Introduction
Study has confirmed that persistent infection with
high-risk type human papilloma virus (HPV) may result in the
gradual alteration of normal cervical epithelial tissue to cervical
intraepithelial neoplasia (CIN), which may progress to cervical
invasive carcinoma (1). However,
the underlying pathogenetic mechanism of cervical cancer, which may
be a multigene, multifactor, multistep and multistage complex
process, remains unclear. Previous studies have demonstrated that
the coordinated regulation of cytoskeletal proteins is pivotal in
motility, invasion and metastasis (2–4).
Metastasis suppressor 1 (MTSS1), also termed missing in metastasis
(MIM), is a newly identified actin binding protein that is mainly
involved in cytoskeletal remodeling, signal transduction and
transcriptional activation, and is closely associated with tumor
growth and invasion (5). However,
the association between MTSS1 and cervical lesions has not yet been
reported. In the present study, the role of MTSS1 in cervical
carcinogenesis was examined by investigating MTSS1 expression in
pre-cancerous cervical lesions and malignant cervical tissues, and
by analyzing the association between MTSS1 expression and
clinicopathological factors.
Materials and methods
Clinical materials
The study included a total of 60 patients with CIN
(30 with CIN I and 30 with CIN II–III) and 57 patients with
cervical cancer, as well as 30 healthy individuals. The mean (± SD)
age of all participants was 44.36±10.53 years. Cervical
pathological biopsy specimens were surgically removed during
cervical biopsy and radical hysterectomy performed between January
2000 and December 2012 in outpatient services at the Airforce
General Hospital (Beijing, China). All specimens were maintained in
tissue paraffin blocks. The pathological diagnosis was reviewed in
a double-blind manner by two experienced pathologists. The cervical
cancer clinical stage was defined according to the criteria
determined by the International Federation of Gynecology and
Obstetrics in 2009 (6): 23 cases
were early-stage cervical cancer (stage I–IIa) and 34 cases were
advanced-stage (stage IIb–IV). With regard to the degree of
differentiation, 12 cases were well-differentiated, 35 cases were
moderately differentiated and 10 cases were poorly differentiated.
Lymph node metastasis status was examined, and 48 cases without
lymph node metastasis and 9 cases with lymph node metastasis were
detected. A total of 57 patients were diagnosed with cervical
squamous carcinoma and 30 cases of normal cervical epithelium were
selected for comparison. No significant differences between groups
with regard to general patient information, including age and
pregnancy history, were identified. This study was approved by the
ethics committee of Airforce General Hospital (Beijing, China).
Written informed consent was obtained from all patients.
Immunohistochemical staining of MTSS1
protein
MTSS1 expression in the 147 cervical tissue
specimens was detected by immunohistochemistry using the EnVision
two-step immunohistochemical staining method (Dako, Carpinteria,
CA, USA). The anti-MTSS1 primary antibody used was ab56780 (1:130
dilution; Abcam, Cambridge, UK). An EnVision immunohistochemical
kit (secondary antibody, K5007, DAB chromogenic system) was
purchased from Beijing Golden Bridge Biotechnology Company
(Beijing, China). Normal cervical tissues were selected to serve as
a positive control. Phosphate-buffered saline (PBS; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
served as a negative control in place of the primary antibody. For
each case, 3-μm sections were cut from the paraffin-embedded tumor
tissue blocks and placed on slides. The tissues were de-waxed in
xylene and rehydrated in alcohol. Antigen retrieval was performed
by placing the tissues in sodium citrate buffer (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.) and applying a high voltage
for 3 min (pH 6.0), followed by natural cooling. The sections were
then placed in 3% H2O2 (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.) for 10 min to inhibit
endogenous peroxide activity, washed with distilled water and
washed with PBS for 3 min (four times). The samples were then
incubated in MTSS1 antibody at 37°C for 45 min. Subsequently, the
sections were washed with distilled water, then washed with PBS for
3 min (four times). The samples were then incubated with the
secondary antibodies at 37°C for 25 min. Subsequently, the sections
were washed with distilled water and PBS for 3 min (four times).
Freshly prepared DAB chromogen was dropped onto the slides, which
were then incubated at 37°C for between 3 and 5 min. The sections
were then counterstained in hematoxylin (Beijing Yili Fine
Chemicals, Co., Ltd., Beijing, China) (30 sec), washed with
distilled water, differentiated with 1% hydrochloric acid alcohol,
washed with distilled water and dehydrated in ascending grades of
methanol (Beijing Yili Fine Chemicals, Co., Ltd.) prior to clearing
in xylene (Beijing Yili Fine Chemicals, Co., Ltd.) and mounting
under a cover slip.
Immunohistochemical result
determination
Positive reaction products were indicated by yellow,
tan or brown particulates in the cytoplasm. Each section was
analyzed by two pathologists who selected five fields under
high-power magnification and scored each field according to the
staining intensity and the percentage of positive cells (secondary
scoring method). The number, expression intensity, shape and
distribution characteristics of the brown particles were observed
with an optical microscope [Nikon ECLIPSE 80i; Nikon Instruments
(Shanghai) Co., Ltd., Shanghai, China]. The coloring intensity of
cells was graded as 0 (no staining), 1 (faint yellow), 2 (yellow or
deep yellow) or 3 (tan or brown). The specimens were also grouped
into categories as determined by the percentage of positive cells:
0 = 0–4%, 1 = 5–25%, 2 = 26–50%, 3 = 51–75% or 4 = 76–100%. When
the product of the staining intensity and the percentage of
positive cells was >3, the specimen was defined as
immune-positive. The staining results were divided into four grades
according to the product score: − (negative, 0–2), + (weakly
positive, 3–5), ++ (moderately positive, 6–8) or +++ (strong
positive, 9–12).
Statistical analysis
All statistical analyses were performed using SPSS
20.0 software (IBM, Armonk, NY, USA). Comparisons between groups
were analyzed using χ2 tests and Fisher’s exact
probability tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
MTSS1 expression in cervical tissues
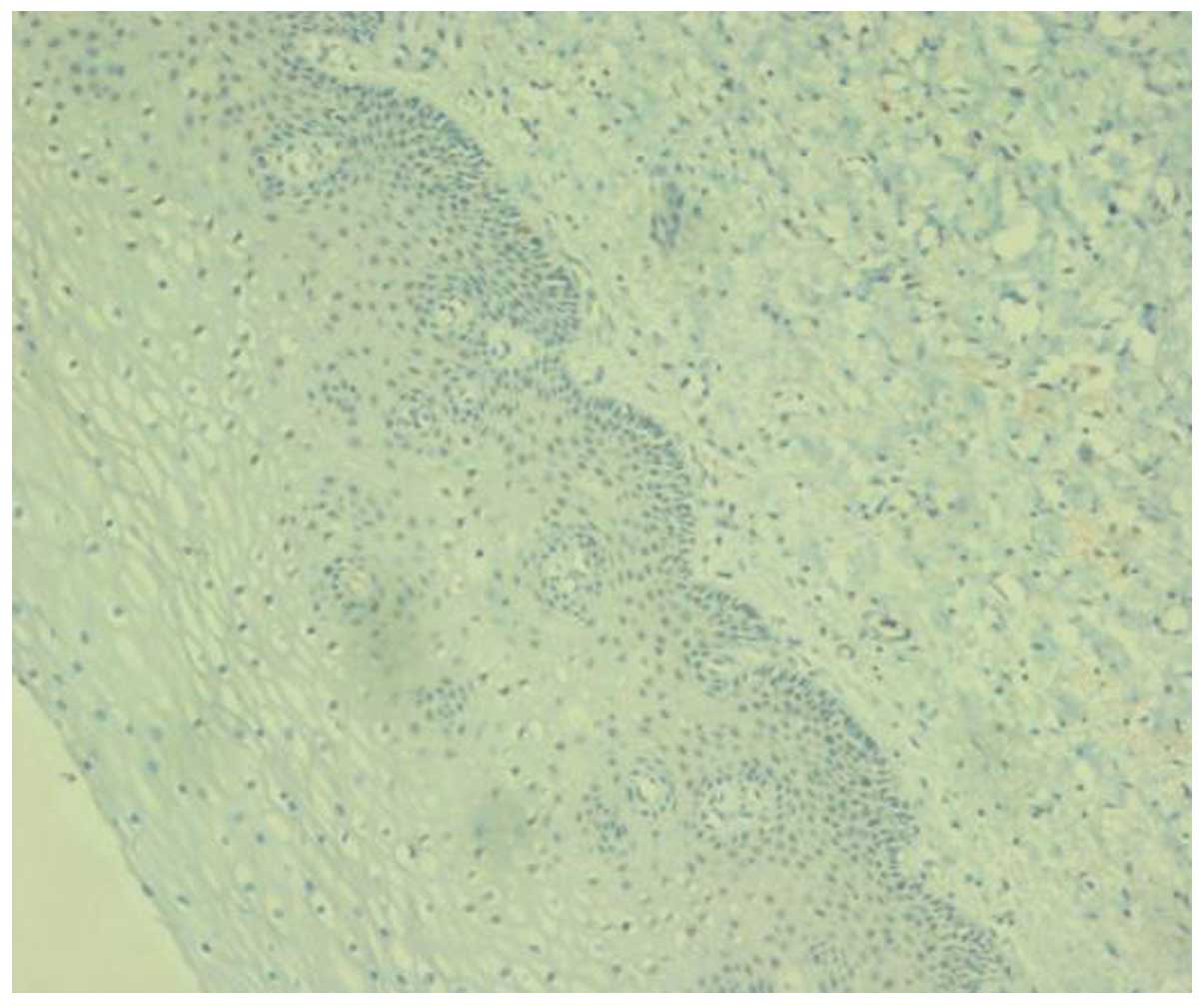
MTSS1 expression in the cytoplasm of benign cervical
epithelial cells was predominantly negative or occasionally weakly
positive. Approximately one-third of the cells in the CIN I
specimens exhibited weakly positive staining for MTSS1 in the
epithelium. The epithelial cells in the CIN II–III specimens
exhibited moderate to strong MTSS1 expression, and expression was
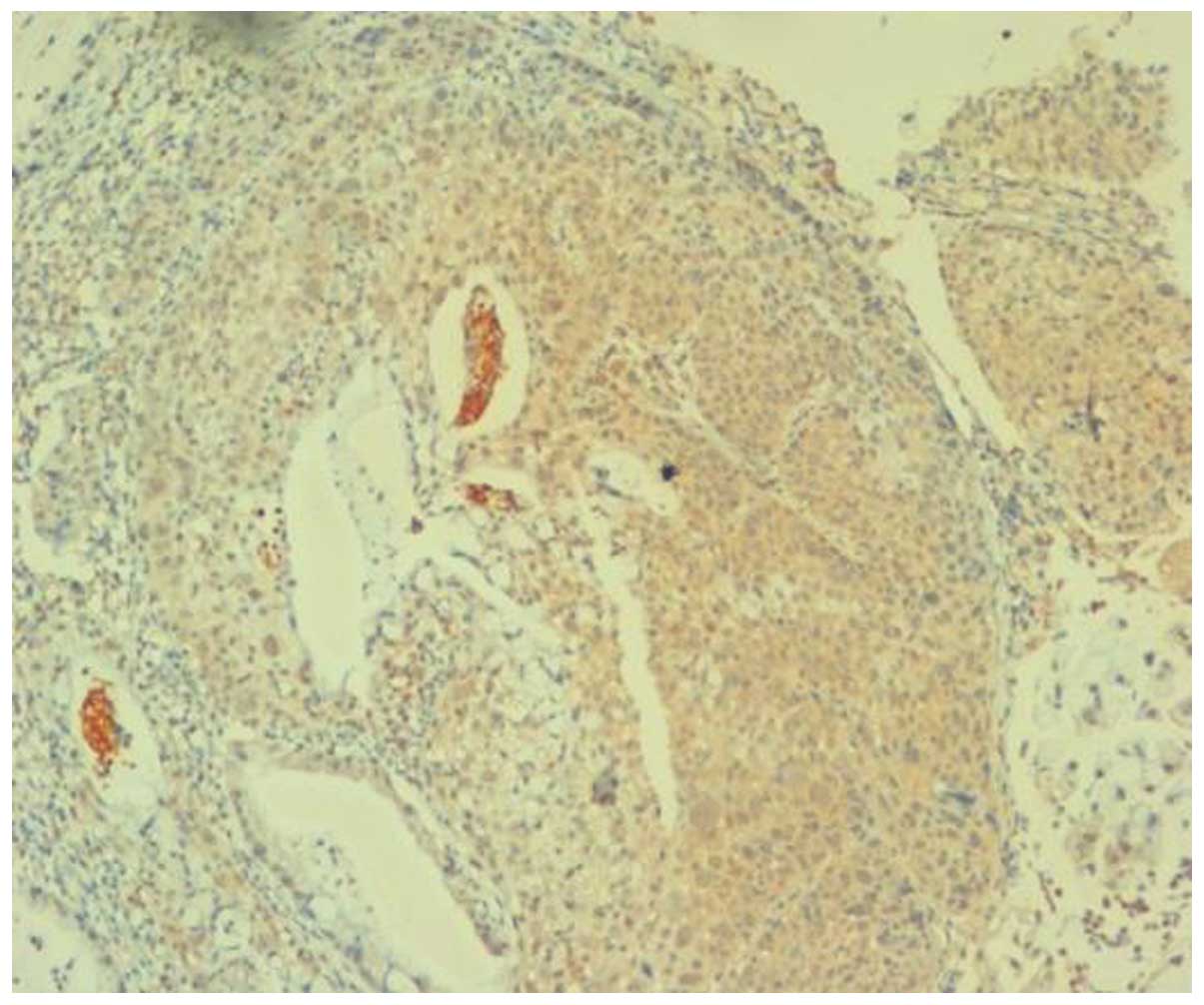
detected over the entire epithelial layer (Figs. 1–5).
Within the cervical cancer group, early-stage cervical cancer
tissues exhibited moderate to strong MTSS1 expression, and middle-
and advanced-stage cervical cancer tissues exhibited strong
positive MTSS1 expression.
Cytoplasmic expression of MTSS1 was significantly
greater in cervical carcinoma and CIN II–III tissues than in normal
cervical tissues (χ2=40.000, P<0.01). The MTSS1
positive expression rate in CIN II–III tissues was also
significantly higher than that of the CIN I tissues
(χ2=23.721, P<0.01). However, no significant
differences in cytoplasmic MTSS1 expression were identified between
CIN I and normal cervical tissues (χ2=3.774, P>0.05;
Table I). However, cytoplasmic
expression of MTSS1 was significantly higher in cervical carcinoma
tissues when compared with normal cervical tissues
(χ2=62.971, P<0.001).
 | Table ICytoplasmic MTSS1 expression in
cervical tissues from each group. |
Table I
Cytoplasmic MTSS1 expression in
cervical tissues from each group.
| | MTSS1 expression |
|---|
| |
|
|---|
| Group | n | −, n | +, n | ++, n | +++, n | Positive rate, % |
|---|
| Normal cervix | 30 | 24 | 6 | 0 | 0 | 20.00a |
| CIN I | 30 | 17 | 11 | 2 | 0 | 43.33b |
| CIN II–III | 30 | 0 | 2 | 15 | 13 | 100.00c |
| Cervical cancer | 57 | 0 | 0 | 20 | 37 | 100.00d |
Correlation between MTSS1 expression and
clinicopathological characteristics of cervical cancer
Among the 57 cases of cervical squamous carcinoma,
the strong positive MTSS1 expression rate was significantly higher
in middle- and advanced-stage cervical cancer specimens than in
early stage cervical cancer specimens (χ2=42.043,
P<0.05). However, the strong positive expression rate of MTSS1
was not correlated with patient age, tumor differentiation or the
presence of lymphatic metastasis (P>0.05, Table II).
 | Table IIAssociations between MTSS1 expression
and clinicopathological factors in cervical cancer patients. |
Table II
Associations between MTSS1 expression
and clinicopathological factors in cervical cancer patients.
| | MTSS1 expression |
|---|
| |
|
|---|
| Clinicopathological
factor | n | −, n | +, n | ++, n | +++, n | Strong positive
expression, % |
|---|
| Age, years |
| <60 | 35 | 0 | 0 | 13 | 22 | 62.86a |
| ≥60 | 22 | 0 | 0 | 7 | 15 | 68.18 |
| Clinical stage |
| I–IIa | 23 | 0 | 0 | 14 | 9 | 39.13b |
| IIb–IV | 34 | 0 | 0 | 6 | 28 | 82.35 |
| Differentiation |
| Well | 12 | 0 | 0 | 5 | 7 | 58.33c |
| Moderate to low | 45 | 0 | 0 | 15 | 30 | 66.67 |
| Lymphatic
metastasis |
| Negative | 48 | 0 | 0 | 17 | 31 | 64.58d |
| Positive | 9 | 0 | 0 | 3 | 6 | 66.67 |
Discussion
MTSS1, which maps to the 8q24.1 chromosomal region,
is a gene that was first identified by Lee et al (7) when mRNA differential display
technology was used to investigate the metastatic mechanism of
bladder cancer. MTSS1 is expressed in normal bladder tissue and
non-metastatic bladder cancer, but is deleted in metastatic bladder
cancer and, therefore, was originally defined as a metastasis
suppressor gene. Studies examining prostate cancer have also
revealed that the overexpression of MTSS1 clearly inhibits cell
metastasis, growth and adherence (8,9). Xie
et al (10) demonstrated
that MTSS1 expression levels in esophageal squamous cell carcinoma
patients were significantly lower in high TNM stage tumors than in
low TNM stage tumors, and MTSS1 expression levels were also lower
in patients with lymph node metastasis than in those without. A
gastric cancer study in a Chinese population also revealed that the
expression levels of MTSS1 were increased in tissues adjacent to
carcinoma, but were clearly reduced in cancer tissues (11). In addition, the MTSS1 expression
levels were observed to be gradually reduced with decreasing
degrees of histological differentiation. Decreased MTSS1 expression
levels were significantly correlated with larger tumor size, deeper
invasion, increased lymphatic metastasis and advanced tumor stage
(11). In a study on human breast
cancer, strong positive expression of MTSS1 was detected in normal
breast tissue, which was significantly reduced or even completely
absent in breast cancer tissues, and increased levels of MTSS1
expression were found to inhibit breast cancer cell growth,
invasion and metastatic potential (12). These studies suggest that MTSS1 acts
as a tumor metastasis suppressor gene in these malignancies.
In recent years, studies have found that MTSS1
exhibits tissue-specific expression, and increased MTSS1 expression
has been associated with tumorigenesis in certain other types of
malignant tumor, such as hepatocellular carcinoma (13), colorectal cancer (14), and head and neck squamous cell
carcinoma (15). Ma et al
(13) proposed that MIM-B (a MIM
homologous isomer) may be an early predictor of liver cancer.
Mattila et al (16)
suggested that, instead of acting as a tumor metastasis suppressor
gene, MTSS1 is a type of scaffolding protein that interacts with
the oncogene Rac, actin and pseudopodia formation-related proteins.
Lee et al (7) observed low
expression levels of MTSS1 in the uterus. However, the expression
and significance of MTSS1 have not been reported in precancerous
cervical lesions or cervical cancer. In the present study, MTSS1
protein expression in CIN, cervical cancer and normal cervical
tissues was examined by immunohistochemistry. The results revealed
negative or weak expression of MTSS1 in the majority of normal
cervical specimens. The MTTS1 positive expression rate and MTSS1
intensity were gradually increased along with an increased degree
of precancerous cervical lesions. The positive expression rate of
MTSS1 was significantly higher in CIN II–III and cervical cancer
tissues than in CIN I and normal cervical tissues (P<0.05),
suggesting that MTSS1 may be important in cervical carcinogenesis.
Furthermore, the strong positive expression of MTSS1 was
significantly higher in middle- and advanced-stage cervical cancer
than in early stage cancer (P<0.05). This indicates that MTSS1
may be involved in cervical cancer invasion and metastasis, a
finding consistent with those from studies regarding hepatocellular
carcinoma and colorectal cancer tissues (13,14).
Studies examining hepatocellular carcinoma and colon cancer also
revealed that the expression of MTSS1 was positively associated
with lymph node metastasis (9,10), but
the results from the present study demonstrate no statistically
significant difference in the MTSS1 expression levels between
patients with lymphatic metastasis and those without. The lack of
significance may be due to the limited number of cervical cancer
samples; therefore, further confirmation in a larger sample group
is required.
The mechanism by which MTSS1 is involved in cervical
cancer development remains unclear. However, certain previous
studies have provided insight into the possible underlying
mechanisms. Wang et al (17)
observed that tyrosine phosphorylation of the MTSS1 protein may be
involved in platelet-derived growth factor-mediated cell shape
changes. Therefore, MTTS1 may be part of a novel signaling pathway
that connects platelet-derived growth factor signaling and the
actin cytoskeleton through the tyrosine kinase, Src, which
regulates novel actin-related proteins, such as MTSS1 (18). Another possible mechanism involves
the potential association of MTSS1 expression in cervical cancer
with hedgehog (Hh)-Gli signaling pathways. A previous study
demonstrated that MTSS1 is a novel Hh-Gli signal response gene
implicated in Gli regulation of tumorigenesis (19). Furthermore, Xuan et al
(20) demonstrated active Hh-Gli
signaling in cervical cancer and that expression levels of the
Hh-Gli signaling protein were higher in CIN than in normal cervical
tissue, suggesting that MTSS1 may be involved in the Hh-Gli
signaling pathway through interaction with Gli, allowing the
occurrence and development of cervical lesions. In addition, in
head and neck squamous cell carcinoma cells, MTSS1 was found to
interact with the epidermal growth factor (EGF), strengthen the
localization of the EGF receptor in the cell membrane, prolong the
Erk signal and promote cell proliferation (15). In cervical cancer, EGF was found to
promote lamellipodial stretching by autocrine or paracrine
mechanisms, thus promoting cervical cancer cell invasion (21). Elucidation of the specific mechanism
of MTSS1 involvement in cervical cancer requires further studies
using cervical cancer cell lines.
In conclusion, in the present study, MTSS1 was found
to be highly expressed in CIN II–III and cervical cancer tissues,
and MTSS1 expression levels were positively correlated with the
clinical stage of cervical cancer. This provides a basis for
further investigation of the biological role of MTSS1 in the
development and progression of cervical cancer. MTSS1 may be a
novel diagnostic biomarker or a therapeutic target in cervical
cancer.
References
|
1
|
Dell G and Gaston K: Human
papillomaviruses and their role in cervical cancer. Cell Mol Life
Sci. 58:1923–1942. 2001.
|
|
2
|
Kelley LC, Shahab S and Weed SA: Actin
cytoskeletal mediators of motility and invasion amplified and
overexpressed in head and neck cancer. Clin Exp Metastasis.
25:289–304. 2008.
|
|
3
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophys Acta. 1773:642–652. 2007.
|
|
4
|
Haslehurst AM, Koti M, Dharsee M, Nuin P,
Evans K, et al: EMT transcription factors snail and slug directly
contribute to cisplatin resistance in ovarian cancer. BMC Cancer.
12:912012.
|
|
5
|
Huang XY, Huang ZL, Tang ZY, Zheng Q and
Ye SL: Role of proteins of missing in metastasis in cancer
initiation and progression. Tumor. 30:170–172. 2010.
|
|
6
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009.
|
|
7
|
Lee YG, Macoska JA, Korenchuk S and Pienta
KJ: MIM, a potential metastasis suppressor gene in bladder cancer.
Neoplasia. 4:291–294. 2002.
|
|
8
|
Du P, Ye L, Ruge F, Yang Y and Jiang WG:
Metastasis suppressor-1, MTSS1, acts as a putative tumour
suppressor in human bladder cancer. Anticancer Res. 31:3205–3212.
2011.
|
|
9
|
Mustafa N, Martin TA and Jiang WG:
Metastasis tumour suppressor-1 and the aggressiveness of prostate
cancer cells. Exp Ther Med. 2:157–162. 2011.
|
|
10
|
Xie F, Ye L, Chen J, et al: The impact of
Metastasis Suppressor-1, MTSS1, on oesophageal squamous cell
carcinoma and its clinical significance. J Transl Med.
9:952011.
|
|
11
|
Liu K, Wang G, Ding H, et al:
Downregulation of metastasis suppressor 1(MTSS1) is associated with
nodal metastasis and poor outcome in Chinese patients with gastric
cancer. BMC Cancer. 10:4282010.
|
|
12
|
Parr C and Jiang WG: Metastasis suppressor
1 (MTSS1) demonstrates prognostic value and anti-metastatic
properties in breast cancer. Eur J Cancer. 45:1673–1683. 2009.
|
|
13
|
Ma S, Guan XY, Lee TK and Chan KW:
Clinicopathological significance of missing in metastasis B
expression in hepatocellular carcinoma. Hum Pathol. 38:1201–1206.
2007.
|
|
14
|
Wang D, Xu MR, Wang T, Li T and Zhu JW:
MTSS1 overexpression correlates with poor prognosis in colorectal
cancer. J Gastrointest Surg. 15:1205–1212. 2011.
|
|
15
|
Dawson JC, Timpson P, Kalna G and Machesky
LM: MTSS1 regulates epidermal growth factor signaling in head and
neck squamous carcinoma cells. Oncogene. 31:1781–1793. 2012.
|
|
16
|
Mattila PK, Salminen M, Yamashiro T and
Lappalainen P: Mouse MIM, a tissue specific regulator of
cytoskeletal dynamics interacts with ATP-actin monomers through its
C-terminal WH2 domain. J Biol Chem. 278:8452–8459. 2003.
|
|
17
|
Wang Y, Zhou K, Zeng X, Lin J and Zhan X:
Tyrosine phosphorylation of missing in metastasis protein is
implicated in platelet-derived growth factor-mediated cell shape
changes. J Biol Chem. 282:7624–7631. 2007.
|
|
18
|
Murata T, Mizushima H, Chinen I, et al:
HB-EGF and PDGF mediate reciprocal interactions of carcinoma cells
with cancer-associated fibroblasts to support progression of
uterine cervical cancers. Cancer Res. 71:6633–6642. 2011.
|
|
19
|
Callahan CA, Ofstad T, Horng L, et al:
MIM/BEG4, a Sonic hedgehog-responsive gene that potentiates
Gli-dependent transcription. Genes Dev. 18:2724–2729. 2004.
|
|
20
|
Xuan YH, Jung HS, Choi YL, et al: Enhanced
expression of hedgehog signaling molecules in squamous cell
carcinoma of uterine cervix and its precursor lesions. Mod Pathol.
19:1139–1147. 2006.
|
|
21
|
Narayanan R, Kim HN, Narayanan NK, Nargi D
and Narayanan B: Epidermal growth factor-stimulated human cervical
cancer cell growth is associated with EGFR and cyclin D1
activation, independent of COX-2 expression levels. Int J Oncol.
40:13–20. 2012.
|