Introduction
Immune evasion is an important mechanism in cancer
progression. Several studies have identified abnormal cellular
immunity in cancer patients, thus indicating potential new
strategies for cancer treatment (1). However, successful immunotherapy
requires an improved understanding of the changes in the immune
system of cancer patients.
Acute leukemia (AL) is a malignant tumor of the
hematological system, characterized by malignant clones of leukemia
cells in the bone marrow (BM). AL can be classified as acute
myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL),
according to the French-American-British system (2). Chemotherapy and stem cell
transplantation (SCT) represent the main treatments for AL.
Long-term survival may require SCT, which is based on the
rebuilding of the immune system to produce a graft-versus-leukemia
effect (3). However, the high cost
and the significant side effects and mortality limit the
applicability of BM transplantations in China. Therefore, novel
immunotherapies are currently used and investigation into the
abnormalities of the immune system in AL patients has become a hot
topic. Several studies have shown changes in the number and
function of T-lymphocyte subsets in chronic lymphocytic leukemia
(4–7).
CD3+CD56+ T lymphocytes were
first described as a distinct subset of T cells more than one
decade ago (8,9). This subset expresses surface receptors
that are also found on conventional T cells (such as CD3), together
with receptors characteristic of natural killer (NK) cells
(including CD56) and, therefore, are also referred to as NKT cells.
These cells have already been shown to exhibit antitumor
cytotoxicity (10,11); however, unlike classical T cells,
which recognize peptides presented by highly polymorphic major
histocompatibility complex (MHC) molecules, NKT cells recognize
glycolipids via MHC-like, non-polymorphic CD1d molecules (12–21).
CD1d-restricted T-cell populations have a physiological role in
tumor immunosurveillance, which is mediated at least partly through
the maturation of antigen-presenting cells (APCs; including
monocytes, macrophages and dendritic cells) and IL-12 induction via
NK and CD8+ T cells (22–30).
In addition, immunity against a number of tumor models has been
observed with the therapeutic activation of NKT by selective
agonist α-galactosylceramide presented by CD1d+ APCs
(22–24).
As a number of studies have shown that cancer
progression may be associated with the dysfunction of abnormal APCs
(31–34), APCs may be abnormal in AL patients.
In our previous study, the number and cytotoxicity of
CD3+CD56+ T lymphocytes were found to change
in AL patients. In particular, the cytotoxicity of
CD3+CD56+ T lymphocytes was decreased in AL
patients. Therefore, we questioned whether APCs may be abnormal in
AL patients, particularly the levels of CD1d. At present, no
studies have examined the levels of CD1d on the monocytes and
lymphocytes in the peripheral blood (PB) of AL patients. The
present study compared the levels of CD1d on the monocytes and
lymphocytes in patients with primary AL and healthy controls, as
well as in AL patients who had achieved complete remission (CR)
following chemotherapy. Simultaneously, the correlation between the
number of CD3+CD56+ T lymphocytes and levels
of CD1d was analyzed.
Materials and methods
Patients
Fresh PB samples were collected from 56 randomly
selected patients with primary AL (32 AML and 24 ALL, with the
exclusion of acute promyelocytic leukemia) and 28 CR-AL patients
(14 AML and 14 ALL, with the exclusion of acute promyelocytic
leukemia) who visited the Department of Hematology at the Second
Affiliated Hospital of Wenzhou Medical College (Wenzhou, China) for
treatment. In total, 18 AML and 10 ALL patients exhibited increased
(>10×109/l) white blood cell (WBC) counts at the time
of diagnosis (termed AML-1 and ALL-1, respectively), and 14 AML and
14 ALL patients exhibited low (<10×109/l) WBC counts
at diagnosis (termed AML-2 and ALL-2, respectively). The patient
characteristics are shown in Table
I. Normal fresh PB samples were obtained from 20 healthy
volunteers. No individuals in the control group were administered
any medication or suffered from any known acute or chronic disease.
All patients and volunteers provided written informed consent to
participate in the study. The study was approved by the ethics
committee of the Second Affiliated Hospital & Yuying Children’s
Hospital of Wenzhou Medical University (Wenzhou, China).
 | Table ICharacteristics of AML/ALL and
CR-AML/-ALL patients. |
Table I
Characteristics of AML/ALL and
CR-AML/-ALL patients.
| Patients | n |
|---|
| AML | 32 |
| WBC,
>10×109/l | 18 |
| WBC,
<10×109/l | 14 |
| ALL | 24 |
| WBC,
>10×109/l | 10 |
| WBC,
<10×109/l | 14 |
| CR-AMLa | 14 |
| CR-ALLa | 14 |
Reagents
Monoclonal allophycocyanin mouse anti-human CD45,
PerCP-Cy5.5 mouse anti-human CD3, phycoerythrin (PE) mouse
anti-human CD1d and fluorescein isothiocyanate (FITC) mouse
anti-human CD56 were purchased from eBioscience (San Diego, CA,
USA).
Lymphocyte membrane phenotype and
expression of CD1d on monocytes and lymphocytes
Triple-labeling experiments were performed using
EDTA-anticoagulated PB samples (AL, CR-AL and healthy controls).
Aliquots of 100 μl were incubated for 30 min at room temperature
with pretitered dilutions of allophycocyanin-, FITC-, PE- and
PerCP-Cy5.5-conjugated monoclonal antibodies against CD45 (HI30),
CD3 (OKT3; clone 25; IgG1) and CD56 (IgG1), respectively.
Isotype-matched control antibodies conjugated with FITC, PE and
PerCP-Cy5.5 were included to establish background fluorescence.
Erythrocytes were subsequently lysed by adding 3 ml of
NH4Cl for 10 min at room temperature. The cells were
then washed in phosphate-buffered saline supplemented with 0.1 mM
EDTA and 0.02% NaN2, and kept on ice until flow
cytometric examination.
Flow cytometry
A flow cytometer (FACSCalibur; BD Biosciences, San
Jose, CA, USA) was used for data acquisition and FlowJo software
(TreeStar Inc., Ashland, OR, USA) was used for analysis.
Statistical analysis
Data are presented as the mean ± standard deviation.
The significant differences were analyzed by one-way analysis of
variance. The correlation between the number of
CD3+CD56+ T lymphocytes and levels of CD1d
was analyzed by Pearson’s correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Levels of CD1d in the PB of patients
presenting with AL
The levels of CD1d on monocytes were assessed in the
PB of 56 primary AL and 28 CR-AL patients, as well as 20 healthy
volunteers. A significant decrease was identified in the levels of
CD1d on monocytes in the PB of AL patients (AML and ALL) compared
with the healthy controls (P<0.05; Table II and Fig. 1B–D and G). Simultaneously, a
difference was observed between the levels of CD1d on monocytes in
CR-AML and CR-ALL patients. The levels of CD1d on monocytes
remained lower in CR-AML patients than in the healthy controls
(P<0.0.5; Table II and Fig. 1E and G), while the levels of CD1d on
monocytes recovered in the CR-ALL patients (P>0.0.5; Table II and Fig. 1F and G). However, no difference was
observed in the changing levels of CD1d on monocytes in AML and ALL
patients with high (>10×109/l) WBC counts
(P>0.0.5; Table III). The
levels of CD1d on lymphocytes were then assessed in the PB of
primary AL and CR-AL patients, as well as the healthy volunteers,
but no significant difference was identified (P>0.0.5, Table IV, Fig.
2). In addition, no significant difference was observed in the
levels of CD1d on lymphocytes in AML and ALL patients with high
(>10×109/l) WBC counts (P>0.05; data not
shown).
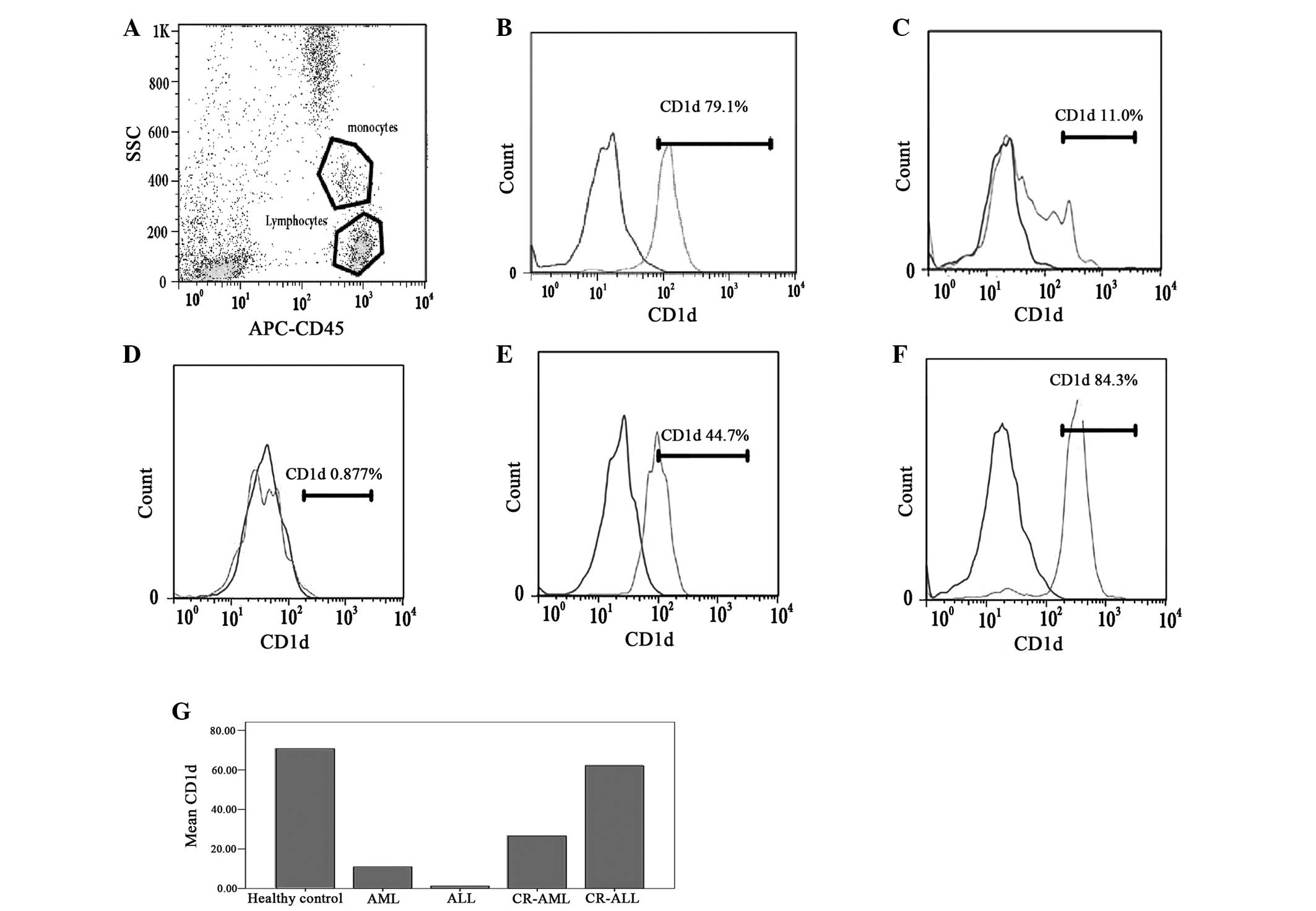 | Figure 1Levels of CD1d on the monocytes in the
PB of patients with AL and CR-AL, and healthy controls. The levels
of CD1d on the monocytes in the PB of AL patients (AML and ALL)
were lower than those in the healthy controls. The change in the
levels of CD1d on the monocytes was different in CR-AML and CR-ALL
patients. The levels of CD1d on the monocytes remained low in
CR-AML patients, but the levels of CD1d on the monocytes recovered
in CR-ALL patients. (A) Monocytes and lymphocytes; (B) healthy
control, (C) AML, (D) ALL, (E) CR-AML and (F) CR-ALL groups; and
(G) the levels of CD1d in the different groups. PB, peripheral
blood; AL, acute leukemia; CR, complete remission; AML, acute
myeloid leukemia; ALL, acute lymphoblastic leukemia; APC,
antigen-presenting cell; SCC, side scatter. |
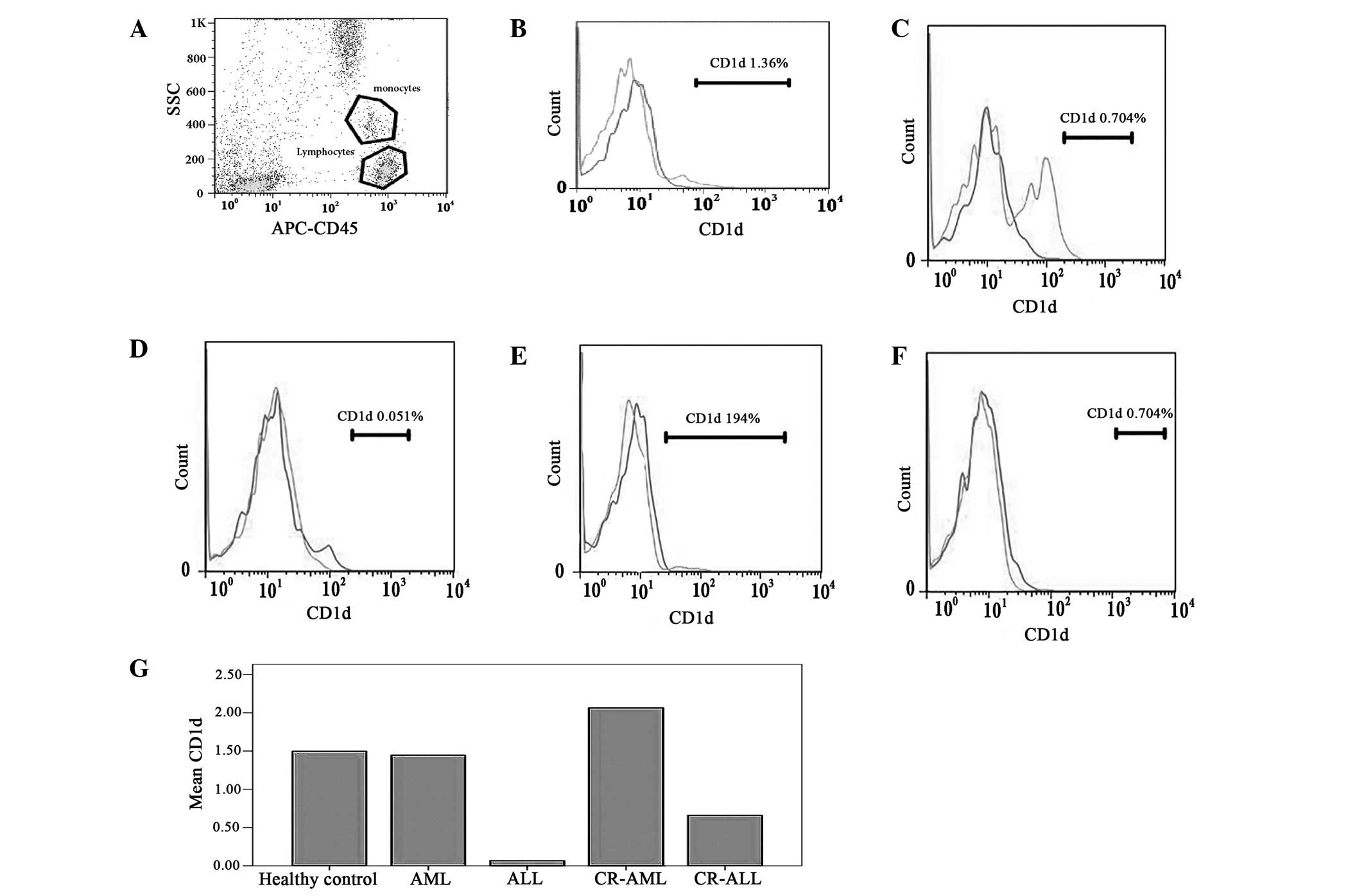 | Figure 2Levels of CD1d on the lymphocytes in
the peripheral blood of patients with AL and CR-AL, and of healthy
controls. The results showed no significant deviation between AL
and AL-CR patients, and the healthy controls. (A) Monocytes and
lymphocytes; (B) healthy control, (C) AML, (D) ALL, (E) CR-AML and
(F) CR-ALL groups; and (G) the levels of CD1d in the different
groups. AL, acute leukemia; CR, complete remission; AML, acute
myeloid leukemia; ALL, acute lymphoblastic leukemia; APC,
antigen-presenting cell; SCC, side scatter. |
 | Table IILevels of CD1d on the monoctyes in the
peripheral blood of patients with AML, ALL, CR-AML, CR-ALL and
healthy controls. |
Table II
Levels of CD1d on the monoctyes in the
peripheral blood of patients with AML, ALL, CR-AML, CR-ALL and
healthy controls.
| Patients | CD1d, % | P-valuea |
|---|
| Healthy controls | 70.63±18.07 | <0.05 |
| AML | 10.96±3.36 | <0.05 |
| ALL | 1.21±0.57 | <0.05 |
| CR-AML | 26.50±4.81 | <0.05 |
| CR-ALL | 62.03±16.57 | >0.05 |
 | Table IIILevels of CD1d on the monoctyes in
the peripheral blood of patients with AML and ALL in relation to
the WBC count at diagnosis. |
Table III
Levels of CD1d on the monoctyes in
the peripheral blood of patients with AML and ALL in relation to
the WBC count at diagnosis.
| Patients | CD1d, % | P-valuea |
|---|
| AML-1 | 17.63±8.69 | >0.05 |
| AML-2 | 2.62±1.69 | >0.05 |
| ALL-1 | 2.49±0.47 | >0.05 |
| ALL-2 | 0.62±0.36 | >0.05 |
 | Table IVLevels of CD1d on the lymphocytes in
the peripheral blood of patients with AML, ALL, CR-AML, CR-ALL and
healthy controls. |
Table IV
Levels of CD1d on the lymphocytes in
the peripheral blood of patients with AML, ALL, CR-AML, CR-ALL and
healthy controls.
| Patients | CD1d, % | P-valuea |
|---|
| Healthy
controls | 1.50±0.25 | >0.05 |
| AML | 1.44±0.69 | >0.05 |
| ALL | 0.07±0.04 | >0.05 |
| CR-AML | 2.06±0.77 | >0.05 |
| CR-ALL | 0.65±0.61 | >0.05 |
Correlation between the number of
CD3+CD56+ T lymphocytes and levels of CD1d on
monocytes
In our previous study, the number and function of
CD3+CD56+ T lymphocytes were found to change
in AL patients (35). In the
present study, the function of CD3+CD56+ T
lymphocytes was decreased in AL patients (Tables V and VI). Therefore, the current study analyzed
the correlation between the number of
CD3+CD56+ T lymphocytes and levels of CD1d on
monocytes. The results showed that the number of
CD3+CD56+ T lymphocytes was increased in
primary AL patients (AML and ALL patients), while the levels of
CD1d on monocytes were decreased in primary AL patients. Therefore,
a negative correlation was identified between the number of
CD3+CD56+ T lymphocytes and the levels of
CD1d on monocytes (P>0.05; Table
VI). When the AL patients achieved CR, the number of
CD3+CD56+ T lymphocytes returned to normal;
however, the levels of CD1d exhibited two types of change. In AML
patients who had achieved CR, the levels of CD1d remained lower
than that in the healthy controls. Whereas in ALL patients who had
achieved CR, the levels of CD1d recovered. Therefore, in ALL-CR
patients, a negative correlation was observed between the number of
CD3+CD56+ T lymphocytes and the levels of
CD1d on monocytes (P<0.05; Table
VI). The correlation between CD3+CD56+ T
lymphocyte function and the levels of CD1d on monocytes was also
analyzed. The results showed that the function of
CD3+CD56+ T lymphocytes and the levels of
CD1d on monocytes were decreased in the primary AL patients,
showing a positive correlation (P<0.05; Table VII). In AL patients who had
achieved CR, the function of the CD3+CD56+ T
lymphocytes remained lower than that of healthy controls. Although
the levels of CD1d were significantly different between the AML and
ALL patients, no significant difference was identified between
CD3+CD56+ T lymphocyte cytotoxicity and the
levels of CD1d (P>0.05; Table
VII).
 | Table VNumber of
CD3+CD56+ T lymphocytes in the peripheral
blood in patients with AML, ALL, CR-AML, CR-ALL and healthy
controls. |
Table V
Number of
CD3+CD56+ T lymphocytes in the peripheral
blood in patients with AML, ALL, CR-AML, CR-ALL and healthy
controls.
|
CD3+CD56+ T
lymphocytes | Healthy
controls | AML | ALL | CR-ALL | CR-ALL |
|---|
| Proportion (%) | 2.72±1.58 | 6.05±1.83a | 7.08±3.70a | 3.58±1.01 | 3.26±1.53 |
| Number
(x106/l) | 58.9±34.7 | 162.4±54.1a | 183.3±91.7a | 52.4±14.4 | 43.5±3.9 |
 | Table VICorrelation between the levels of
CD1d and number of CD3+CD56+ T lymphocytes in
the peripheral blood of patients with AML, ALL, CR-AML and
CR-ALL. |
Table VI
Correlation between the levels of
CD1d and number of CD3+CD56+ T lymphocytes in
the peripheral blood of patients with AML, ALL, CR-AML and
CR-ALL.
CD1d correlation
coefficient
P-value |
|---|
| AML | −0.278 | 0.041a |
| ALL | −0.273 | 0.048a |
| CR-AML | −0.021 | 0.881 |
| CR-ALL | −0.313 | 0.049a |
 | Table VIICorrelation between the levels CD1d
and perforin on the CD3+CD56+ T lymphocytes
in the peripheral blood of patients with AML, ALL, CR-AML and
CR-ALL. |
Table VII
Correlation between the levels CD1d
and perforin on the CD3+CD56+ T lymphocytes
in the peripheral blood of patients with AML, ALL, CR-AML and
CR-ALL.
| Patients | CD1d, % | P-value |
|---|
| AML | 0.685 | 0.000a |
| ALL | 0.627 | 0.001a |
| CR-AML | −0.171 | 0.220 |
| CR-ALL | 0.001 | 0.500 |
Discussion
The antitumor effect of NKT cells has been reported
in several studies (10,11) analyzing the expression of CD1d on
APCs (22–30,36).
The results of the current study demonstrated that the levels of
CD1d on monocytes were decreased in AML and ALL patients compared
with healthy controls. These results also showed that the deficient
antigen presentation in AML and ALL patients may be one of the
reasons for AML and ALL progression. According to our previous
study, a negative correlation exists between the number of
CD3+CD56+ T lymphocytes and the levels of
CD1d on monocytes (35). This
indicated that the reason for the increase in the number of
CD3+CD56+ T lymphocytes may be compensation
for the deficient antigen presentation, similar to the increasing
number of erythrocytes observed in patients with anoxia. However,
the compensation of an increase in the number of
CD3+CD56+ T lymphocytes does not prevent
disease progression due to the lack of cytotoxicity of
CD3+CD56+ T lymphocytes. According to other
studies, T cells reactive against self-peptides are in an ignorant
state known as peripheral tolerance and require activation by
professional APCs in a process termed cross-priming to exert their
effector functions (37–40), particularly the CD1d on APCs, which
affect the function of NKT cells (41,42).
Therefore, the aim of the present study was to investigate the
correlation between the levels of CD1d on monocytes and the
cytotoxicity of CD3+CD56+ T lymphocytes in
AML and ALL patients. The results identified a positive correlation
between the cytotoxicity of CD3+CD56+ T
lymphocytes and the levels of CD1d on monocytes in AML and ALL
patients. Therefore, the low levels of CD1d on monocytes may cause
the lack of cytotoxicity of CD3+CD56+ T
lymphocytes. In addition, the results showed no significant
deviation between the levels of CD1d on monocytes in the AML and
ALL patients with varying WBC counts. However, according to the
results of our previous (35) and
current study comparing the number of
CD3+CD56+ T lymphocytes with varying WBC
counts, no explanation has been reached concerning the compensated
CD3+CD56+ T lymphocyte number in AML-1 and
ALL-2 patients only. Therefore, further studies with larger sample
sizes are required to clarify the clinical significance of these
findings.
When AML and ALL patients achieved CR by
chemotherapy, the levels of CD1d differed between the AML and ALL
patients. In CR-AML patients, the levels of CD1d increased a
little, but remained lower than those of the healthy controls,
while levels returned to normal in CR-ALL patients. This was
similar to the change in the number CD3+CD56+
T lymphocytes in CR-ALL patients. A previous review of ALL showed
that young ALL patients (particularly children) may exhibit a good
prognosis without SCT in certain conditions (43). As a result, the normalization of the
levels of CD1d on monocytes in ALL patients may be one of the
reasons for the good prognosis. However, a study by Fais et
al (44) showed that the CD1d
expression on B-precursor acute lymphoblastic leukemia subsets has
poor prognosis. Further studies with the follow-up of patients who
exhibit the normalization of the levels of CD1d on monocytes are
required to clarify the prognosis. Our previous study showed that
the levels of perforin remained low in the
CD3+CD56+ T lymphocytes of CR-AML and -ALL,
however, no significant correlation was identified between the
levels of CD1d and perforin (35).
Therefore, we consider the levels of CD1d on monocytes and the lack
of cytotoxicity to be two independent prognosis factors for AML and
ALL patients who have received chemotherapy.
By reviewing the literature, it was found that the
levels of CD1d may also be expressed on the surface of lymphocytes
(14,15). As a result, the current study tested
the levels of CD1d on lymphocytes to investigate whether there was
a difference between AL patients and healthy controls. However, no
significant deviation was observed between the AL patients and
healthy controls and, therefore, we do not consider the levels of
CD1d on lymphocytes to influence the disease progression.
In conclusion, the decreasing levels of CD1d on
monocytes may contribute to AML and ALL progression, as a
correlation was observed between the levels of CD1d on monocytes
and the number/cytotoxicity of CD3+CD56+ T
lymphocytes in AML and ALL patients. Furthermore, a correlation may
exist between the normalization of the levels of CD1d on monocytes
in AML and ALL patients and disease prognosis. These findings
suggest that the reinforcement or repair of APCs for immunocytes
with antitumor effects may prolong survival in AL patients unable
to undergo SCT, and may thus represent a useful strategy for
treating AL.
Acknowledgements
The authors would like to thank Professor Chen Hui
and Professor Yang Junjun for their skillful technical assistance,
as well as Zhu Xueqiong for funding assistance. This study was
supported by the program of WenZhou Science and Technology Bureau
(grant no. Y20100260).
References
|
1
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011.
|
|
2
|
Bennett JM, Catovsky D, Daniel MT, et al:
Proposals for the classification of the acute leukaemias.
French-American-British (FAB) co-operative group. Br J Haematol.
33:451–458. 1976.
|
|
3
|
Horowitz MM, Gale RP, Sondel PM, et al:
Graft-versus-leukemia reactions after bone marrow transplantation.
Blood. 75:555–562. 1990.
|
|
4
|
Görgün G, Holderried TA, Zahrieh D,
Neuberg D and Gribben JG: Chronic lymphocytic leukemia cells induce
changes in gene expression of CD4 and CD8 T cells. J Clin Invest.
115:1797–1805. 2005.
|
|
5
|
Görgün G, Ramsay AG, Holderried TA, et al:
E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of
chronic lymphocytic leukemia-induced T-cell dysfunction. Proc Natl
Acad Sci USA. 106:6250–6255. 2009.
|
|
6
|
Dustin ML and Cooper JA: The immunological
synapse and the actin cytoskeleton: molecular hardware for T cell
signaling. Nat Immunol. 1:23–29. 2000.
|
|
7
|
Ramsay AG, Johnson AJ, Lee AM, et al:
Chronic lymphocytic leukemia T cells show impaired immunological
synapse formation that can be reversed with an immunomodulating
drug. J Clin Invest. 118:2427–2437. 2008.
|
|
8
|
Godfrey DI, Hammond KJ, Poulton LD, Smyth
MJ and Baxter AG: NKT cells: facts, functions and fallacies.
Immunol Today. 21:573–583. 2000.
|
|
9
|
Joyce S: CD1d and natural T cells: how
their properties jump-start the immune system. Cell Mol Life Sci.
58:442–469. 2001.
|
|
10
|
Pittet MJ, Speiser DE, Valmori D,
Cerottini JC and Romero P: Cutting edge: cytolytic effector
function in human circulating CD8+ T cells closely correlates with
CD56 surface expression. J Immunol. 164:1148–1152. 2000.
|
|
11
|
Ohkawa T, Seki S, Dobashi H, et al:
Systematic characterization of human CD8+ T cells with natural
killer cell markers in comparison with natural killer cells and
normal CD8+ T cells. Immunology. 103:281–290. 2001.
|
|
12
|
Kronenberg M: Toward an understanding of
NKT cell biology: progress and paradoxes. Annu Rev Immunol.
23:877–900. 2005.
|
|
13
|
Bendelac A, Savage PB and Teyton L: The
biology of NKT cells. Annu Rev Immunol. 25:297–336. 2007.
|
|
14
|
Taniguchi M, Tashiro T, Dashtsoodol N,
Hongo N and Watarai H: The specialized iNKT cell system recognizes
glycolipid antigens and bridges the innate and acquired immune
systems with potential applications for cancer therapy. Int
Immunol. 22:1–6. 2010.
|
|
15
|
Godfrey DI and Kronenberg M: Going both
ways: immune regulation via NKT cells. J Clin Inv.
114:13792004.
|
|
16
|
Van der Vliet HJ, Molling JW, von Blomberg
BM, et al: The immunoregulatory role of CD1d-restricted natural
killer T cells in disease. Clin Immunol. 112:8–23. 2004.
|
|
17
|
Nowak M and Stein-Streilein J: Invariant
NKT cells and tolerance. Int Rev Immunol. 26:95–119. 2007.
|
|
18
|
Matsuda JL, Mallevaey T, Scott-Browne J
and Gapin L: CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of
the immune system. Curr Opin Immunol. 20:358–368. 2008.
|
|
19
|
Parekh VV, Wilson MT and Van Kaer L:
iNKT-cell responses to glycolipids. CritRevImmunol. 25:183–213.
2005.
|
|
20
|
Salio M, Silk JD and Cerundolo V: Recent
advances in processing and presentation of CD1 bound lipid
antigens. Curr Opin Immunol. 22:81–88. 2010.
|
|
21
|
Venkataswamy MM and Porcelli SA: Lipid and
glycolipid antigens of CD1d-restricted natural killer T cells.
Semin Immunol. 22:68–78. 2010.
|
|
22
|
Swann JB, Coquet JM, Smyth MJ and Godfrey
DI: CD1-restricted T cells and tumor immunity. Curr Top Microbiol
Immunol. 314:293–323. 2007.
|
|
23
|
Berzofsky JA and Terabe M: The contrasting
roles of NKT cells in tumor immunity. Curr Mol Med. 9:667–672.
2009.
|
|
24
|
Dhodapkar MV: Harnessing human CD1d
restricted T cells for tumor immunity: progress and challenges.
Front Biosci. 14:796–807. 2009.
|
|
25
|
Hegde S, Fox L, Wang X and Gumperz JE:
Autoreactive natural killer T cells: promoting immune protection
and immune tolerance through varied interactions with myeloid
antigen-presenting cells. Immunology. 130:471–483. 2010.
|
|
26
|
Exley M, Garcia J, Wilson SB, et al: CD1d
structure and regulation on human thymocytes, peripheral blood T
cells, B cells and monocytes. Immunology. 100:37–47. 2000.
|
|
27
|
Kitamura H, Iwakabe K, Yahata T, et al:
The natural killer T(NKT) cell ligand alpha-galactosylceramide
demonstrates its immunopotentiating effect by inducing interleukin
(IL)-12 production by dendritic cells and IL-12 receptor expression
on NKT cells. J Exp Med. 189:1121–1128. 1999.
|
|
28
|
Tomura M, Yu WG, Ahn HJ, et al: A novel
function of Valpha14+CD4+NKT cells:
stimulation of IL-12 production by antigen-presenting cells in the
innate immune system. J Immunol. 163:93–101. 1999.
|
|
29
|
Hayakawa Y, Takeda K, Yagita H, et al:
Differential regulation of Th1 and Th2 functions of NKT cells by
CD28 and CD40 costimulatory pathways. J Immunol. 166:6012–6018.
2001.
|
|
30
|
Berzins SP, Smyth MJ and Baxter AG:
Presumed guilty: natural killer T cell defects and human disease.
Nat Rev Immunol. 11:131–142. 2011.
|
|
31
|
Troy AJ, Summers KL, Davidson PJT,
Atkinson CH and Hart DNJ: Minimal recruitment and activation of
dendritic cells within renal cell carcinoma. Clin Cancer Res.
4:585–593. 1998.
|
|
32
|
Enk AH, Jonuleit H, Saloga J and Knop J:
Dendritic cells as mediators of tumor-induced tolerance in
metastatic melanoma. Int J Cancer. 73:309–316. 1997.
|
|
33
|
Nestle FO, Burg G, Fäh J, Wrone-Smith T
and Nickoloff BJ: Human sunlight-induced
basal-cell-carcinoma-associated dendritic cells are deficient in T
cell co-stimulatory molecules and are impaired as
antigen-presenting cells. Am J Pathol. 150:641–651. 1997.
|
|
34
|
Gabrilovich DI, Corak J, Ciernik IF,
Kavanaugh D and Carbone DP: Decreased antigen presentation by
dendritic cells in patients with breast cancer. Clin Cancer Res.
3:483–490. 1997.
|
|
35
|
Guo W, Xing C, Dong A, et al: Numbers and
cytotoxicities of CD3+CD56+ T lymphocytes in peripheral blood of
patients with acute myeloid leukemia and acute lymphocytic
leukemia. Cancer Biol Ther. 14:916–921. 2013.
|
|
36
|
Spada FM, Borriello F, Sugita M, et al:
Low expression level but potent antigen presenting function of CD1d
on monocyte lineage cells. Eur J Immunol. 30:3468–77. 2000.
|
|
37
|
Kurts C, Robinson BW and Knolle PA:
Cross-priming in health and disease. Nat Rev Immunol. 10:403–414.
2010.
|
|
38
|
Matzinger P: The danger model: a renewed
sense of self. Science. 296:301–5. 2002.
|
|
39
|
Burgdorf S, Schölz C, Kautz A, Tampé R and
Kurts C: Spatial and mechanistic separation of cross-presentation
endogenous antigen presentation. Nature Immunology. 9:558–566.
2008.
|
|
40
|
East JE, Sun W and Webb TJ: Artificial
antigen presenting cell (aAPC) mediated activation and expansion of
natural killer T cells. J Vis Exp. 29:43332012.
|
|
41
|
Webb TJ, Bieler JG, Schneck JP and Oelke
M: Ex vivo induction and expansion of natural killer T cells by
CD1d1-Ig coated artificial antigen presenting cells. J Immunol
Methods. 31:38–44. 2009.
|
|
42
|
Webb TJ, Giuntoli RL II, Rogers O, Schneck
J and Oelke M: Ascites specific inhibition of CD1d-mediated
activation of natural killer T cells. Clin Cancer Res. 1:7652–7658.
2008.
|
|
43
|
Inaba H, Greaves M and Mullighan CG: Acute
lymphoblastic leukaemia. Lancet. 381:1943–1955. 2013.
|
|
44
|
Fais F, Tenca C, Cimino G, et al: CD1d
expression on B-precursor acute lymphoblastic leukemia subsets with
poor prognosis. Leukemia. 19:551–556. 2005.
|
















