CFPAC-1, CFPAC-1 control RNAi and
CFPAC-1 EphB2 RNAi cells of the subcutaneous tumor. QYHJ
significantly increased the mRNA and protein level of EphrinB1 in
these three cell lines and a statistically significant increase was
identified in the EphrinB1 mRNA (P<0.05, P<0.01 and
P<0.05) and protein (P<0.05, P<0.05 and P<0.05) level
between the corresponding QYHJ and control groups. Furthermore, the
QYHJ treatment resulted in the statistically significant
downregulation of CDK6 mRNA (P<0.05) and protein (P<0.05)
levels in the CFPAC-1 and CFPAC-1 control RNAi cells, however, did
not affect cyclin D1 expression (Fig.
4). Cell cycle-related CDK6 and cyclin D1 did not show a
statistically significant change following QYHJ treatment in the
CFPAC-1 EphB2 RNAi cells (Fig. 4).
In addition, QYHJ treatment did not result in a statistically
significant change in cell apoptosis-related Bcl-2 expression in
cells that were expressing different levels of EphB2 (Fig. 4A and B). Consequently, the mechanism
of the different cancer growth inhibition responses following QYHJ
treatment did not necessarily arise as a result of the upregulation
of EphB2, rather, it is the critical upregulation of EphrinB1 that
stimulates EphB2-expressing cells to inhibit cancer cell growth by
downregulating CDK6 expression.
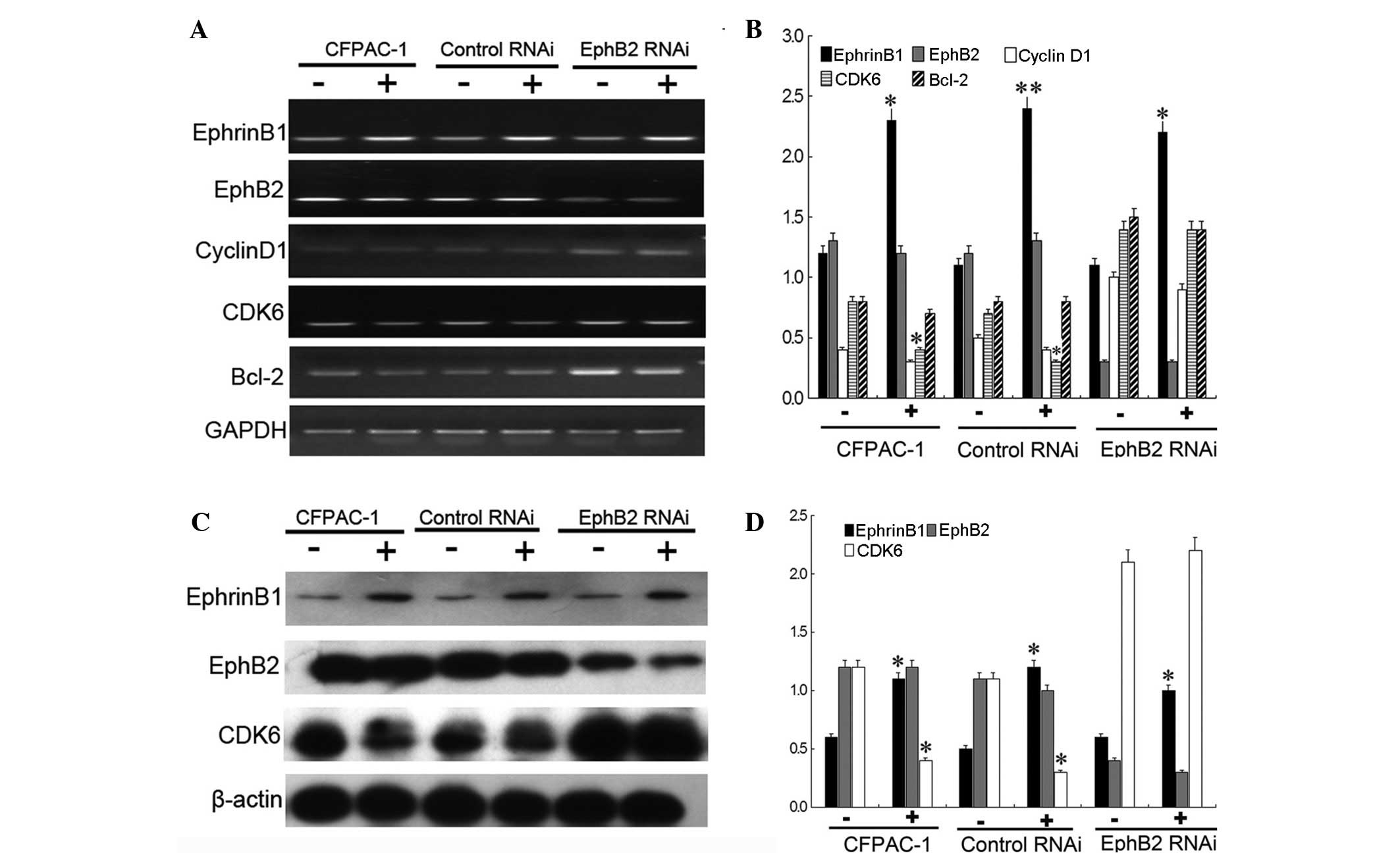 | Figure 4High expression of EphB2 predicts a
superior response to QYHJ treatment through the EphrinB1-EphB2-CDK6
pathway in CFPAC-1 cells. QYHJ resulted in an unclear change of
EphB2 (A and B) mRNA and (C and D) protein level, however, a
statistically significant increase was identified in the EphrinB1
(A and B) mRNA (P<0.05, P<0.01 and P<0.05) and (C and D)
protein (P<0.05, P<0.05 and P<0.05) level following QYHJ
treatment in CFPAC-1, CFPAC-1 control RNAi cells and CFPAC-1 EphB2
RNAi cells. QYHJ also resulted in a statistically significant
decrease in the CDK6 (A and B) mRNA (P<0.05) and (C and D)
protein (P<0.05) level in CFPAC-1 and CFPAC-1 control RNAi cells
(P<0.05), however, no change was identified in CFPAC-1 EphB2
RNAi cells. EphB2, erythropoietin-producing hepatoma cell line-B2;
QYHJ, Qingyihuaji formula; CDK6, cyclin-dependent kinase 6;
EphrinB1, Eph receptor-interacting B1. *P<0.05 and
**P<0.01 compared with the NS group. |
Discussion
Pancreatic cancer is one of the most malignant types
of cancer worldwide. In 2012, pancreatic cancer resulted in ~85.13%
of cancer-related mortality in the USA. In addition, the five-year
survival rate was ≤5% and the median survival rate was less than
six months (1,2). Only 20% of patients are diagnosed at
early stage and undergo surgical treatment (23). Currently, chemotherapy or radiation
are the best treatment options for >80% of advanced-stage
patients, however, a number of these cases are found to be highly
resistant to these treatments (24,25).
Despite the rapid advancement over the last decade in cancer
therapy, pancreatic cancer patients benefit the least with regard
to treatment products and survival rate due to the high degree of
malignancy, and susceptibility to chemoradiation resistance.
Therefore, it is imperative to identify the mechanism of the
treatment resistance, which results in a poor prognosis, and to
identify novel treatment strategies to prolong patient survival
time.
Ephrin receptors are one of the largest subfamilies
of receptor tyrosine kinases (RTKs). EphB2 is one of the two
subgroups, which preferentially binds transmembrane EphrinB ligands
(26–28). Previously, it was verified that
EphB2 is a prognostic factor for several types of cancer; it has
been associated with histological grade, stage, and overall and
disease-free survival (7–12). Numerous studies have demonstrated
that EphB2 preferentially acts as a tumor suppressor in various
cancers, including colorectal (CRC) (13), gastric (15) and prostate (14) cancer, which is unlike other RTKs
that are generally regarded as oncogenes. In CRC, the loss of EphB2
expression is observed in >50% patients, its downregulation
accelerates the progression of CRC (21) and is associated with a poor
prognosis (9,11,29). A
complete loss of EphB2 expression is observed in 52.5% of gastric
cancer and 82% of nodal metastases patients. Therefore, loss of
EphB2 expression is significantly associated with advanced T stage,
nodal metastasis, advanced disease stage and poor histological
differentiation. The frequent deletion and decreased expression of
EphB2 indicates that it may be a negative biomarker for gastric
cancer and a potential predictor of the final outcome (15). In addition, EphB2 has been
identified as a tumor suppressor gene in prostate cancer (14). Despite the clear link between EphB2
and a number of cancers, little is known concerning the correlation
between EphB2 and pancreatic cancer prognosis. Our previous study
was designed to investigate this correlation by eliminating EphB2
expression using lentivirus-based RNAi to observe the biological
characteristic changes in pancreatic cancer CFPAC-1 cells. The
results demonstrated that silencing EphB2 promoted cancer growth by
stimulating cell proliferation through a mechanism of G1/S phase
breakthrough, which was dependent on a cyclin D1/CDK6 cell cycle
regulating signal. Similarly, EphB2 inhibition also reduced the
apoptosis of CFPAC-1 cells by increasing Bcl-2 expression, with
EphB2 acting as a tumor suppressor in the cell proliferation and
apoptosis in pancreatic cancer (16).
For thousands of years, TCM has been widely used for
cancer treatment in China (30).
Integrative TCM and Western medicine for cancer treatment has been
broadly approved by governments and patients, including for
pancreatic cancer. The authors of the present study have
accumulated a wealth of experience in pancreatic cancer integrative
therapy and obtained certain promising results. A total of 164
pancreatic cancer patients with liver metastases that were treated
with chemotherapy, radiation therapy and/or QYHJ were analyzed. The
results demonstrated an overall median survival time of 4.7 months
and a one-year survival time of 14%, with clinical outcomes more
effective than those identified by other studies (31). Multivariate analysis showed that
chemotherapy and QYHJ were protective factors (3). Notably, certain patients experienced
long survival times when treated with QYHJ, however, disease
continued to progress in a number of patients even though QYHJ
treatment had been received in clinical practice. These differences
in patient reaction to QYHJ treatment require investigation; there
may be effective and ineffective populations for QYHJ treatment,
therefore, a method to distinguish them must be identified. TCM is
regarded as a multi-target therapy through immune alteration, tumor
microenvironment transforming, oncogenes or tumor suppressor gene
regulation, and is comparable to target therapy in modern medicine
(17,18). Previous studies have identified that
not all patients benefit from target therapies since patients have
their own effective population according to their distinctive
predictors. Several types of treatment response predictors have
been identified for target therapy, and gene expression predictors
have been regarded as the most appropriate and valuable as they
organically link the molecular biology and pharmacology (32–34).
Aberrant activation or mutations in RTKs are responsible for tumor
progression and development, and a number of RTKs have been
validated as prognostic factors and therapeutic targets in human
cancers, which are caused by activated RTKs (35–37).
In addition, specific RTKs have been verified as able to show a
predictive value for target therapy (38,39).
Epidermal growth factor receptor mutations appear to identify
distinct subsets of patients with an increased response to
gefitinib in non-small cell lung carcinoma (38). In CRC, a KRAS mutation has
previously been associated with resistance to cetuximab and a
poorer survival in metastatic CRC patients that were treated with
cetuximab (39). In pancreatic
cancer, histone levels are a survival predictor for patients who
have received adjuvant fluorouracil; furthermore, histone
modification patterns predicted the prognosis and the treatment
response (33).
EphB2 functions as a positive prognostic factor and
tumor suppressor in pancreatic cancer growth and our previous
studies identified that different pancreatic cancer cell lines
appear to exhibit different responses to QYHJ, as cells expressed
different levels of EphB2 (6). By
acting as a positive prognostic factor in pancreatic cancer, or a
distinctive predictive factor for QYHJ treatment, partially reveals
the mechanism of the different responses to QYHJ treatment in cells
that express different levels of EphB2. Subsequently, a series of
experiments were performed in the present study to verify this
hypothesis. The results showed that tumor weight was 0.14±0.04,
0.16±0.04 and 0.40±0.10 g for CFPAC-1, CFPAC-1 control RNAi and
CFPAC-1 EphB2 RNAi cells, respectively, and the tumor weight
inhibitory rate was 31.40, 31.33 and 18.36%, respectively. A
statistically significant decrease was identified in tumor weight
following the QYHJ intervention in CFPAC-1 (P<0.05) and CFPAC-1
control RNAi (P<0.01) cells. However, a statistically
significant difference was predicted to appear in CFPAC-1 EphB2
RNAi cells following the QYHJ treatment. Previously, a high
expression of EphB2 was found to indicate advantageous tumor growth
suppression with QYHJ treatment in CFPAC-1 cells. This is
comparable to the clinical results, which were identified in the
present study, that QYHJ acts as a protective factor and prolongs
survival time for certain patients, however, not all patients. Cell
cycle analyses showed that QYHJ treatment did not change the cell
cycle distribution in CFPAC-1 EphB2 RNAi cells, however, a
statistically significant increase in the G0/G1 phase (P<0.05),
and a significant decrease in the S phase (P<0.05) populations
was identified in CFPAC-1 and CFPAC-1 control RNAi cells following
QYHJ treatment. QYHJ treatment did not result in a statistically
significant increase in the proportion of dead cells in CFPAC-1
cell lines that were expressing different levels of EphB2.
Accordingly, a higher expression of EphB2 acted as a positive
predict factor for QYHJ treatment, with the exception of being a
prognostic factor in pancreatic cancer; an additional experiment
was performed to explore this mechanism. Previous studies revealed
that the overexpression or loss of EphB2 influenced the cell cycle
and apoptosis (12,40,41)
and that EphB2 emanated intracellular signals by binding EphrinB1
ligands to form the EphB2-EphrinB1 complex (20,21,27).
The results of the current study showed that the mRNA and protein
level of EphB2 changed indistinctly following QYHJ treatment. In
addition, QYHJ significantly increased the mRNA and protein level
of EphrinB1 in CFPAC-1 cell lines that were expressing different
levels of EphB2. The QYHJ treatment resulted in a statistically
significant decrease in the S phase population, which was
associated with a decrease in the CDK6 mRNA (P<0.05) and protein
(P<0.05) levels in CFPAC-1 and CFPAC-1 control RNAi cells. No
evident change was observed in the CFPAC-1 EphB2 RNAi cells.
Furthermore, QYHJ treatment did not result in a statistically
significant change in Bcl-2 expression in cells that were
expressing different levels of EphB2. The mechanism of the higher
expression of EphB2 acting as a predictive factor for QYHJ
treatment arose as a result of the upregulation of EphrinB1, which
stimulated the EphB2-expressing cells to inhibit cancer cell growth
by downregulating the CDK6 expression.
In conclusion, a high expression of EphB2 predicts a
superior response to QYHJ treatment through a mechanism that is
dependent on inhibiting the cell cycle via an
EphrinB1-EphB2-induced CDK6 decrease in CFPAC-1 cells. Therefore,
EphB2 may act as a predictive factor for QYHJ treatment in
pancreatic cancer CFPAC-1 cells.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant nos. 81173461, 81072942
and 30901911).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012.
|
|
2
|
Dai M, Ren JS, Li N, Li Q, Yang L and Chen
YH: Estimation and prediction on cancer related incidence and
mortality in China, 2008. Zhonghua Liu Xing Bing Xue Za Zhi.
33:57–61. 2012.(In Chinese).
|
|
3
|
Ouyang H, Wang P, Meng Z, et al:
Multimodality treatment of pancreatic cancer with liver metastases
using chemotherapy, radiation therapy, and/or Chinese herbal
medicine. Pancreas. 40:120–125. 2011.
|
|
4
|
Shen YH, Liu LM, Chen Z, et al: Study on
Chinese medicine combined with chemotherapy for treatment of 32
cases of advanced pancreatic cancer. Zhong Yi Za Zhi. 47:115–117.
2006.(In Chinese).
|
|
5
|
Shen YH, Liu LM, Meng ZQ, et al: Survival
analysis on 64 cases of advanced pancreatic cancer treated by
integrated Western and traditional Chinese medicine mainly with
Qingyi Huaji formula. Zhong Yi Za Zhi. 50:39–42. 2009.(In
Chinese).
|
|
6
|
Shen YH, Liu LM, Lu Y, et al: Impact of
Qingyi Xiaoji Decoction on gene expression of experimental
pancreatic cancer in vivo. Zhongguo Ai Zheng Za Zhi. 15:454–457.
2005.(In Chinese).
|
|
7
|
Kataoka H, Tanaka M, Kanamori M, et al:
Expression profile of EFNB1, EFNB2, two ligands of EPHB2 in human
gastric cancer. J Cancer Res Clin Oncol. 128:343–348. 2002.
|
|
8
|
Wu Q, Suo Z, Risberg B, Karlsson MG,
Villman K and Nesland JM: Expression of Ephb2 and Ephb4 in breast
carcinoma. Pathol Oncol Res. 10:26–33. 2004.
|
|
9
|
Guo DL, Zhang J, Yuen ST, et al: Reduced
expression of EphB2 that parallels invasion and metastasis in
colorectal tumours. Carcinogenesis. 27:454–464. 2006.
|
|
10
|
Hafner C, Schmitz G, Meyer S, et al:
Differential gene expression of Eph receptors and ephrins in benign
human tissues and cancers. Clin Chem. 50:490–499. 2004.
|
|
11
|
Jubb AM, Zhong F, Bheddah S, et al: EphB2
is a prognostic factor in colorectal cancer. Clin Cancer Res.
11:5181–5187. 2005.
|
|
12
|
Genander M, Halford MM, Xu NJ, et al:
Dissociation of EphB2 signaling pathways mediating progenitor cell
proliferation and tumor suppression. Cell. 139:679–692. 2009.
|
|
13
|
Batlle E, Bacani J, Begthel H, et al: EphB
receptor activity suppresses colorectal cancer progression. Nature.
435:1126–1130. 2005.
|
|
14
|
Huusko P, Ponciano-Jackson D, Wolf M, et
al: Nonsense-mediated decay microarray analysis identifies
mutations of EPHB2 in human prostate cancer. Nat Genet. 36:979–983.
2004.
|
|
15
|
Yu G, Gao Y, Ni C, et al: Reduced
expression of EphB2 is significantly associated with nodal
metastasis in Chinese patients with gastric cancer. J Cancer Res
Clin Oncol. 137:73–80. 2011.
|
|
16
|
Hua YQ, Ouyang HQ, Chen Z, et al: Promoted
cancer growth by stimulating cell proliferation and decreasing
apoptosis using a lentivirus-based EphB2 RNAi in pancreatic
carcinoma CFPAC-1 cells. Biomed Pharmacother. 65:123–131. 2011.
|
|
17
|
McCulloch M, See C, Shu XJ, et al:
Astragalus-based Chinese herbs and platinum-based chemotherapy for
advanced non-small-cell lung cancer: meta-analysis of randomized
trials. J Clin Oncol. 24:419–430. 2006.
|
|
18
|
Guo H, Liu JX, Xu L, Madebo T and Baak
JPA: Traditional Chinese medicine herbal treatment may have a
relevant impact on the prognosis of patients with stage IV
adenocarcinoma of the lung treated with platinum-based chemotherapy
or combined targeted therapy and chemotherapy. Integr Cancer Ther.
10:127–137. 2011.
|
|
19
|
Schoumacher RA, Ram J, Iannuzzi MC, et al:
A cystic fibrosis pancreatic adenocarcinoma cell line. Proc Natl
Acad Sci USA. 87:4012–4016. 1990.
|
|
20
|
Pasquale EB: Eph-ephrin bidirectional
signaling in physiology and disease. Cell. 133:38–52. 2008.
|
|
21
|
Noberini R and Pasquale EB: Proliferation
and tumor suppression: not mutually exclusive for Eph receptors.
Cancer Cell. 16:452–454. 2009.
|
|
22
|
Nakada M, Niska JA, Tran NL, McDonough WS
and Berens ME: EphB2/R-Ras signaling regulates glioma cell
adhesion, growth, and invasion. Am J Pathol. 167:565–576. 2005.
|
|
23
|
Wade TP, Halaby IA, Stapleton DR, Virgo KS
and Johnson FE: Population-based analysis of treatment of
pancreatic cancer and Whipple resection: Department of Defense
hospitals, 1989–1994. Surgery. 120:680–687. 1996.
|
|
24
|
Masui T, Hosotani R, Ito D, et al: Bcl-XL
antisense oligonucleotides coupled with antennapedia enhances
radiation-induced apoptosis in pancreatic cancer. Surgery.
140:149–160. 2006.
|
|
25
|
Reni M, Pasetto L, Aprile G, et al:
Raltitrexed-eloxatin salvage chemotherapy in gemcitabine-resistant
metastatic pancreatic cancer. Br J Cancer. 94:785–791. 2006.
|
|
26
|
Lemke G: A coherent nomenclature for Eph
receptors and their ligands. Mol Cell Neurosci. 9:331–332.
1997.
|
|
27
|
Himanen JP and Nikolov DB: Eph signaling:
a structural view. Trends Neurosci. 26:46–51. 2003.
|
|
28
|
Gale NW, Holland SJ, Valenzuela DM, et al:
Eph receptors and ligands comprise two major specificity subclasses
and are reciprocally compartmentalized during embryogenesis.
Neuron. 17:9–19. 1996.
|
|
29
|
Lugli A, Spichtin H, Maurer R, et al:
EphB2 expression across 138 human tumor types in a tissue
microarray: high levels of expression in gastrointestinal cancers.
Clin Cancer Res. 11:6450–6458. 2005.
|
|
30
|
Li X, Yang G, Zhang Y, et al: Traditional
Chinese medicine in cancer care: a review of controlled clinical
studies published in chinese. PLoS One. 8:e603382013.
|
|
31
|
DeWitt J, Yu M, Al-Haddad MA, Sherman S,
McHenry L and Leblanc JK: Survival in patients with pancreatic
cancer after the diagnosis of malignant ascites or liver metastases
by EUS-FNA. Gastrointest Endosc. 71:260–265. 2010.
|
|
32
|
Jiang T, Kambadakone A, Kulkarni NM, Zhu
AX and Sahani DV: Monitoring response to antiangiogenic treatment
and predicting outcomes in advanced hepatocellular carcinoma using
image biomarkers, CT perfusion, tumor density, and tumor size
(RECIST). Invest Radiol. 47:11–17. 2012.
|
|
33
|
Manuyakorn A, Paulus R, Farrell J, et al:
Cellular histone modification patterns predict prognosis and
treatment response in resectable pancreatic adenocarcinoma: results
from RTOG 9704. J Clin Oncol. 28:1358–1365. 2010.
|
|
34
|
Lundin KB, Henningson M, Hietala M, Ingvar
C, Rose C and Jernström H: Androgen receptor genotypes predict
response to endocrine treatment in breast cancer patients. Br J
Cancer. 105:1676–1683. 2011.
|
|
35
|
Krause DS and Van Etten RA: Tyrosine
kinases as targets for cancer therapy. N Engl J Med. 353:172–187.
2005.
|
|
36
|
Shawver LK, Slamon D and Ullrich A: Smart
drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell.
1:117–123. 2002.
|
|
37
|
Chow LQ and Eckhardt SG: Sunitinib: from
rational design to clinical efficacy. J Clin Oncol. 25:884–896.
2007.
|
|
38
|
Bell DW, Lynch TJ, Haserlat SM, et al:
Epidermal growth factor receptor mutations and gene amplification
in non-small-cell lung cancer: molecular analysis of the
IDEAL/INTACT gefitinib trials. J Clin Oncol. 23:8081–8092.
2005.
|
|
39
|
Lièvre A, Bachet JB, Boige V, et al: KRAS
mutations as an independent prognostic factor in patients with
advanced colorectal cancer treated with cetuximab. J Clin Oncol.
26:374–379. 2008.
|
|
40
|
Senior PV, Zhang BX and Chan ST: Loss of
cell-surface receptor EphB2 is important for the growth, migration,
and invasiveness of a colon cancer cell line. Int J Colorectal Dis.
25:687–694. 2010.
|
|
41
|
Kandouz M, Haidara K, Zhao J, Brisson ML
and Batist G: The EphB2 tumor suppressor induces autophagic cell
death via concomitant activation of the ERK1/2 and PI3K pathways.
Cell Cycle. 9:398–407. 2010.
|