Introduction
Schwannomas, also known as neurilemmomas, are
common, benign soft tissue tumors of nerve sheath origin. These
slow-growing lesions arise from the peripheral, spinal or cranial
nerves and commonly present several years prior to diagnosis
(1–3). Characteristic bone scalloping of the
spinal cord, including foramen enlargement, may develop when these
tumors are located adjacent to a bone (4). Bone scalloping has been reported to
occur in the bones of the extremities, as well as in vertebral
bodies (5–8).
However, little has been reported on the periosteal
reactions or sclerotic changes in the bones that are in contact
with schwannomas. Bone scalloping is considered to be a
radiologically benign indication of the prolonged existence of a
tumor; however, the molecular mechanism underlying this process has
yet to be elucidated.
To investigate the possible underlying mechanism of
schwannoma-induced bone scalloping, it was hypothesized, in the
present study that a specific extracellular factor, for example an
inhibitor of bone formation, may be secreted from the tumor.
Noggin, a potent antagonist of bone morphogenetic protein (BMP),
inhibits BMP signal transduction through binding to ligands and
consequently prevents the bone formation that is induced by BMP
(9–12). In addition, noggin is expressed
during the early development of the central nervous system
(13) and has a major role in
neural induction via the inhibition of BMPs (14–16).
Although the expression of noggin in neurogenic tumors, including
schwannomas, has yet to be investigated, this type of tumor may
produce noggin given their neurogenic cellular origin.
In the present study the expression of noggin in
soft tissue tumor samples, including schwannomas, was analyzed. The
expression of noggin mRNA and protein was examined and the effect
of the tissue extract from a noggin-producing schwannoma for
BMP-induced osteoblastic differentiation in vitro was
investigated. The present study proposes a possible pathomechanism
of bone resorption by schwannomas.
Materials and methods
Tumor tissues
Tumor samples were obtained from the primary tumors
of five patients with schwannoma and 30 patients with other soft
tissue tumors (five hemangiomas, five lipomas, five malignant
fibrous histiocytomas, five malignant schwannomas, five synovial
sarcomas and five liposarcomas) at the Department of Orthopedic
Surgery, Osaka University and the Osaka Medical Center for Cancer
and Cardiovascular Diseases (Osaka, Japan). The histological
diagnoses and subtypes were established via routine pathological
evaluation according to the criteria, which followed the World
Health Organisation classification system (17). Clinical data, including age, gender,
location of the lesion and the radiological findings were obtained
for the schwannoma samples. Written informed consent based on the
Ethical Committees of Osaka University Graduate School of Medicine
and the Osaka Medical Center for Cancer and Cardiovascular Diseases
was obtained from each patient. The study was approved by the
ethics committee of Osaka University (Suita, Japan).
Reverse transcription (RT)-polymerase
chain reaction (PCR) and quantitative (q)PCR
Tumor tissues were frozen immediately following
surgical excision and stored at −80°C until the RNA extraction was
performed. The total RNA was isolated using TRIzol®
Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s instructions. Complementary (c)DNA was
generated using the Transcriptor First Strand cDNA Synthesis kit
(Roche Diagnostics, Mannheim, Germany). The transcripts of noggin
and the BMP antagonists, chordin and sclerostin, were
analyzed in all of the tumor tissues. RT-PCR analysis was performed
using a PCR Master Mix (Promega Corporation, Madison, WI, USA) with
the following primer sequences: Forward, 5′-CTCGGGGGCCACTACGAC-3′
and reverse, 5′-GCACGAGCACTTGCACTCG-3′ for noggin; forward,
5′-AACACATGCTTCTTCGAGG-3′ and reverse, 5′-CTGTGGTTCCCAGAGGTAGTG-3′
for chordin; forward, 5′-CCGGAGCTGGAGAACAACAAG-3′ and reverse,
5′-GCACTGGCCGGAGCACACC-3′ for sclerostin; and forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCACCTGTTGCTGTA-3′
for GAPDH. The PCR products were separated using agarose gel
electrophoresis and detected using ethidium bromide. For the qPCR
analysis, the expression of each mRNA was quantified using the
LightCycler® TaqMan® Master kit (Roche
Diagnostics). The Universal ProbeLibrary (UPL) probes used were as
follows: Forward, 5′-GAAGCTGCGGAGGAAGTTAC-3′ and reverse,
5′-TACAGCACGGGGCAGAAT-3′ for noggin (UPL probe no. 5); and forward,
5′-AGACACATCGCTCAGACAC-3′ and reverse, 5′-GCCCAATACGACCAAATCC-3′
for GAPDH (UPL probe no. 60). The expression of noggin was
normalized to that of GAPDH.
Western blot analysis for noggin protein
expression
The total protein extracted from the schwannoma
samples was used for western blot analysis. Tumor tissue was
homogenized in tissue protein extraction reagent buffer (Pierce
Biotechnology, Inc., Rockford, IL, USA) containing a protease
inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA) to
avoid protein degradation and was solubilized using a 2× SDS-PAGE
sample buffer. Samples were subjected to 4–12% SDS-PAGE and
transferred onto nitrocellulose membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Subsequent to blocking with 0.1% Tween 20
in phosphate-buffered saline (PBS) containing 3% bovine serum
albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) the membranes were
incubated with specific rabbit polyclonal primary antibodies
against noggin (ab16054; Abcam PLC, Cambridge, UK) or β-actin (Cell
Signaling Technology, Inc., Beverly, MA, USA). Membranes were
subsequently incubated with horseradish peroxidase-conjugated
secondary antibodies (GE Healthcare, Little Chalfont, UK) and
enhanced chemiluminescence reagents (GE Healthcare).
Immunohistochemistry for noggin
expression
Tissue sections were deparaffinized using xylene,
dehydrated using graded alcohol and immersed in 70% methanol with
H2O2 to block endogenous peroxidase activity.
Antigen retrieval for noggin was performed using a microwave oven
for 10 min in 10 mM citrate buffer (pH 7.0). Sections were
incubated with 1% goat serum for 1 h at room temperature, washed in
PBS and incubated with anti-noggin antibodies (ab16054) in 2% (w/v)
BSA/PBS overnight at 4°C. Sections were washed three times with
0.1% (v/v) Tween 20/PBS followed by incubation and were analyzed
using the EnVision™ system (Dako, Glostrup, Denmark). The staining
intensity was scored according to the following scale: −, <10%;
+, 10–45% positive cells; and ++, 46–95% positive cells.
Effect of schwannoma tissue extract on
the osteoblastic differentiation of MC3T3-E1 cells
Mouse preosteoblastic MC3T3-E1 cells were obtained
from Riken Cell Bank (Tsukuba, Japan). The MC3T3-E1 cells were
maintained in α-minimal essential medium (Invitrogen Life
Technologies) and supplemented with 10% fetal bovine serum
(Hyclone, Road Logan, UT, USA) in a humidified atmosphere of 5%
CO2 at 37°C. For each assay, the growth medium was
replaced with differentiation medium and supplemented with 0.2 mM
ascorbic acid (Sigma-Aldrich) and 4 mM β-glycerophosphate
(Sigma-Aldrich).
Alkaline phosphatase (ALP) staining and
activity in MC3T3-E1 cells
MC3T3-E1 cells were plated onto 24-well plates
(Becton-Dickinson, Franklin Lakes, NJ, USA) at a density of
4×104 cells/well. After 24 h, the cells were treated
with various concentrations of homogenized schwannoma extract. The
culture media was replaced with growth medium. Following three days
of culture, cells were washed with PBS and fixed for 15 min with
10% formalin at room temperature. Following fixation, cells were
incubated with the ProtoBlot AP System with Stabilized Substrate
(Promega Corpration) for 1 h at room temperature. To measure ALP
activity, the cells were washed with PBS and lysed in mammalian
protein extraction reagent (Pierce Biotechnology, Inc.) according
to the manufacturer’s instructions. ALP activity was measured using
LabAssay™ ALP (Wako Pure Chemicals Industries, Ltd., Osaka, Japan)
with p-Nitrophenyl phosphate as a substrate. To normalize the
enzyme activity, the protein content was measured using a
bicinchoninic acid protein assay kit (Pierce Biotechnology,
Inc.).
RT-PCR analysis for osteoblastic
markers
The total RNA was isolated from the cells using
TRIzol® Reagent (Invitrogen Life Technologies) according
to the manufacturer’s instructions. cDNA synthesis was performed
using a cDNA synthesis kit (Roche Diagnostics) and RT-PCR analysis
was performed using a PCR master mix (Promega Corporation) and the
appropriate primer pairs. The specific primer sequences used for
RT-PCR analysis were as follows: Forward, 5′-GCCCTCTCCAAGACATATA-3′
and reverse, 5′-CCATGATCACGTCGATATCC-3′ for ALP; forward,
5′-CAAGTCCCACACACAGCAGCTT-3′ and reverse,
5′-AAAGCCGAGCTGCCAGAGAGTT-3′ for osteocalcin; forward,
5′-GCAATCGGGATCAGTACGAA-3′ and reverse, 5′-CTTTCACGCCTTTGAAGCCA-3′
for collagen I; and forward, 5′-TGAACGGGAAGCTCACTGG-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′ for GAPDH. The PCR products were
separated using agarose gel electrophoresis and detected using
ethidium bromide.
Proliferation assay of MC3T3-E1
cells
MC3T3-E1 cells were cultured on 96-well plates
(Becton-Dickinson) at a concentration of
2×104/cm2. After three days of culture, cell
proliferation was assessed using the Premix WST-1 cell
proliferation assay system (Takara Bio, Inc., Otsu, Japan)
according to the manufacturer’s instructions. This assay was
performed every 24 h.
Statistical analysis
All data are presented as the mean ± standard
deviation and a minimum of three independent experiments were
performed for each assay. Statistical analysis was performed using
a two-sided unpaired Student’s t-test or analysis of variance for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Detection of noggin mRNA in soft tissue
tumors
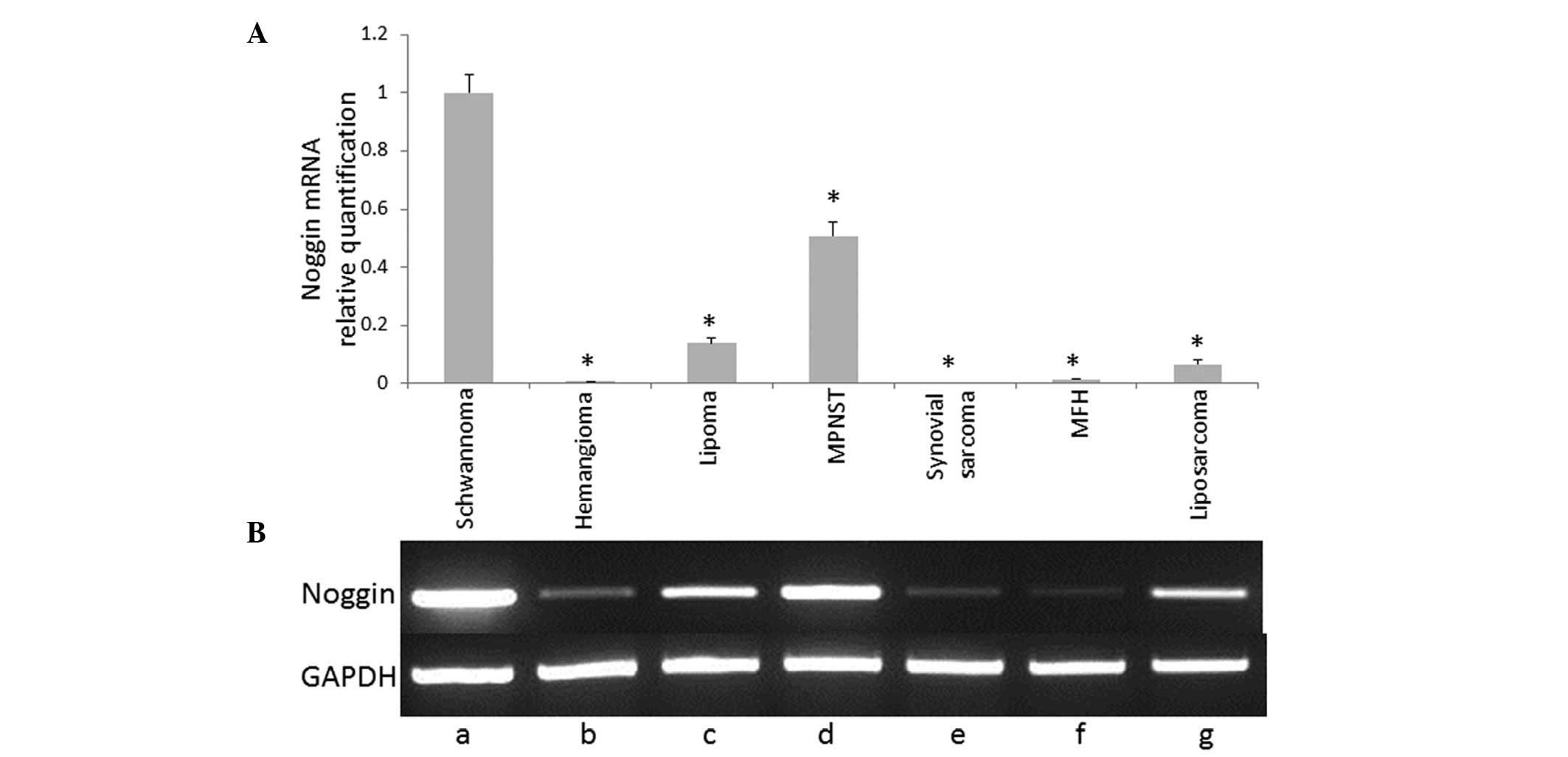
Fig. 1 shows the
mRNA expression profile of noggin in soft tissue tumors detected
using RT-PCR analysis. Noggin mRNA expression was determined using
qPCR analysis. Data are presented as relative quantification values
against GAPDH. Noggin mRNA expression was found to be significantly
increased in the schwannoma tissue compared with the other soft
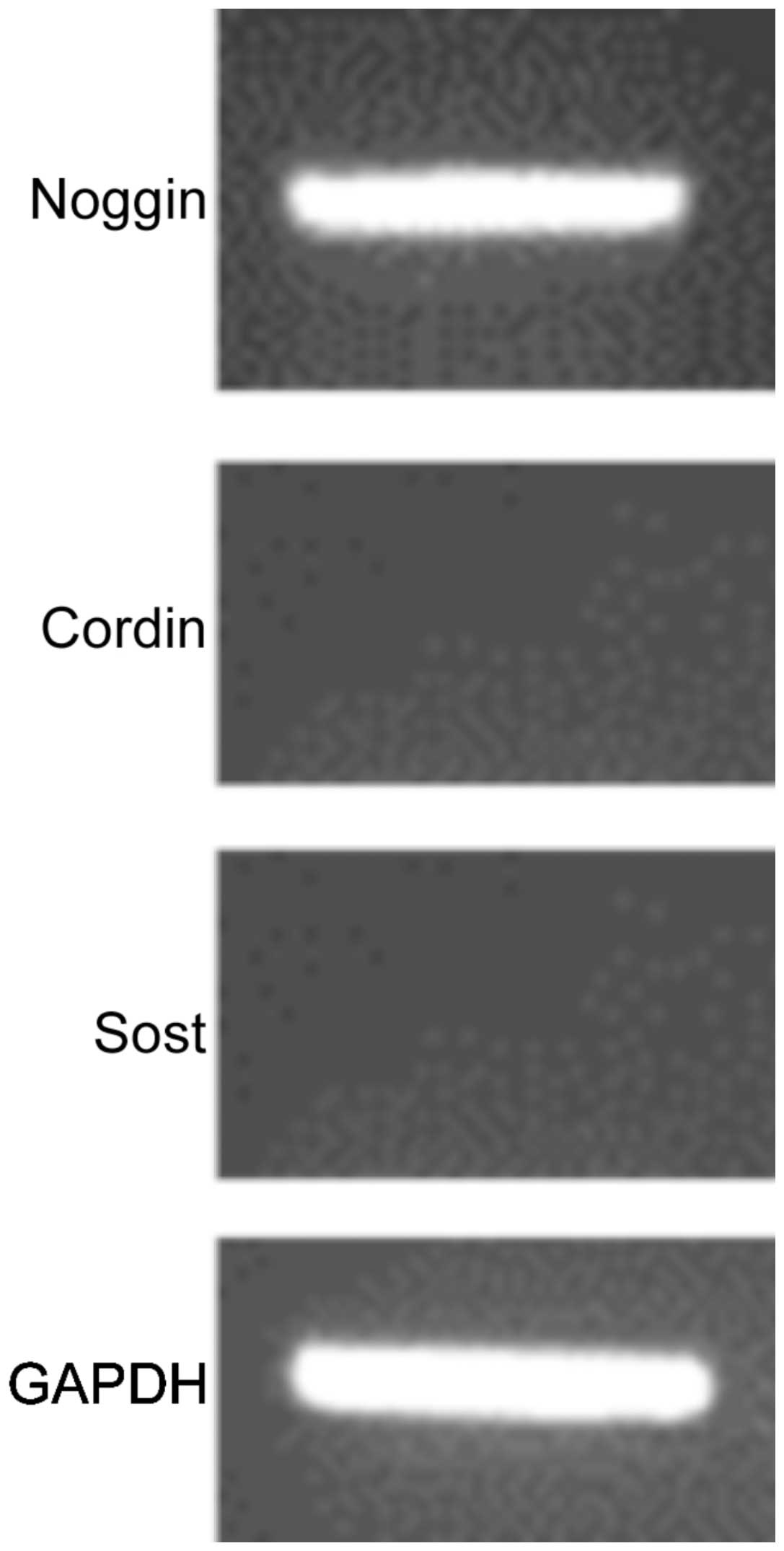
tissue tumors (P<0.05). The BMP antagonists, chordin and
sclerostin, were not found to be expressed in schwannoma (Fig. 2) or other soft tissue tumors (data
not shown).
Noggin protein expression in soft tissue
tumors
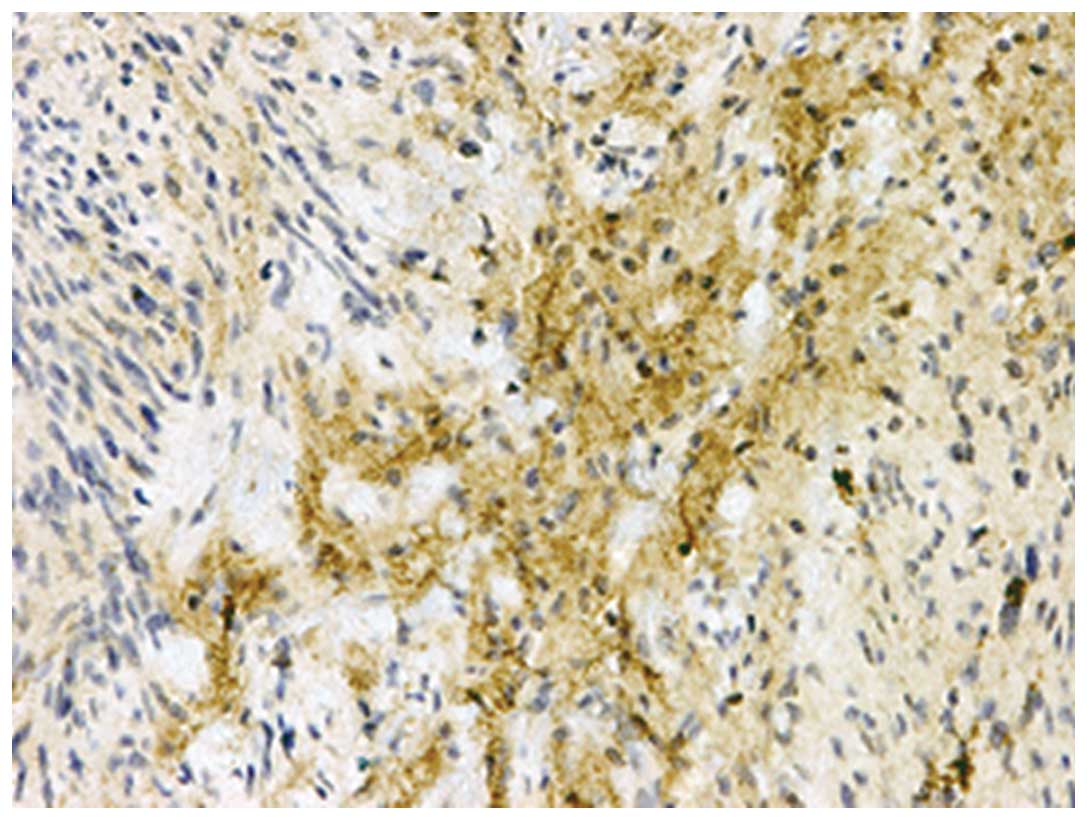
Table I shows the
immunohistochemical analyses of the noggin protein in the soft
tissue tumors. Noggin expression was detected in the schwannoma
tissue, however, it was not detected in the other soft tissue
tumors. In the schwannoma tissue samples, various levels of noggin
immunoreactivity were observed in two of the five tissues. The
immunostaining for noggin was localized to the cytoplasm of the
spindle tumor cells, primarily demonstrating an Antoni B tissue
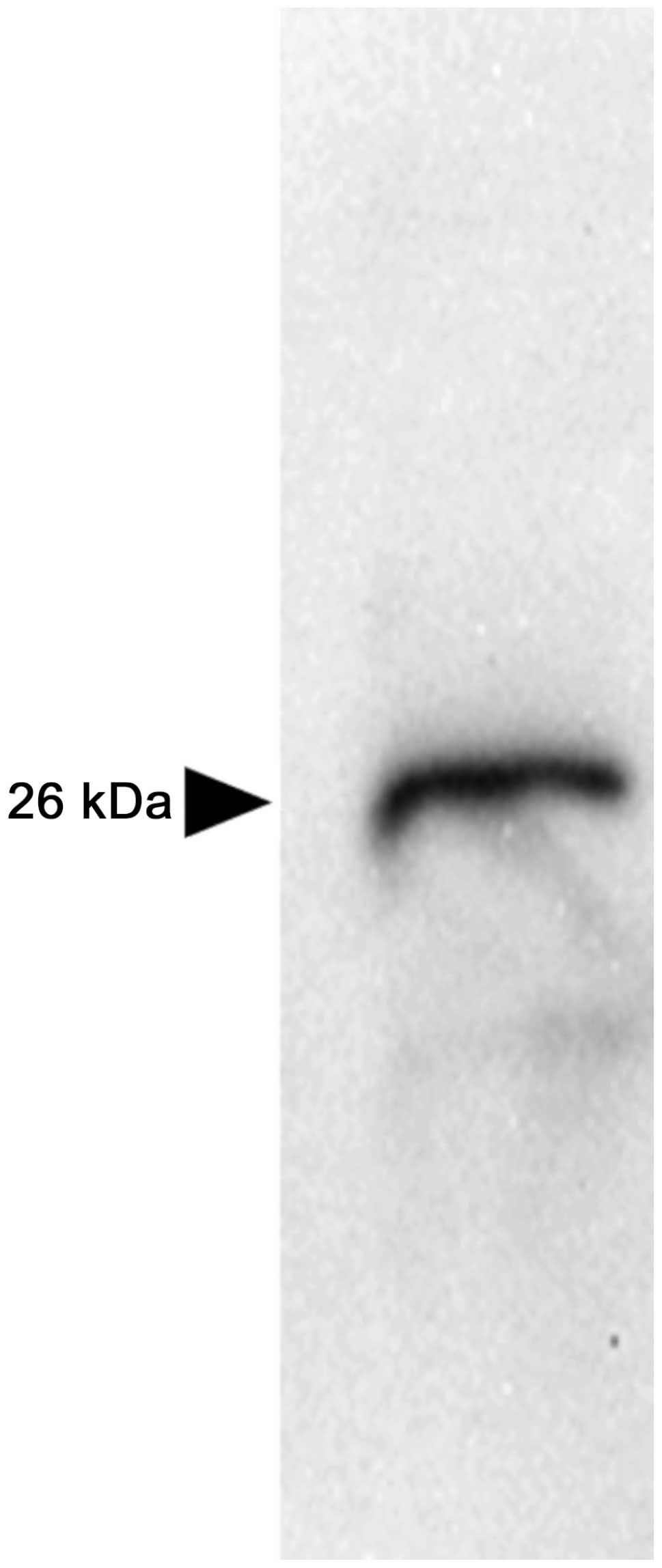
pattern (Fig. 3). Western blot
analysis in the schwannoma tissue revealed a single immunoreactive
band corresponding with the size of the noggin protein, with a
molecular mass of 26 kDa (Fig.
4).
 | Table IImmunohistochemical analyses of noggin
in soft tissue tumors. |
Table I
Immunohistochemical analyses of noggin
in soft tissue tumors.
| | Noggin
expression |
|---|
| |
|
|---|
| Diagnosis | No. | ++ | + | − | Rate (%) |
|---|
| Schwannoma | 5 | 1 | 1 | 3 | 40 |
| Hemangioma | 5 | 0 | 0 | 5 | 0 |
| Lipoma | 5 | 0 | 0 | 5 | 0 |
| MPNST | 5 | 0 | 0 | 5 | 0 |
| MFH | 5 | 0 | 0 | 5 | 0 |
| Synovial sarcoma | 5 | 0 | 0 | 5 | 0 |
| Liposarcoma | 5 | 0 | 0 | 5 | 0 |
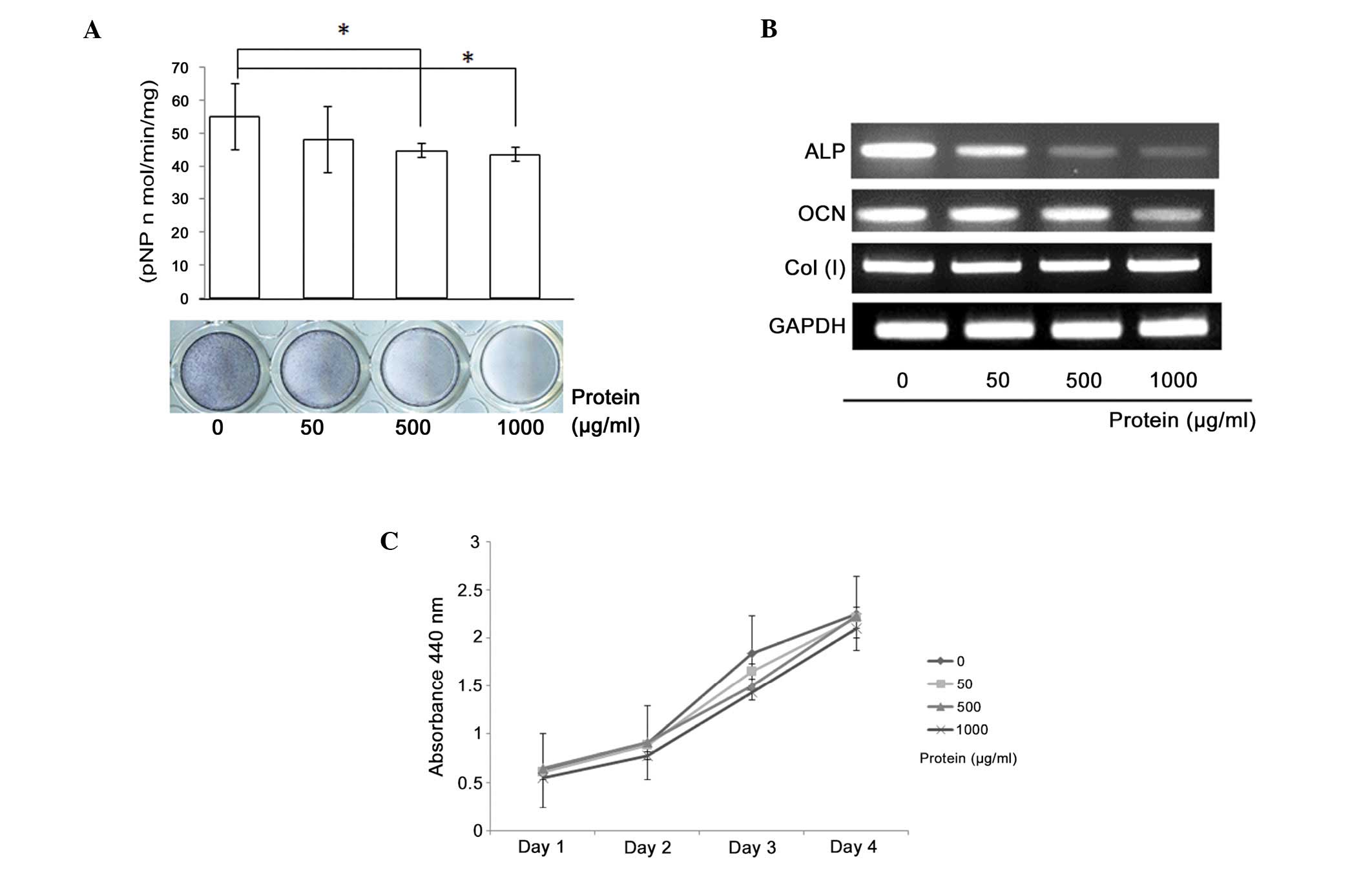
Effect of schwannoma tissue extract on
the differentiation and proliferation of mouse MC3T3-E1 cells
Schwannoma tissue extracts containing the noggin
protein were found to inhibit osteoblastic differentiation in
MC3T3-E1 cells, resulting in a dose-dependent reduction in ALP
activity (Fig. 5A). The ALP
staining results correlated with the ALP activity results. RT-PCR
analysis revealed a suppression of ALP and osteocalcin mRNA
expression with increasing extract concentration (Fig. 5B). However, the proliferation of the
MC3T3-E1 cells was not affected by the addition of the tissue
extract (Fig. 5C).
Clinical data of the patients with
schwannoma and noggin expression patterns
Table II shows the
clinical data of the patients with schwannomas and the noggin
expression patterns in the schwannoma samples obtained from these
patients. The tumor from case 1 was in contact with the bone and
the patient exhibited typical bone scalloping. The tumors in the
other cases were not in contact with the bone and no bone
scalloping was observed. The immunoreactivity for noggin was
positive in cases 1 and 2 and noggin mRNA was expressed in cases 1,
2, 3 and 4. Western blot analysis revealed that noggin protein
expression was only detected in case 1.
 | Table IIClinical data of patients with
schwannoma and expression patterns of noggin in the tumors. |
Table II
Clinical data of patients with
schwannoma and expression patterns of noggin in the tumors.
| | | | | Expression of
noggin |
|---|
| | | | |
|
|---|
| No. | Gender/Age | Location | Contact with
bone | Bone scalloping | IHC | RT-PCR | Western blot
analysis |
|---|
| 1 | F/59 | Spine | (+) | (+) | (++) | (+) | (+) |
| 2 | F/63 | Spine | (−) | (−) | (+) | (+) | (+) |
| 3 | F/49 | Supraclavicular
fossa | (−) | (−) | (−) | (+) | (−) |
| 4 | F/31 | Elbow | (−) | (−) | (−) | (+) | (−) |
| 5 | F/51 | Foot | (−) | (−) | (−) | (−) | (−) |
Discussion
Noggin, an extracellular homodimeric glycoprotein,
is a bone morphogenetic protein antagonist, which binds to BMP-2/4
with high affinity; therefore, noggin interferes with BMP-receptor
binding (18,19). Noggin is significant in the negative
regulation of bone formation, including fracture healing (20,21).
For example, a transgenic mouse overexpressing noggin exhibited
decreased trabecular bone volume and osteopenia (9). In noggin-null mice, augmented BMP
activity has been reported to evoke a series of developmental
abnormalities, including dysmorphogenesis of the axial skeleton and
joint lesions (10,22). Noggin was initially discovered due
to its capacity to induce secondary axis formation in Xenopus
embryos (15,23,24).
Furthermore, the expression of noggin has been reported in the
early development of the central nervous system, which indicates
that noggin may be produced by neurogenic cells. Additionally,
noggin regulates a BMP gradient-directed dorsal-ventral patterning
with subsequent germ layer formation (25). However, it has also been reported
that noggin is more widely expressed throughout the adult central
nervous system and has been proposed to have an important role in
the adult brain (26).
The present study detected the expression of noggin
in schwannomas using RT-PCR analysis, immunohistochemistry and
western blot analysis. Notably, the sample that exhibited vertebral
bone scalloping also exhibited increased noggin mRNA and protein
expression. Furthermore, RT-PCR analysis revealed that noggin mRNA
levels were greatest in the schwannoma tissue and the second
highest in the malignant neurogenic tumor tissue. These findings
are in accordance with a previous report of noggin expression in
the central nervous system (16).
In addition, in the present study, osteoblastic
differentiation in MC3T3-E1 cells was found to be inhibited by
schwannoma tissue extracts, which indicates that these extracts may
include certain factors, which inhibit bone formation. As noggin is
the most potent inhibitor of BMP, it may be the factor within the
extract that is responsible for this inhibition.
Clinically, the most common imaging findings in
spinal schwannoma include pedicle erosion, vertebral body
scalloping and widening of the neural foramen (7,27–29).
The pathomechanism of shwannnoma-induced foramen enlargement and
vertebral scalloping has yet to be elucidated; however, it has been
proposed that pressure erosion on the bone adjacent to the
schwannoma may occur due to the gradual increase in schwannoma size
(30,31). The findings of the present study
indicate that schwannoma-derived noggin may induce a negative
balance of bone remodeling via its BMP antagonist activity,
resulting in local bone resorption.
In conclusion, the present study has detected the
expression of noggin in schwannoma tissue samples. The analysis of
noggin expression in a subset of schwannomas may provide a novel
diagnostic tool for schwannoma. Noggin may be a useful molecular
marker for the differential diagnosis of soft tissue tumors in
pathology. Furthermore, the radiological bone scalloping and
erosion observed in schwannoma patients may be caused by
schwannoma-derived noggin.
References
|
1
|
Chick G, Alnot JY and Silbermann-Hoffman
O: Benign solitary tumors of the peripheral nerves. Rev Chir Orthop
Reparatrice Appar Mot. 86:825–834. 2000.(In French).
|
|
2
|
Knight DM, Birch R and Pringle J: Benign
solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br.
89:382–387. 2007.
|
|
3
|
Amirjamshidi A, Hashemi SM and Abbassioun
K: Schwannoma of the greater superficial petrosal nerve. J
Neurosurg. 113:1093–1098. 2010.
|
|
4
|
Ikushima K, Ueda T, Kudawara I, Nakanishi
K and Yoshikawa H: Plexiform schwannoma of the foot. Eur Radiol.
9:1653–1655. 1999.
|
|
5
|
Agarwal K, Agarwal C, Agarwal M and
Harbhajanka A: Plexiform schwannoma of scalp: a case report with
brief review of literature. Indian J Pathol Microbiol. 50:797–799.
2007.
|
|
6
|
Singson RD, Dee G and Quader MA: Case
report 265. Scalloping and destruction of pedicles of lumbar
vertebral bodies on the right side secondary to venous collaterals,
associated with thrombosis of the inferior ±vena cava. Skeletal
Radiol. 11:293–295. 1984.
|
|
7
|
Inaoka T, Takahashi K, Hanaoka H, et al:
Paravertebral neurinoma associated with aggressive intravertebral
extension. Skeletal Radiol. 30:286–289. 2001.
|
|
8
|
Singrakhia MD, Parmar H, Maheshwari M and
Fehlings M: Cervical schwannoma presenting as an expansile
vertebral body lesion: report of two cases with a technical note on
the surgical management. Surg Neurol. 66:192–196. 2006.
|
|
9
|
Nakamura Y, Wakitani S, Nakayama J,
Wakabayashi S, Horiuchi H and Takaoka K: Temporal and spatial
expression profiles of BMP receptors and noggin during
BMP-2-induced ectopic bone formation. J Bone Miner Res.
18:1854–1862. 2003.
|
|
10
|
Tylzanowski P, Mebis L and Luyten FP: The
Noggin null mouse phenotype is strain dependent and
haploinsufficiency leads to skeletal defects. Dev Dyn.
235:1599–1607. 2006.
|
|
11
|
Devlin RD, Du Z, Pereira RC, Kimble RB,
Economides AN, Jorgetti V and Canalis E: Skeletal overexpression of
noggin results in osteopenia and reduced bone formation.
Endocrinology. 144:1972–1978. 2003.
|
|
12
|
Gazzerro E, Gangji V and Canalis E: Bone
morphogenetic proteins induce the expression of noggin, which
limits their activity in cultured rat osteoblasts. J Clin Invest.
102:2106–2114. 1998.
|
|
13
|
Bachiller D, Klingensmith J, Kemp C, et
al: The organizer factors Chordin and Noggin are required for mouse
forebrain development. Nature. 403:658–661. 2000.
|
|
14
|
Krause C, Guzman A and Knaus P: Noggin.
Int J Biochem Cell Biol. 43:478–481. 2011.
|
|
15
|
Bonaguidi MA, Peng CY, McGuire T, et al:
Noggin expands neural stem cells in the adult hippocampus. J
Neurosci. 28:9194–9204. 2008.
|
|
16
|
Li W and LoTurco JJ: Noggin is a negative
regulator of neuronal differentiation in developing neocortex. Dev
Neurosci. 22:68–73. 2000.
|
|
17
|
Fletcher CDM, Unni KK and Mertens F:
Pathology and Genetics of Tumours of Soft Tissue and Bone. 4. World
Health Organisation; Lyon: 2002
|
|
18
|
Miyazono K, Kamiya Y and Morikawa M: Bone
morphogenetic protein receptors and signal transduction. J Biochem.
147:35–51. 2010.
|
|
19
|
Takayama K, Suzuki A, Manaka T, et al: RNA
interference for noggin enhances the biological activity of bone
morphogenetic proteins in vivo and in vitro. J Bone
Miner Metab. 27:402–411. 2009.
|
|
20
|
Yoshimura Y, Nomura S, Kawasaki S,
Tsutsumimoto T, Shimizu T and Takaoka K: Colocalization of noggin
and bone morphogenetic protein-4 during fracture healing. J Bone
Miner Res. 16:876–884. 2001.
|
|
21
|
Nakase T, Nomura S, Yoshikawa H, et al:
Transient and localized expression of bone morphogenetic protein 4
messenger RNA during fracture healing. J Bone Miner Res. 9:651–659.
1994.
|
|
22
|
Brunet LJ, McMahon JA, McMahon AP and
Harland RM: Noggin, cartilage morphogenesis, and joint formation in
the mammalian skeleton. Science. 280:1455–1457. 1998.
|
|
23
|
Holley SA, Neul JL, Attisano L, et al: The
Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by
preventing DPP from activating its receptor. Cell. 86:607–617.
1996.
|
|
24
|
Iemura S, Yamamoto TS, Takagi C, et al:
Direct binding of follistatin to a complex of bone-morphogenetic
protein and its receptor inhibits ventral and epidermal cell fates
in early Xenopus embryo. Proc Natl Acad Sci USA. 95:9337–9342.
1998.
|
|
25
|
Zimmerman LB, De Jesús-Escobar JM and
Harland RM: The Spemann organizer signal noggin binds and
inactivates bone morphogenetic protein 4. Cell. 86:599–606.
1996.
|
|
26
|
Mikawa S and Sato K: Noggin expression in
the adult rat brain. Neuroscience. 184:38–53. 2011.
|
|
27
|
Asahara H, Kawai A, Harada Y, Senda M and
Inoue H: Spinal schwannomas: a review of 42 cases. Acta Med
Okayama. 50:25–28. 1996.
|
|
28
|
Chibbaro S, Mirone G, Makiese O, Bresson D
and George B: Dumbbell-shaped jugular foramen schwannomas: surgical
management, outcome and complications on a series of 16 patients.
Neurosurg Rev. 32:151–159. 2009.
|
|
29
|
Yokota H, Isobe K, Murakami M, Kubosawa H
and Uno T: Dumbbell-shaped nonpsammomatous malignant melanotic
schwannoma of the cervical spinal root. Spine J. 12:e14–e17.
2012.
|
|
30
|
Piek J: Giant schwannoma of the cauda
equina without neurological deficits - case report and review of
the literature. Wien Klin Wochenschr. 122:645–648. 2010.
|
|
31
|
Jankowski R, Szmeja J, Nowak S, Sokół B
and Blok T: Giant schwannoma of the lumbar spine. A case report.
Neurol Neurochir Pol. 44:91–95. 2010.
|