Introduction
Ménétrier’s disease (MD) is a rare type of
hypertrophic gastropathy involving the body of the stomach, which
was initially described in 1888 (1). It is characterized by thickening of
the gastric mucosa in the form of giant rugal folds,
hypochlorhydria and protein loss. The classic symptoms of MD
include abdominal pain, nausea, vomiting and peripheral oedema
(2). It occurs in two forms,
depending on the patient’s age; in adult males aged ~55 years, it
presents as a progressive disease with an asymptomatic onset.
However, in early childhood, it develops abruptly and resolves
spontaneously. While the development of MD is associated with the
cytomegalovirus (CMV) infection in the case of infants, the cause
of MD in adults remains unknown (3–5).
Previous studies demonstrate the coexistence of MD with infections
(Helicobacter pylori, CMV, herpes simplex, human
immunodeficiency virus [HIV], Mycoplasma pneumoniae)
(4,6–9) as
well as non-specific inflammatory diseases (including ulcerative
colitis) (10). However, the
administration of targeted therapy in these disorders has not
provided any benefits concerning the treatment of MD. Furthermore,
the coexistence of MD with different types of cancer has been
documented in >50 cases. It remains unknown as to whether cancer
develops from MD, whether the disease is a premalignant condition
and what the possible mechanism of carcinogenesis is. In the
current study, a patient with MD and advanced gastric cancer is
described and a review of the literature is presented, which
indicates that MD should be recognized as a premalignant condition.
The studywas approved by the ethics committee of the Medical
University of Białystok, (Białystok, Poland) and the patient
provided written informed consent.
Case report
History
A 51-year-old male was admitted to the Second
Department of Surgery and Gastroenterology (Białystok, Poland) for
planned surgery and was diagnosed with stomach cancer by
performance of a gastroscopy. The description of the preoperative
endoscopy, obtained by the patient from another center,
demonstrated a nodular change with an irregular friable ulceration,
localized on the border of the body and the fundus on the posterior
wall of the stomach. The histopathological examination of the
biopsy material identified that it was an adenocarcinoma. The
patient’s medical history revealed only abdominal pain without
weight loss, normal peristalsis and regular stools. The
preoperative blood parameters showed no abnormalities. Only the
α1-globulin level was marginally elevated to 0.24 g/dl, and the
total protein concentration and albumin level were normal. The
tumour markers, carcinoembryonic antigen and cancer antigen 19-9,
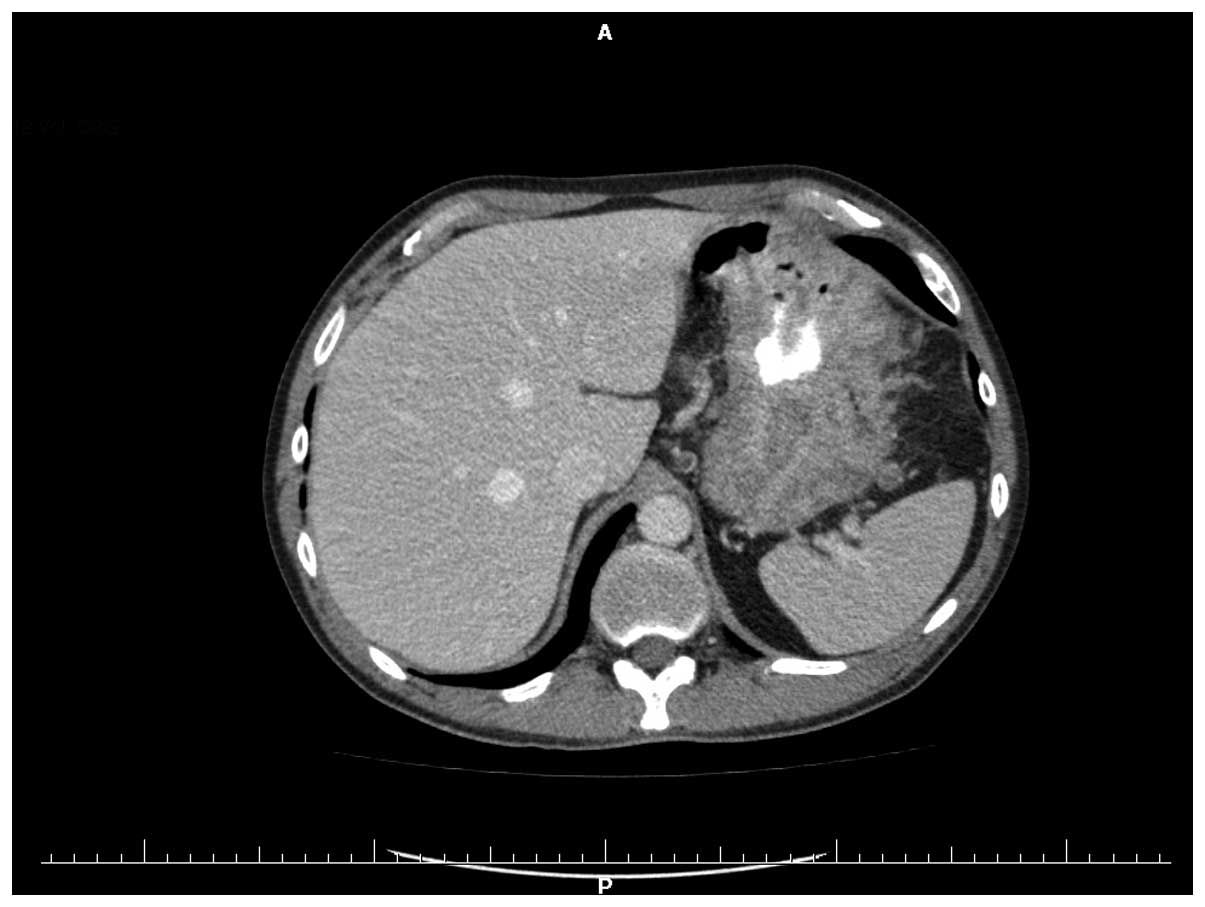
were also normal. Multi-slice computed tomography of the stomach
was performed preoperatively and demonstrated that the heterogenic
wall within the corpus and the prepyloric part had thickened to
34.5 mm (Fig. 1). Intraoperatively,
a large neoplastic infiltration was identified, ~5 cm in diameter,
with a 35-mm crater ulceration at the greater curvature of the
stomach, in the upper third, infiltrating the pancreatic tail and
involving the splenic flexure. The gastric wall was thickened and
showed massive, oversized rugal folds of the mucous membrane. In
addition, the regional lymph nodes of the stomach were markedly
enlarged. The splenic flexure was separated from the tumour. A
total gastrectomy was conducted to remove the spleen and pancreatic
tail and the lymph nodes were removed by D2 lymphadenectomy. The
digestive tract was reconstructed using the double tract
reconstruction technique that enables the passage of chyme through
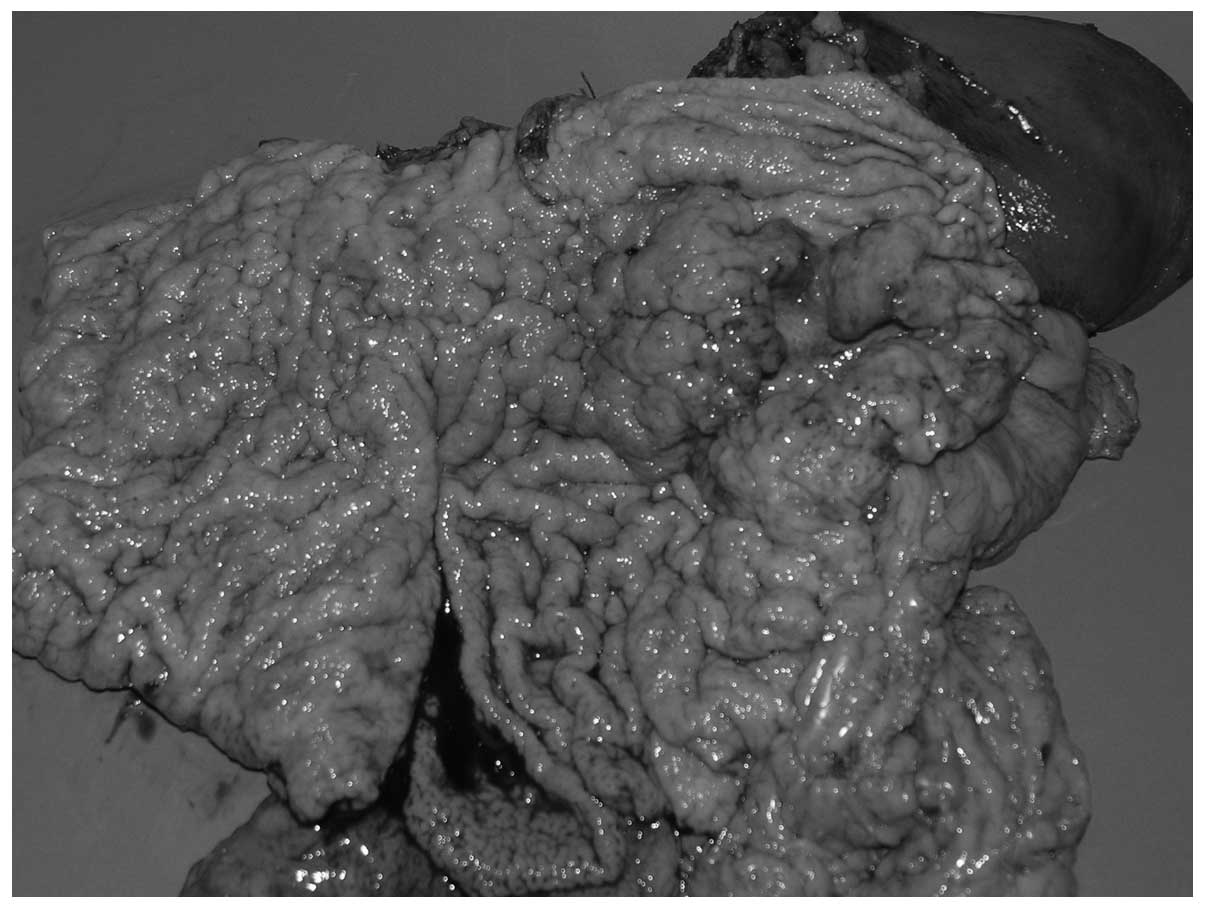
the duodenum. The postoperative period was uneventful (Fig. 2).
Histopathology
Macroscopically, the postoperative formalin-fixed
sample showed a tumour situated on the anterior wall of the
stomach, partly on the lesser curvature, which was 10 cm at the
greatest diameter. An additional lesion was identified on the
posterior wall of the stomach, located 3 cm away from the other
tumour. The tumours were characterized by exophytic growth, were
poorly demarcated and the surrounding mucous membrane was
infiltrated.
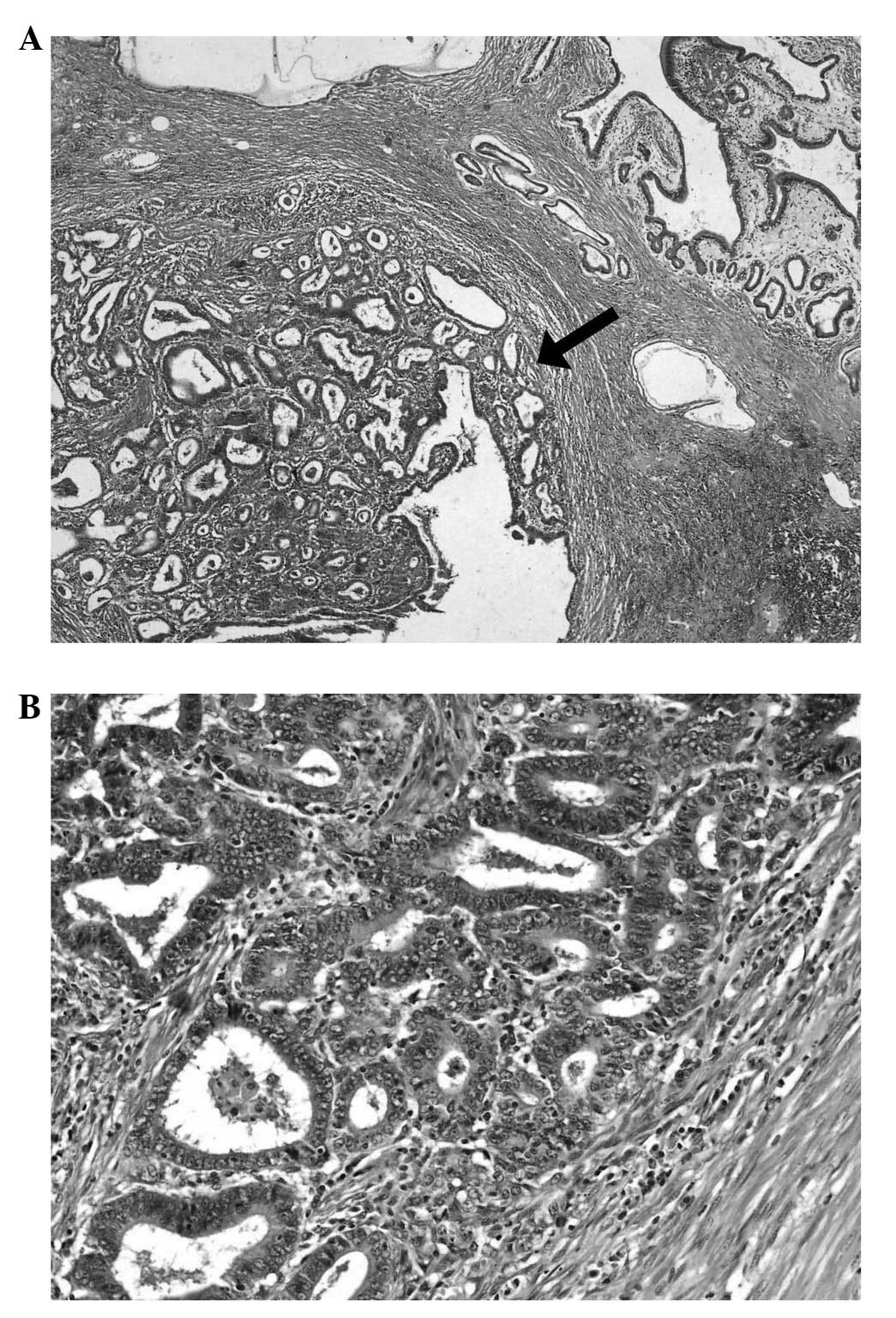
The microscopic examination of the first lesion
demonstrated tubular and papillary adenocarcinoma and was
classified histologically using the 7th edition of the Union for
International Cancer Control classification (11) as extending to the serosal mucosa
(pT3) with a moderately differentiated malignancy (G2) (12). The tumour was classified as an
intestinal type, according to Lauren’s classification, and as type
I according to the Goseki criteria (numerous glandular structures
and a small quantity of mucus in the cancer cells). The
immunohistochemical analysis for human epidermal growth factor
receptor 2 was negative, and metastases to 21/78 lymph nodes and
tumour infiltration of the pancreatic tail were observed (Fig. 3).
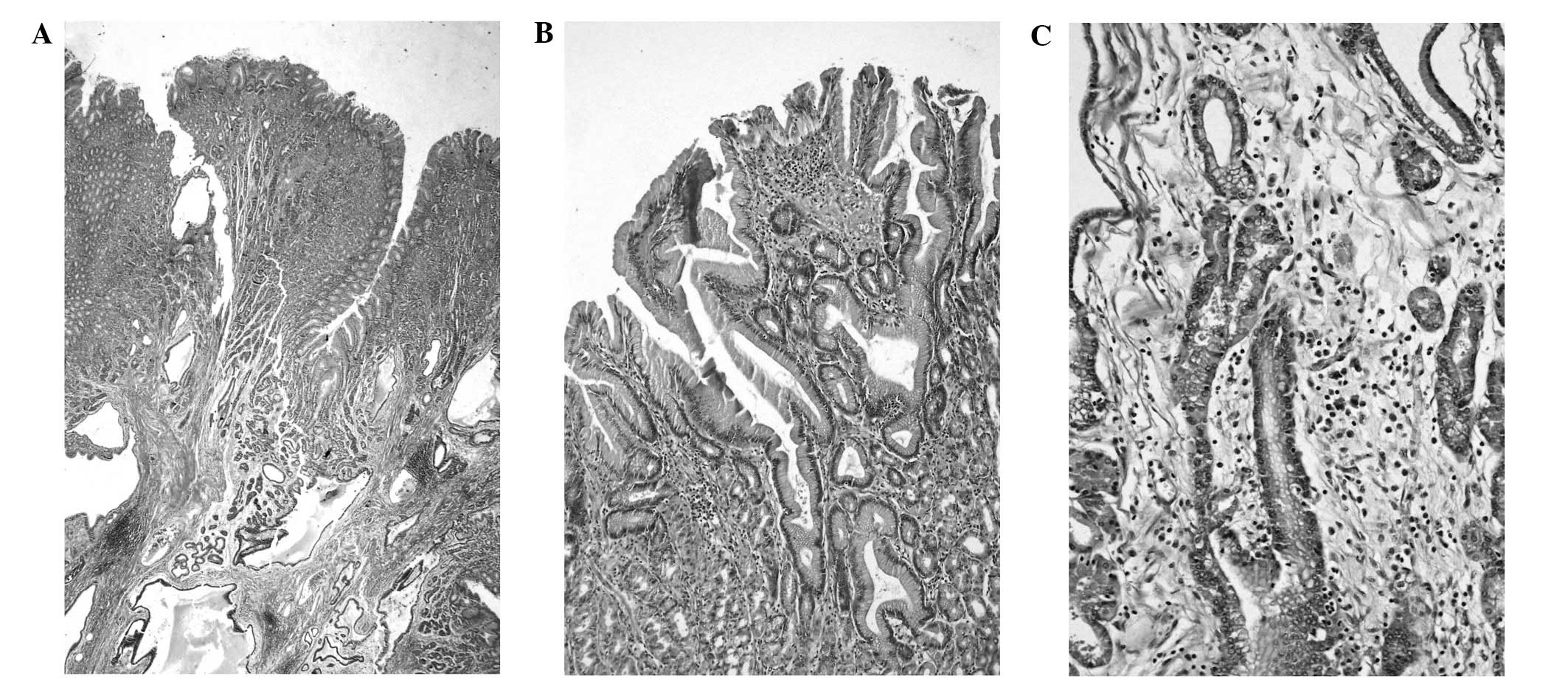
The microscopic image of the smaller lesion
substantiated the diagnosis of MD. The gastric mucosa was thickened
and appeared polyp-like. The mucosal architecture was normal with
glands remaining parallel at the surface. However, minimal
architectural disorder was identified in the deep third of the
mucosa. Foveolar hyperplasia was poorly marked and the tortuosity
of the glands was localized in the upper and middle third of the
mucosa. Cystic dilatation of the glands was distinct in the lower
regions of the mucosa and submucosa. There was an increase in the
number of parietal cells observed, and inflammatory infiltration
predominantly consisted of numerous eosinophils and plasma cells.
In addition, strongly pronounced smooth muscle hyperplasia and
slight oedema were observed. The H. pylori infection was not
detected (Fig. 4).
Discussion
Since MD is rare and difficult to discriminate from
other hypertrophic gastropathies, Rich et al (2) proposed an algorithm for its
recognition. According to the algorithm, the diagnosis of MD should
be based on a comprehensive collection of data concerning clinical,
endoscopic, laboratory and histopathological findings. The most
common symptoms of MD include abdominal pain, nausea, vomiting and
oedema, in addition to serum albumin loss. Endoscopically,
histopathological examination demonstrates a thickened gastric
mucosa. Additionally, the gastric pH must be evaluated; in MD the
pH is often alkaline. The characteristic microscopic features are
as follows: Parallel mucosal gland ducts, tortuosity and
dilatation, foveolar hyperplasia, a reduced number of parietal
cells, infiltration of eosinophils and/or plasma cells, smooth
muscle hyperplasia and oedema. The laboratory assessments,
including complete blood count, serum protein, serum gastrin as
well as serologic tests for H. pylori and CMV should also be
performed (2). In the present case,
the patient belonged to a group of MD patients with less pronounced
clinical symptoms, who did not exhibit protein loss. The elevated
number of parietal cells observed in the histopathological
examination was the predominant difference. However, other
characteristic features facilitated the diagnosis of MD.
As yet it has been impossible to determine the
number of diagnosed cases of MD due to a lack of accurate incidence
data. However, >50 cases of MD presenting with coexisting
gastric cancer have been documented since 1983. Until 1990, ~30
cases had been described and Hsu et al (13) documented that a higher percentage of
up to 18 cases of MD coexisted with early gastric cancer. Cases of
MD coexisting with cancer documented after 1991 were collected as
part of the present study; out of 16 MD cases, three were with
early stage cancer, seven were with advanced stage cancer and six
contained no evidence of the cancer stage (Table I) (14–24).
Such statistics may be due to the patients’ origin. The majority of
cases described by Hsu et al (13) originate from Japan, where screening
for cancer is widely developed and early changes are more
frequently diagnosed. Histologically, a predominance of poorly
differentiated adenocarcinomas were identified during the
literature search in the present study. In addition, in 2007, Choi
et al (20) demonstrated a
case of MD coexisting with squamous cell carcinoma. In the studies
identified during the literature search, the patients were aged
>40 years and nine out of the 12 cases described were male.
 | Table ICases of Ménétrier’s disease
presenting with gastric carcinoma since 1990. |
Table I
Cases of Ménétrier’s disease
presenting with gastric carcinoma since 1990.
| | | Patient
characteristics |
|---|
| | |
|
|---|
| Case | Author (ref.) | Year | Age (years) | Gender | Protein loss | Histology | Depth of invasion and
metastases |
|---|
| 1 | Mosnier et al
(14) | 1991 | 63 | M | + | Adenocarcinoma,
G2 | T - No data, N0 |
| 2 | Johnson et al
(15) | 1995 | 73 | M | No data | Adenocarcinoma | Early |
| 3 | Charton-Bain et
al (16) | 2000 | 62 | F | No data | Adenocarcinoma,
G1 | Advanced - T2,
N0 |
| 4 | Kim et al
(17) | 2004 | 47 | M | + | Adenocarcinoma,
G1 | Early - T1, N0 |
| 5–8 | Sâadia et al
(18) | 2005 | 58–81a | No data | No data | Adenocarcinoma | No data |
| 9 | Ramia et al
(19) | 2007 | 66 | M | + | Adenocarcinoma | Advanced, M1
(liver) |
| 10 | Choi et al
(20) | 2007 | 40 | M | − | Squamous cell
carcinoma, G3 | Advanced - T4,
N0 |
| 11 | Salinas Martín et
al (21) | 2008 | 40 | F | No data | Adenocarcinoma,
G3 | Advanced, N1 |
| 12 | Salinas Martín et
al (21) | 2008 | 61 | M | No data | Adenocarcinoma,
G3 | Advanced, N1 |
| 13 | Mellado-Castillero
et al (22) | 2008 | 40 | F | No data | Adenocarcinoma,
G3 | Advanced, N1 |
| 14 | Pereyra et al
(23) | 2011 | 72 | M | No data | Adenocarcinoma,
G3 | Early |
| 15 | Famularo et al
(24) | 2011 | 73 | M | + | Poorly differentiated
carcinoma | No data |
| 16 | Present case | 2014 | 51 | M | − | Adenocarcinoma,
G2 | Advanced - T3,
N1 |
The risk of malignancy in MD was described in 1991
as 6–10% of the incidence rate (13). A premalignant condition is defined
as a disease that may develop into a malignancy. This percentage is
considered to be adequate to regard MD as a premalignant condition.
Currently, it is not possible to determine and confirm this rate
due to the low incidence of the disease and a lack of registration
of all cases of MD.
In MD it is also unclear whether a tumour is formed
on hyperplastic growth or develops as a de novo lesion.
Usually, and in the cases that were presented in the literature,
the two changes are diagnosed simultaneously during histological
imaging. However, it was 1983 when Wood et al (25) described a man with MD who developed
early gastric cancer 3.5 years following diagnosis. Furthermore,
Ramia et al (19) reported a
patient with advanced gastric cancer and MD, who was diagnosed with
MD 13 years prior to the cancer diagnosis. In the abovementioned
cases, the cancer developed subsequent to the diagnosis of MD. In
the patient in the present study, the cancer was identified in the
biopsy material, whereas MD was confirmed later in the
postoperative specimen. Macroscopically, there were two distinct
gastric tumours ~3 cm apart, which were connected to each other by
infiltration that indicated independent growth. A morphological
similarity of tumour growth to the cystic gland ducts in MD was
observed. Histologically, the cancerous glands appeared to be
enormous cystic formations with ingrowing papillary structures of
cancer cells (Fig. 4). This
provides further evidence for the hypothesis that cancer may arise
from MD.
The poorly understood pathogenesis of MD that
results in a lack of appropriate treatment strategies is another
point in favour of recognising MD as a premalignant lesion.
Currently, symptomatic treatment, anticholinergic agents, acid
suppression, octreotides and prednisone are predominantly
administered for its treatment (5).
Furthermore, theories of bacterial (H. pylori, Mycoplasma
pneumoniae) and viral infections (CMV, herpes simplex, HIV)
have been proposed, which are associated with chronic inflammation
of the gastric mucosa and result in further abnormal recovery in
the form of hyperplasia (4–9). However, due to a lack of clinical
trials, the benefits of targeted treatment for these infections in
adults are not well known. A theory of increased epidermal growth
factor receptor signalling associated with transforming growth
factor-α overproduction has also been presented and treatment with
monoclonal antibodies has been proposed (2). However, the optimum therapeutic
procedure for MD is a gastrectomy, specifically in those patients
with uncontrolled protein loss, bleeding and a high risk for
coexisting cancer. A partial or total gastrectomy is performed
depending on anastomosis, clinical observations and the cancer
stage (17).
In conclusion, MD should be treated with particular
attention and regarded as a premalignant condition due to the
previously documented cases of its coexistence with gastric cancer,
as well as the lack of knowledge regarding its pathogenesis and
effective therapeutic management. This condition should not be
overlooked, and the clinician and the patient should be aware that
it is necessary to monitor the lesion via regular endoscopic biopsy
examinations.
References
|
1
|
Ménétrier P: Des polyadenomes gastriques
et de leurs rapports avec le cancer de l’estomac. Arch Physiol Norm
Pathol. 32:236–262. 1888.
|
|
2
|
Rich A, Toro TZ, Tanksley J, et al:
Distinguishing Ménétrier’s disease from its mimics. Gut.
59:1617–1624. 2010.
|
|
3
|
Scharschmidt BF: The natural history of
hypertrophic gastrophy (Ménétrier’s disease). Report of a case with
16 year follow-up and review of 120 cases from the literature. Am J
Med. 63:644–652. 1977.
|
|
4
|
Eisenstat DD, Griffiths AM, Cutz E, Petric
M and Drumm B: Acute cytomegalovirus infection in a child with
Ménétrier’s disease. Gastroenterology. 109:592–595. 1995.
|
|
5
|
Coffey RJ, Washington MK, Corless CL and
Heinrich MC: Ménétrier disease and gastrointestinal stromal tumors:
hyperproliferative disorders of the stomach. J Clin Invest.
117:70–80. 2007.
|
|
6
|
Badov D, Lambert JR, Finlay M and Balazs
ND: Helicobacter pylori as a pathogenic factor in
Ménétrier’s disease. Am J Gastroenterol. 93:1976–1979. 1998.
|
|
7
|
Jun DW, Kim DH, Kim SH, et al: Ménétrier’s
disease associated with herpes infection: response to treatment
with acyclovir. Gastrointest Endosc. 65:1092–1095. 2007.
|
|
8
|
diSibio G, McPhaul LW, Sarkisian A, Van
Pham B and French SW: Ménétrier’s disease associated with Kaposi’s
sarcoma. Exp Mol Pathol. 85:160–164. 2008.
|
|
9
|
Ben Amitai D, Zahavi I, Dinari G and Garty
BZ: Transient protein-losing hypertrophic gastropathy associated
with Mycoplasma pneumoniae infection in childhood. J Pediatr
Gastroenterol Nutr. 14:237–239. 1992.
|
|
10
|
Nguyen VX, Nguyen CC, Leighton JA, Pasha
SF, Silva AC, Heppell JP and De Petris G: The association of
Ménétrier disease with ulcerative colitis: A case report with
implications on the pathogenesis of Ménétrier disease. Case Rep
Gastroenterol. 4:66–70. 2010.
|
|
11
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer: TNM classification of
malignant tumors. 7th edition. Wiley-Blackwell; New York, NY:
2009
|
|
12
|
Crawford J: The gastrointestinal tract.
Pathologic Basis of Disease. Contran RS, Kumar V, Robbins SL and
Schoen FJ: WB Saunders Co; Philadelphia, PA: pp. 755–783. 1994
|
|
13
|
Hsu CT, Ito M, Kawase Y, Sekine I,
Ohmagari T and Hashimoto S: Early gastric cancer arising from
localized Ménétrier’s disease. Gastroenterol Jpn. 26:213–217.
1991.
|
|
14
|
Mosnier JF, Flejou JF, Amouyal G, Gayet B,
Molas G, Henin D and Potet F: Hypertrophic gastropathy with gastric
adenocarcinoma: Ménétrier’s disease and lymphocytic gastritis? Gut.
32:1565–1567. 1991.
|
|
15
|
Johnson MI, Spark JI, Ambrose NS and Wyatt
JI: Early gastric cancer in a patient with Ménétrier’s disease,
lymphocytic gastritis and Helicobacter pylori. Eur J
Gastroenterol Hepatol. 7:187–190. 1995.
|
|
16
|
Charton-Bain MC, Paraf F and Bruneval P:
Superficial gastric carcinoma developed on localized hypertrophic
lymphocytic gastritis: a variant of localized Ménétrier’s disease?
Pathol Res Pract. 196:125–128. 2000.
|
|
17
|
Kim J, Cheong JH, Chen J, Hyung WJ, Choi
SH and Noh SH: Ménétrier’s disease in Korea: report of two cases
and review of cases in a gastric cancer prevalent region. Yonsei
Med J. 45:555–560. 2004.
|
|
18
|
Sâadia B, Salna BH, Mohamed J, et al:
Ménétrier’s disease and gastric carcinoma. Tunis Med. 83:499–502.
2005.(In French).
|
|
19
|
Ramia JM, Sancho E, Lozano O, Santos JM
and Domínguez F: Ménétrier’s disease and gastric cancer. Cir Esp.
81:153–154. 2007.(In Spanish).
|
|
20
|
Choi SB, Park SS, Oh SY, et al: Primary
squamous cell carcinoma of the stomach that developed with
Ménétrier’s disease. Dig Dis Sci. 52:1722–1724. 2007.
|
|
21
|
Salinas Martín MV, Carranza Carranza A and
Gavilán Carrasco F: Diffuse gastric carcinoma associated with
localized Ménétrier’s disease. Med Clin (Barc). 130:2392008.(In
Spanish).
|
|
22
|
Mellado-Castillero JM, Ibáñez-Delgado F,
Alcántara-Gijón F, Vázquez-Medina A and Hernández de la Torre JM:
Localized Ménétrier’s disease associated with gastric
adenocarcinoma. Rev Esp Enferm Dig. 100:60–61. 2008.(In
Spanish).
|
|
23
|
Pereyra L, Gómez EJ, Mella JM, et al:
Diffuse gastric cancer associated with Ménétrier’s disease. Acta
Gastroenterol Latinoam. 41:142–145. 2011.(In Spanish).
|
|
24
|
Famularo G, Sajeva MR and Gasbarrone L:
Beyond gastritis and before cancer: the strange case of Ménétrier’s
disease. Intern Emerg Med. 6:369–371. 2011.
|
|
25
|
Wood MG, Bates C, Brown RC and Losowsky
MS: Intramucosal carcinoma of the gastric antrum complicating
Ménétrier’s disease. J Clin Pathol. 36:1071–1075. 1983.
|