Introduction
Nasal-type extranodal natural killer (NK)/T-cell
lymphoma (ENKTCL-N) is an independent-type disease classified as a
lymphoid neoplasm by the World Health Organization-European
Organization for Research and Treatment of Cancer (WHO-EORTC) 2005
standard (1) (the 2008 standard
being the final standard). ENKTCL-N most commonly involves the
nasal cavities, paranasal sinuses and nasopharynx (2). This disease is characterized by
clinical rarity, rapid progression and a high mortality rate, but
its uncharacteristic early clinical manifestations and infrequent
skin involvement tend to result in missed diagnoses and
misdiagnosis (3). The present study
reports a case of ENKTCL-N involving the skin that was diagnosed at
the Department of Dermatology, the Second Affiliated Hospital of
Xi’an Jiaotong University (Xi’an, China), combined with a
literature review. Written informed consent was obtained from the
patient for the publication of the present study and any associated
images.
Case report
Patient and case history
A 56-year-old male presented to the Department of
Dermatology, the Second Affiliated Hospital of Xi’an Jiaotong
University with a nodule on the left side of the nose, which had
been progressively enlarging for one year. One year previously, a
pimple approximately the size of a grain of rice had appeared in
the patient’s left nasal cavity without evident inducement. The
pimple caused no itching or pain, and the corresponding external
skin on the nose exhibited no noticeable change, so the patient
paid no attention to the pimple. Subsequently, the nodule gradually
grew. Six months prior to the current presentation, the patient
noticed that the nodule was locally red, swollen and erosive, and
the patient felt mild nasal congestion and had difficulty
breathing. The patient was diagnosed with chronic nasosinusitis at
the Ear, Nose and Throat Department of a local hospital. Subsequent
to receiving cephalosporin antibiotics as an anti-inflammatory
treatment, the redness and swelling subsided, but the nodule
remained. Afterwards, the nodule continued to enlarge progressively
and grew internally and externally, accompanied by hyperemia and
swelling of the corresponding skin outside the nose. Three months
prior to the current presentation, upon agitation by the patient,
the nodule ulcerated and oozed blood, with locally damaged skin,
deteriorated nasal obstruction and a large amount of purulent nasal
discharge. However, the patient did not experience itching and pain
or systematic symptoms, such as fever and emaciation. The patient
returned to the local hospital. This time, the patient was
diagnosed with a nasal fungal infection and received oral
itraconazole (200 mg, twice a day) for two weeks. Although the
local swelling was somewhat alleviated and the bleeding stopped,
the nodule did not significantly decrease in size. The subsequent
external use of topical amphotericin B (twice a day, for one week)
slightly mitigated the condition and the patient arrived at our
hospital for a diagnostic examination. Since the onset of the
symptoms, the patient had experienced no changes in mood, diet,
sleep, urination or defecation, and no marked changes in body
weight. Prior to the onset of the condition, the patient had no
history of local trauma. The patient was normally healthy and had
smoked for 38 years (20 cigarettes/day). The patient’s mother had
previously been diagnosed with colorectal cancer and the patient’s
son had been diagnosed with a pituitary tumor. The remaining family
members were all healthy and no similar diseases were reported.
Physical examination
The patient possessed stable vital signs, the
systemic superficial lymph nodes, liver and spleen were not
involved in the disease, and the systematic examination revealed no
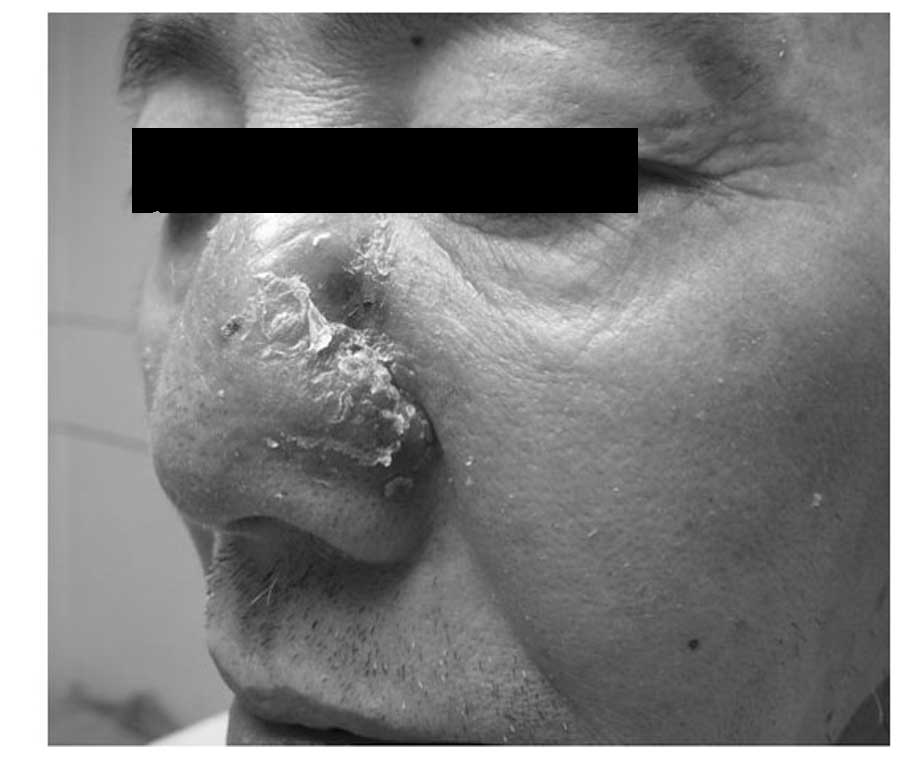
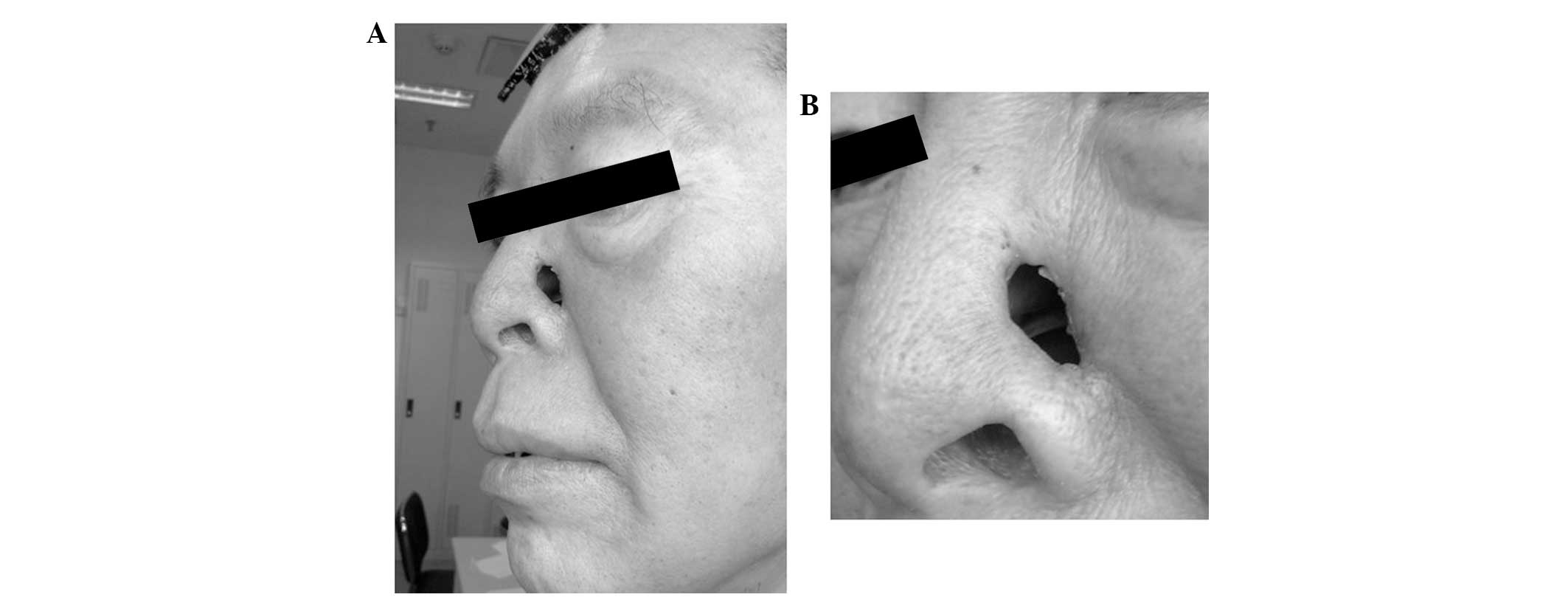
evident abnormalities. A dermatological examination revealed that
the nose was asymmetric and that the right side was normal. Wet red
plaques (~4×5 cm) were present on the left side of the nose, with
scales on the surface that were hard and painful when touched,
without crepitus or fluctuation. Irregular defects, ~1 cm long,
reached the nasal cavity, with a small number of purulent
secretions on the surface (Figs. 1
and 2). The left nasal cavity
exhibited numerous sticky black scabs that were difficult to
remove, tended to bleed and generated malodorous secretions, which
the patient could not detect, and the structure of the nasal cavity
was indiscernible. The right nasal cavity had a small number of
scabs and secretions and exhibited mucosal atrophy. The
nasopharyngeal mucosae were smooth and retained a large number of
mucus secretions. The left maxillary sinus area became swollen and
painful when pressed.
Auxiliary examination
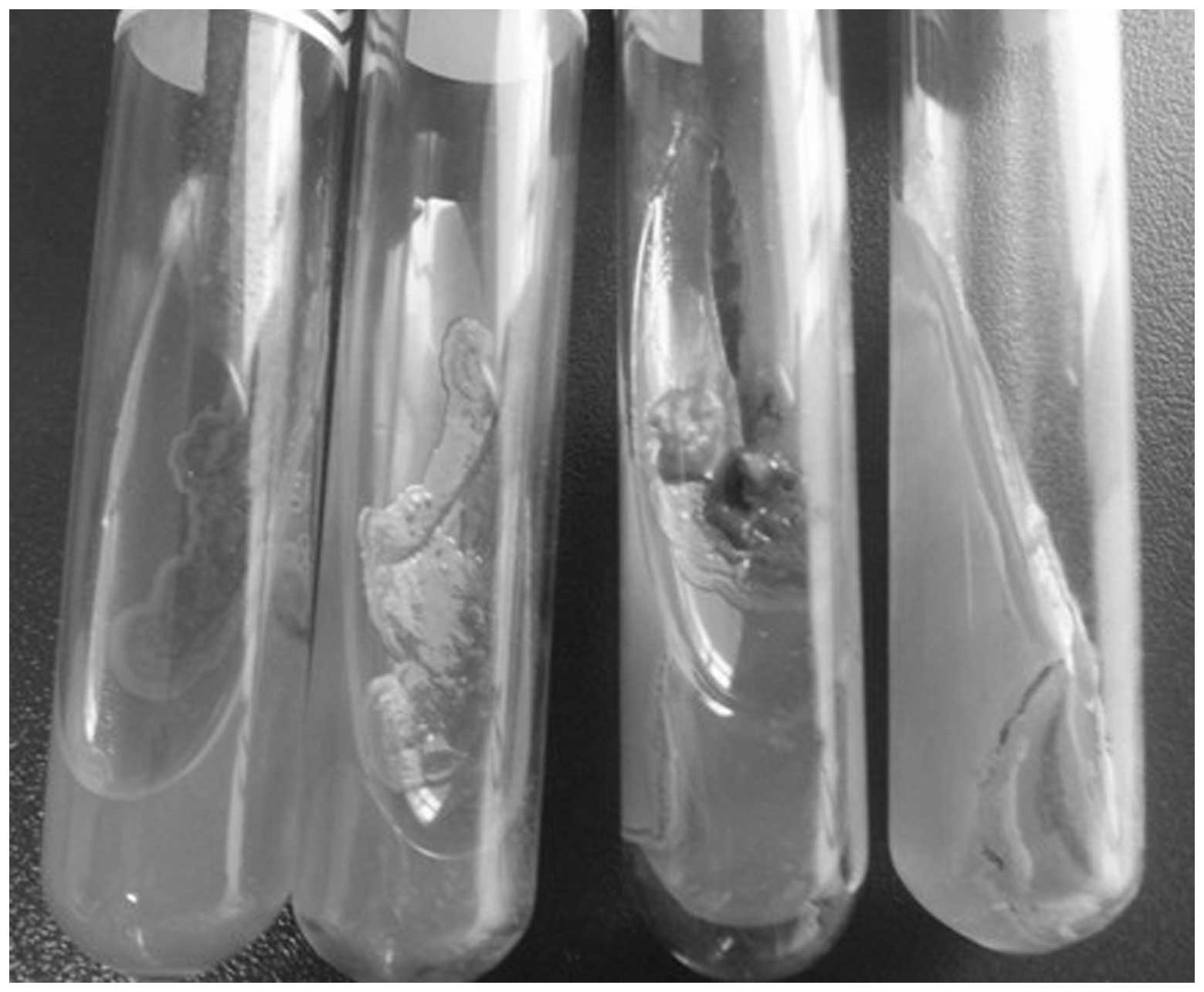
Fungal cultures of the nasal secretions and the
lesion tissue blocks each revealed negative results (Fig. 3). Paranasal sinus computed
tomography (CT) plain scanning revealed that the soft tissue of the
lateral wall of the left nasal cavity was thickened, with a
discontinuous shape, and that its inner density was moderate. The
left middle nasal meatus and a section of the right nasal cavity
were involved. The subcutaneous soft tissues of the left zygomatic
arch was swollen and the mucosae of the left ethmoid sinus and the
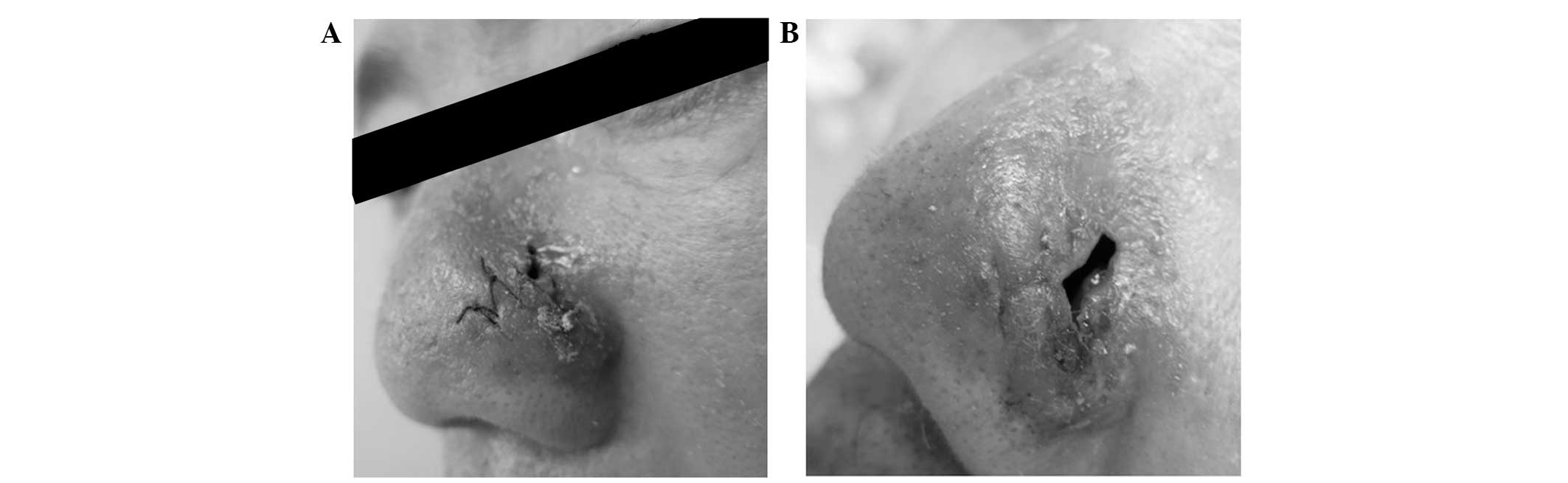
maxillary sinus were thickened. Two weeks later, the lesion had
improved, however the size of the ulcer had increased (Fig. 4).
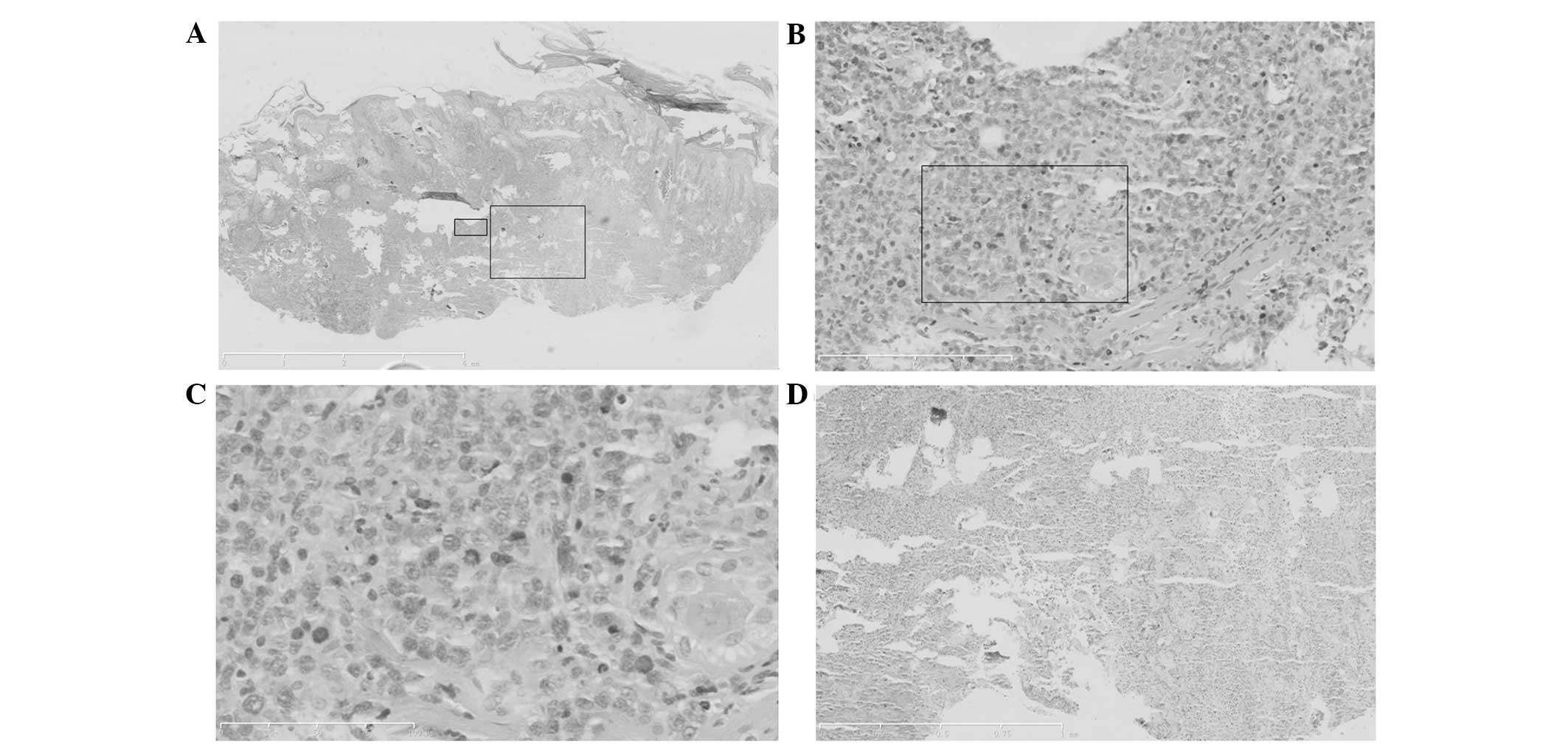
Histopathological changes
A section of the epidermis was necrotic and erosive.
The side with the lesion exhibited hyperkeratosis, parakeratosis
and epidermal cutin extension. Numerous abnormal lymphoid and
plasma cells, and extensive neutrophile granulocyte infiltration
could be observed at the depth of the dermis and the subcutaneous
adipose tissue, together with large areas of necrosis and commonly
observed broken nuclei. Diffuse infiltration was present. The
abnormal lymphoid and plasma cells were of various sizes, but
mainly medium and large. The cells were characterized by a thick
nuclear membrane, fine and smooth chromatin, inconspicuous
nucleoli, commonly observed nuclear fission and an angiotropic
phenomenon (Fig. 5).
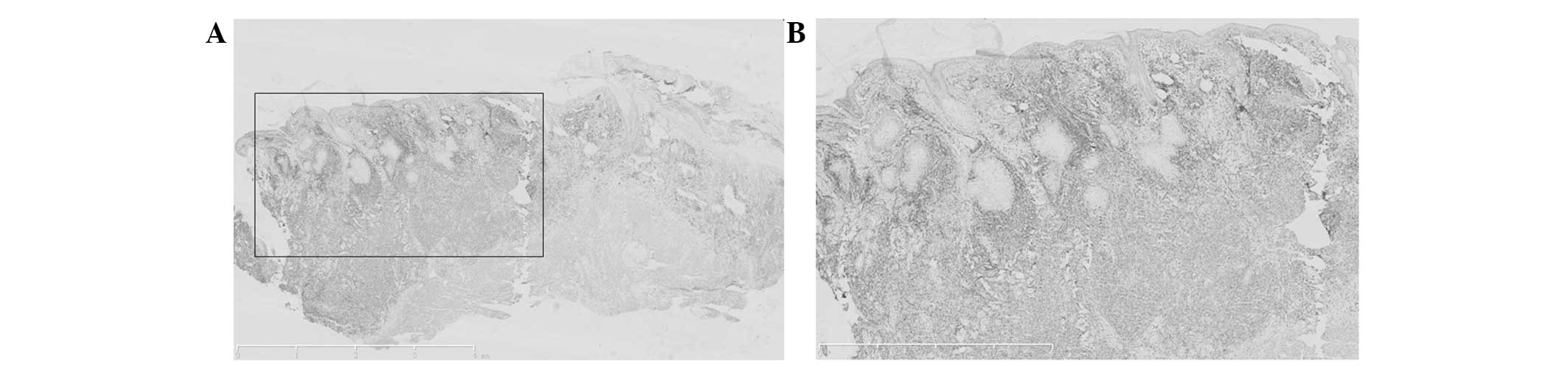
Immunohistochemistry
The tumor cells revealed a positive immunoreaction
for cluster of differentiation (CD)3ɛ, CD56, Epstein-Barr virus
(EBV)-encoded small RNAs, granzyme B and TIA1, with a Ki67 of
60–70%. The immunoreaction was negative for CD5 and CD20 (Figs. 6–12).
Diagnosis
The patient was diagnosed with ENKTCL-N
(super-cavity stage IE) (4,5).
Treatment
Radiotherapy treatment was adopted following the
confirmation of the diagnosis. The radiotherapy program was carried
out using a Siemens ONCOR linear accelerator (Siemens, Munich,
Germany) for image-guided radiotherapy (IGRT). The gross tumor
volume (GTV) consisted of the left nasal cavity lesion visible on
CT. The clinical target volume (CTV) consisted of the GTV and the
right nasal cavity, the wings of the nose, the left maxillary sinus
and the ethmoid sinus. The planning target volume (PTV) consisted
of the CTV and the surrounding 0.5-cm area. Three-field isocentric
irradiation, with the PTV as the target, was conducted at 2
Gy/fraction, 5 times/week, with a total of 60 Gy/30 fractions.
During the treatment, the patient was required to look straight
ahead, with an open mouth and a tongue depressor in place, and with
fillers in the nose to improve the GTV skin dose. During the
radiotherapy, eye cleaning and local washing of the GTV were
intensified. In addition, the patient topically applied mupirocin
(twice a day, for two weeks) to diminish inflammation, received
oral vitamin C (200 mg, twice a day) and vitamin B (5 mg, twice a
day) to alleviate the mucosa reaction and used Traditional Chinese
Medicine to strengthen the body’s resistance and to maintain
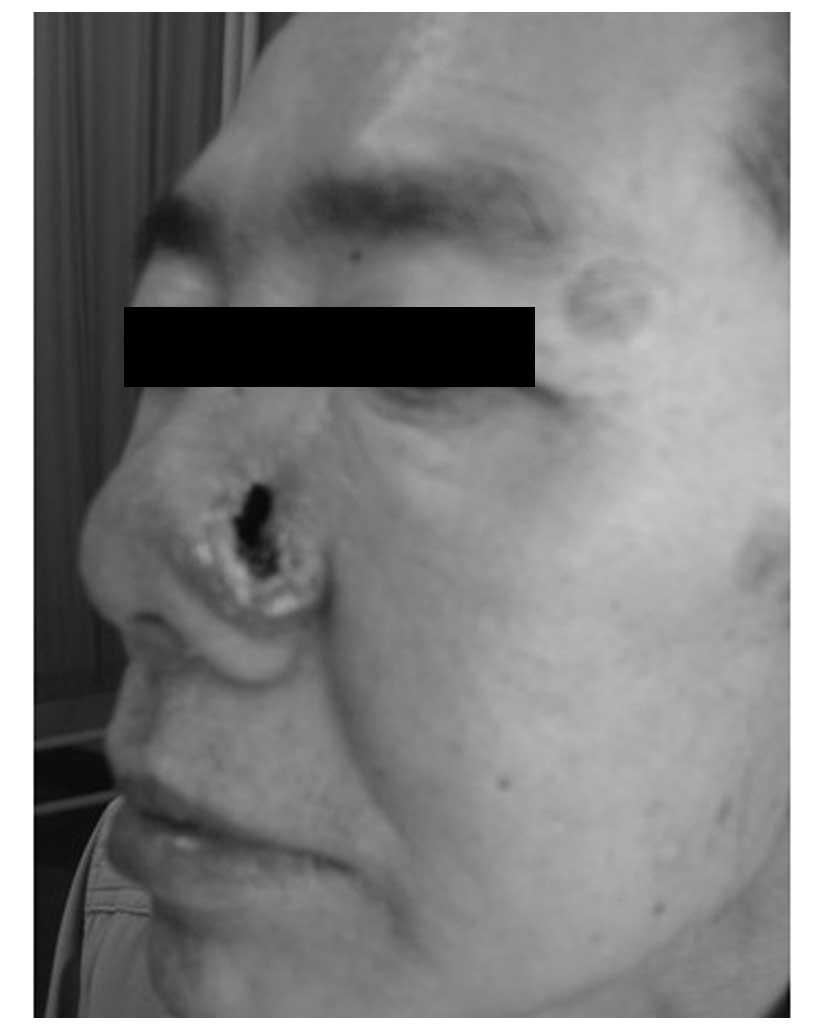
vitality. Following the radiotherapy, the patient experienced
normal health. The skin of the left wing of the nose was normal,
except for a 1×0.5-cm regular defect reaching the nasal cavity,
from which the patient felt no pain when pressure was applied. The
mucosae in each of the nasal cavities were smooth. An oval defect
with an area of ~1×2 cm2 could be observed beneath the
left wing of the nose and the scar had healed two weeks following
treatment (Fig. 13). Following the
treatment, the patient achieved complete remission (CR), with no
relapse during the six months of clinical follow-up.
Discussion
According to the 2008 WHO-EORTC classification of
lymphoid neoplasms (1), ENKTCL-N
belongs to the category of mature T-cell and NK cell lymphomas.
Since the vast majority of tumor cells express a NK
cell phenotype and only a small number of cells express a cytotoxic
T-cell phenotype, the majority of ENKTCL-Ns are considered to be
derived from mature NK cells, and only a fraction of them derive
from NK T cells (6). Therefore, the
condition has been termed NKTCL.
ENKTCL-N occurs predominantly in middle-aged males,
with a male to female ratio of 3:1 and a median age of 44 years.
The disease is more common in Asia, particularly in China and
Japan, and South America, where it accounts for 7–10% of all
non-Hodgkin’s lymphomas. ENKTCL-N is less common in Western
countries, accounting for 1% of all non-Hodgkin’s lymphomas
(7), suggesting a geographic or
ethnic susceptibility to the disease.
EBV is a human DNAγ herpes virus. Latent membrane
protein-1 (LMP-1) is an identified tumor protein encoded by EBV,
which plays a key role in regulating the process of the malignant
transformation of cells (8).
Present studies suggest that patients with NKTCL-Ns have an EBV
infection rate of >90%, while the infection rate of patients
with non-nasal NKTCLs exhibits a downward trend (9,10).
Certain studies have hypothesized that EBV infection is
site-dependent, but there are also findings that are not consistent
with this viewpoint (11–13). NKTCL predominantly occurs in the
nasal cavities, and the upper respiratory tract is the infection
channel of EBV, so the EBV detection rate is relatively high
(14). Therefore, it is necessary
to further investigate and discuss whether EBV infection is
site-dependent or if it has a high affinity for this type of
lymphoma.
Pesticides and chemical solvents can be observed as
possible factors in the pathogenesis of NKTCL, suggesting that
lifestyle and environmental factors are closely associated with
NKTCL (12).
Currently, the focus of the investigation into the
abnormal aspects of the NKTCL-N chromosome includes deletion on the
short arm of chromosome 6, deletion on the long arm of chromosome
1, extension of the short arm of chromosome 2 and occasionally
includes isochromosome 7q (15,16).
In addition, the p53 gene mutation rate in NKTCL-N is 24–48%,
higher than those of the other lymphomas, but there is no
correlation between EBV and p53 mutation (17). Fas is a cell surface receptor that
participates in cell death signaling by binding the Fas ligand, and
mutation of the Fas gene often results in the accumulation of
lymphocytes, thus causing tumors (18). A previous study reported that half
of a group of 14 NKTCL-N cases possessed a mutation in the Fas
gene, suggesting that Fas gene mutation is the pathological basis
of NKTCL and that its mechanism of action involves the inhibition
of the apoptosis of lymphocytes (19). Other genetic abnormalities include
abnormal expression of c-kit, β-catenin and other proto- and
anti-oncogenes, B-cell lymphoma (Bcl-2) protein overexpression, low
or negative expression of the p73 gene, a novel member of the p53
gene family, and upregulated survivin gene expression, which is
negatively correlated with the apoptosis index. The association
between these gene mutations and EBV remains unclear (20).
Ki-67 is highly expressed in ENKTCL-N, with a much
higher expression level compared with the level in follicular
lymphoma (P<0.001). The Ki-67-positive rate of tumor cells in
the majority of ENKTCL-N patients is >60% (19). Certain studies have identified that,
among ENKTCL-N patients, the higher the Ki-67 proliferation index,
the worse the prognosis (22).
Other relevant abnormal expression proteins have also been
reported, including nuclear factor κB, chemokine ligand 9,
G-protein signaling regulator 2, Bcl-2, induced myeloid leukemia
cell differentiation protein, platelet-derived growth factor
receptor and ubiquitin activating enzyme E1 (23–27).
The clinical features of ENKTCL-N occurring in the
nasal cavities, include the clinical symptoms of nasal obstruction,
rhinorrhea with blood or epistaxis, tinnitus, hoarseness,
pharyngalgia and discomfort whilst swallowing. The physical signs
of ENKTCL-N include redness and swelling, ulceration and even
penetration of the local tissues, or tumefied lumps that are often
malodorous (28). Progressive
development may involve the eye sockets, cheeks and frontal bone.
The most prominent facial feature is damage to the midline areas,
including nasal septum perforation, hard palate perforation and
nasal bridge perforation (3). This
disease has rapid clinical progression and extremely high
invasiveness, and the subsequent diffusion may involve the skin,
gastrointestinal tract, testes, central nervous system, spleen and
other organs and tissues, but rarely the lymph nodes (29). The bone marrow and circulatory
system may also be involved; in this situation, the disease
overlaps with aggressive NK-cell leukemia, which is actually the
end-stage manifestation of the disease, and the two have the same
immunophenotype and genotype (28).
In the present study, ENKTCL-N involved the skin, leading to a skin
ulcer and perforation, and the patient only received radiotherapy,
with no relapse occurring within seven months.
ENKTCL-N exhibits numerous histological
characteristics. The tumor cell infiltration position is deep,
mainly in the dermal and subcutaneous adipose tissues, and the
tumor cells exhibit microvascular invasion and damage, with
coagulative necrosis and apoptosis being common. The tumor cells
vary in size, including small-, medium- and large-sized cells, with
the majority of cases being dominated by medium-sized cells
together with varying numbers of small- and large-sized cells
(30). The cells have irregular
nuclei and nuclear fission is commonly observed. There is often
infiltration of the inflammatory cells, including small
lymphocytes, neutrophils, histiocytes and eosinophils mixed in
neoplastic lymphocytes, so the disease tends to be misdiagnosed as
inflammation (2,31). Mraz-Gernhard et al (32) hypothesized that the angiocentric
invasion and pro-epidermal phenomenon was not a feature of NK/T and
that this was the most useful histological characteristic in
distinguishing cutaneous NKTCL from other cutaneous lymphomas
(32).
The immunophenotype of ENKTCL may be primarily
characterized by a lack of CD3 and CD20 expression, positive CD3ɛ,
CD2 and CD56 expression, positive TIA-1, granzyme B and perforin
expression and positive EBER expression.
The main basis for the diagnosis of ENKTCL-N is the
clinical pathogenic locations combined with the histopathological
and immunohistochemical examination results. However, the
uncharacteristic early manifestation of the disease tends to result
in misdiagnosis.
There are several reasons for misdiagnosis. The
first is that the early manifestation has no special features and
is dominated by nasal congestion, rhinorrhea, rhinorrhea with blood
and other chronic nasosinusitis symptoms. Additionally, due to the
small scope of early lesions and the indetectable lesion locations,
doctors and patients may pay no attention to them, and only conduct
simple treatment or even abandon treatment. Another reason is that
during pathological examination, the negative detection rate is
relatively high. The disease is angiotropic and tends to cause
vascular occlusion and consequent tissue necrosis within the scope
of the blood supply; improper sampling may lead to a large number
of necrotic tissues under the microscope. In addition, tumor cells
vary greatly and are not typical in size, including large-, medium-
and small-sized cells. In the case of concurrent infection and
over-small or over-shallow specimens, doctors may only see
inflammatory cells and often diagnose the disease as necrotic
tissue with inflammatory infiltration (33). A final reason is that lymph node
metastasis and distant spread is generally late-stage, and the
patients are in a generally good condition, so the disease-symptom
separation tends to render patients and doctors careless (33,34).
ENKTCL-N must be differentiated from several
diseases, including inflammatory lesions, which are characterized
by extensive inflammatory cell infiltration, with ulceration in
certain lesions, but without abnormal lymphocyte proliferation or
vascular invasion and damage. Hybridization in situ reveals
that the cells are negative for EBER expression. Additionally deep
mycoses must be considered. It is difficult to distinguish clinical
mycoses and deep mycoses. The upper section of the pathological
dermis consists of mixed cell infiltration, but the middle and
lower sections of the dermis have subcutaneous abnormal
lymphocytes, and immunohistochemistry and fungal culture aids
differentiation. There is mixed inflammatory cell infiltration,
with no abnormal cells, and the fungal culture is positive
(35).
ENKTCL-N must also be differentiated from Wegener’s
granulomatosis. The main feature of Wegener’s granulomatosis is
necrotic granulomatous inflammation and true vasculitis.
Inflammatory cells invade the entire vascular wall, causing elastic
fiber damage, vascular occlusion or fibrinoid necrosis, with no
abnormal tumor cells. Elastic fiber staining aids differentiation.
Furthermore, cutaneous γδ T-cell lymphoma requires differentiation.
Immunohistochemical investigation reveals the cells to be CD2-,
CD3- and CD7-positive, T-cell toxic granule-positive, and generally
CD4-, CD8-, and EBV-negative (36).
In addition, ENKTCL-N must be distinguished from aggressive
pro-epidermal CD8-positive cytotoxic T-cell lymphoma. The two
diseases are primary cutaneous peripheral T-cell lymphomas and are
rare. Pro-epidermal lymphoma is negative for EBV and positive for
CD8 expression (37). ENKTCL-N and
blastic plasmacytoid dendritic cell neoplasms must also be
differentiated. Blastic plasmacytoid dendritic cell neoplasms
mainly involve the skin, with leukemoid dissemination. The neoplasm
tumor cells exhibit positive CD4, CD56 and CD123 expression, but
are negative for CD3, TIA-1 and EBV expression. Adult T-cell
leukemia and lymphoma are dominated by peripheral T-cell tumors
composed of highly pleomorphic lymphoid neoplasms. The skin is the
most common site of extranodal involvement. Tumor cells express
CD25 and forkhead box P3 at the same time. Tumor involvement is
common in the peripheral blood, with immunophenotypes primarily
characterized by negative CD56 and EBER expression (38).
Patients with early-stage (Ann Arbor stage I–II)
NKTCL-N are more sensitive to radiotherapy compared with stage
III–IV patients, and single-line radiotherapy has a good effect and
is the main method of treatment (39,40).
The radiotherapy consists of a daily dose of 1.8–2.0/2.5 Gy (5
times/week), a total irradiation dose range of 20–70 Gy and a
median dose of 45 Gy. The irradiation fields include the primary
site and a sufficient edge region, as well as the nose, paranasal
sinuses, nasopharynx and pharyngeal lymph ring (Waldeyer’s ring)
(41,42). If the cervical lymph node is
involved, the irradiation range can be extended to the neck, and
where the nasopharynx or pharyngeal lymph ring is the primary site,
patients should receive conventional irradiation, regardless of
whether the cervical lymph nodes are involved (43). A previous study reported that, soon
after radiotherapy, patients achieved a CR, with a reported CR rate
of 70% and an effective rate of 80% (20). Compared with low-dose radiotherapy,
large-dose irradiation (≥50Gy) has a better effect, with five-year
local control rates of 100 and 67%, respectively; this difference
is statistically significant (P=0.013) (40).
Although radiotherapy has clear short-term effects,
it is not applicable to disseminated or relapsing cases, and a
large number of studies have revealed an extremely high long-term
relapse rate for single-line radiotherapy (range, 17–77%, with 50%
as the most commonly reported value). This indicates that
single-line radiotherapy may be insufficient for even stage I and
II patients, and current attention should be focused on whether the
combination of radiotherapy and chemotherapy can aid the
improvement of the curative effect (40).
For patients with early-stage (stage I and II)
ENKTCL-N, single-line radiotherapy retains high local relapse and
distant tumor dissemination rates (40,44).
Currently, these patients are mainly treated by combining
radiotherapy and chemotherapy, including radiotherapy followed by
chemotherapy, chemotherapy followed by radiotherapy, the sandwich
method (2–4 courses of chemotherapy, followed by radiotherapy and
then chemotherapy for the remaining courses of treatment) and
concurrent radiotherapy and chemotherapy (45). Theoretically, the combination of
radiotherapy and chemotherapy not only administers early-stage
radiotherapy to local lesions, but it also targets distant
potential metastatic lesions. You et al (46) investigated the treatment program for
NKTCL-N in 46 patients. The results revealed that chemoradiotherapy
is more effective compared with radiotherapy and chemotherapy. Yang
Yong et al (47) reviewed
and analyzed 18 cases of patients with early-stage ENKTCL-N. The 18
cases consisted of three cases of induction chemotherapy with
concurrent chemoradiotherapy and adjuvant chemotherapy, 13 cases of
induction chemotherapy with concurrent chemoradiotherapy, one case
of concurrent chemoradiotherapy with adjuvant chemotherapy and one
case of concurrent chemoradiotherapy. The study used sequential
chemoradiotherapy as the control. The results revealed five-year
overall survival rates of 80.8 and 54.3% in the concurrent
chemoradiation therapy and control groups, respectively, suggesting
that concurrent chemoradiotherapy may improve the long-term
survival of early-stage patients (46).
For patients with early-stage (stage III and IV)
ENKTCL-N, the treatment programs are dominated by systemic
chemotherapy, and radiotherapy is only used as an auxiliary control
for local lesions. However, for advanced-stage tumors, numerous
types of treatments are of poor curative effect, and stronger or
novel effective treatment programs require consideration (48).
The treatment of NKTCL by hematopoietic stem cell
transplantation (HSCT) remains in the exploratory stage. In recent
years, the method of high-dose chemotherapy followed by auto-HSCT
has achieved good results. Pre-HSCT high-dose chemotherapy can
mobilize a sufficient number of hematopoietic stem cells, kill
in vivo tumors, avoid the reinfusion of stem cells
contaminated by tumors and realize in vivo ‘purification’
(49). Niu et al (50) treated a female patient with invasive
ENKTCL-N by auto-HSCT. During the 67 months of follow-up, the
patient remained in CR.
Currently, the investigation into NK/T-cell lymphoma
targeted therapy has just begun; there has been no targeted drug
entering clinical trials, and bortezomib, a type of proteasome
inhibitor, has demonstrated curative effects in certain preliminary
studies (51). However, Kim et
al (52) reported the treatment
of 46 T-cell lymphoma patients using bortezomib combined with the
cyclophosphamide, doxorubicin, vincristine and prednisolone
chemotherapy program, including 10 cases of ENKTCL-N, eight cases
of angioimmunoblastic T-cell lymphoma, six cases of anaplastic
lymphoma kinase-negative anaplastic large cell lymphoma, five cases
of cutaneous T-cell lymphoma, one case of hepatorenal T-cell
lymphoma and 16 cases of non-independently classified peripheral
T-cell lymphoma. The results revealed that a total of 30 patients
(CR rate, 65%) achieved a CR following treatment, but of the 10
cases of ENKTCL-N patients, only three cases (CR rate, 30%)
achieved a CR following treatment. This rate was far lower than
that for other subtypes of T-cell lymphoma (CR rate, 73%). The
aforementioned results are different from in vitro studies
(44), which indicate that
bortezomib has an anti-NK lymphoma cell effect, suggesting that the
targeted therapy for ENKTCL-N requires further study (52).
Nasal-type NKTCL is a highly aggressive tumor with a
poor prognosis, a median survival time of <12 months and a
previous five-year survival rate of 30–40%. However, this five-year
survival rate has increased to 71% (28) in recent years with the application
of intensive treatment methods. Studies have demonstrated that the
prognosis of patients is associated with numerous factors,
including bone marrow infiltration, tumor invasion, existence of
group B symptoms (fever >38°C for >3 days, night sweats and
weight loss of >10% in six months), EBV DNA level of circulating
blood (53), granzyme B, CD94,
Ki-67 expression and hemoglobin concentration prior to treatment
(54). Clinically, the most
commonly used risk assessment system with a proven prognosis value
is the international prognostic index (55) and certain Korean scholars have
introduced the Korean prognostic index, which includes group B
symptoms, Ann Arbor staging, lactate dehydrogenase levels and the
involvement of 1–3 lymph nodes, but without distant transfer
(56). All the aforementioned
methods provide guidance for the prognosis assessment of ENKTCL-N
patients (55).
The patient in the present study was a middle-aged
male with a long history of heavy smoking. Starting with a nodule
on the nose, the patient only experienced nasal congestion,
shortness of breath and other non-specific symptoms in the early
stages, so paid no attention to the condition for a long time.
Later, since the patient had the habit of picking at his nose, the
progressive development of the disease and frequent agitation of
the nodule led to local redness, swelling and erosion, and then
even skin ulceration and bleeding. However, there was no itching,
pain or lymphoma group B symptoms, which include a fever >38°C
for >3 days, night sweats and weight loss of >10% in six
months (4,5). Since the patient was in a good general
condition, the symptoms were non-specific, a local secondary
infection had occurred and the antibiotics and antifungal agents
had exhibited a limited effect, the patient was misdiagnosed with
nasosinusitis and nasal fungal infection several times. However,
the tumor maintained its progressive development instead of being
effectively controlled. Combining the results of histopathological
and immunohistochemical examinations, the Department of
Dermatology, the Second Affiliated Hospital of Xi’an Jiatong
University eventually diagnosed the patient with ENKTCL-N. In
addition to the primary site, the lesions also invaded the local
skin, and left maxillary, left ethmoid and right ethmoid sinuses,
without lymph node or distant metastasis. The nodule was an
early-stage lymphoma (super-cavity IE period) and the patient was
sensitive to radiotherapy, so high-dose (60 Gy) IGRT was adopted.
In order to prevent long-term relapse and achieve better long-term
survival, additional systemic chemotherapy was suggested, but the
patient refused due to the possible side-effects. The radiotherapy
achieved a good response and the patient achieved a CR following
the treatment. During the six months of clinical follow-up, no
relapse occurred. It was recommended that the patient regularly
undergo follow-up examinations, including blood routine
examinations, paranasal sinus CT, chest CT, abdominal
ultrasonography and pelvic CT, in order to detect a local relapse
or distant metastasis early and then undergo chemotherapy.
References
|
1
|
Swerdlow SH: WHO classification of tumours
of haematopoietic and lymphoid tissues. 2. 4th edition. World
Health Organization; 2008
|
|
2
|
Zhao B: China clinical skin disease
science. Jiangsu Science and Technology Press. 1661:1679–1681.
2009.
|
|
3
|
Li S, Feng X, Li T, et al: Extranodal
NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson
Cancer Center. Am J Surg Pathol. 37:14–23. 2013.
|
|
4
|
Li S, Feng X, Li T, et al: Extranodal
NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson
Cancer Center. Am J Surg Pathol. 37:14–23. 2013.
|
|
5
|
Lister TA, Crowther D, Sutcliffe SB, et
al: Report of a committee convened to discuss the evaluation and
staging of patients with Hodgkin’s disease: Cotswolds meeting. J
Clin Oncol. 7:1630–1636. 1989.
|
|
6
|
Wang Hai ZS and Shi MT: Advances in NK/T
cell lymphoma, nasal type. Chinese Journal of Oncology.
15:1909–1912. 2008.
|
|
7
|
Tlholoe MM, Kotu M, Khammissa RA, Bida M,
Lemmer J and Feller L: Extranodal natural killer/T-cell lymphoma,
nasal type: ‘midline lethal granuloma’. A case report. Head Face
Med. 9:42013.
|
|
8
|
Zhao S, Liu WP, Zhang WY and Li GD:
Extranodal nasal type NK/T-cell lymphoma: the expression of
Epstein-Barr virus latent membrane protein 1 and its significance
of prognosis. Sichuan Da Xue Xue Bao Yi Xue Ban. 36:338–340.
2005.(In Chinese).
|
|
9
|
Kanavaros P, De Bruin PC, Briere J, Meijer
CJ and Gaulard P: Epstein-Barr virus (EBV) in extranodal T-cell
non-Hodgkin’s lymphomas (T-NHL). Identification of nasal T-NHL as a
distinct clinicopathological entity associated with EBV. Leuk
Lymphoma. 18:27–34. 1995.
|
|
10
|
Zhang Y, Peng J, Tang Y, et al: The
prevalence of Epstein-Barr virus infection in different types and
sites of lymphomas. Jpn J Infect Dis. 63:132–135. 2010.
|
|
11
|
Jaffe ES: Nasal and nasal-type T/NK cell
lymphoma: a unique form of lymphoma associated with the
Epstein-Barr virus. Histopathology. 27:581–583. 1995.
|
|
12
|
Jiang Lu ZW and Saijuan Chen: Research on
NK/T cell lymphoma extranodal nasal type. Diagnostic Progress of
Theory and Practice. 11:191–194. 2012.
|
|
13
|
Ohtsuka R, Abe Y, Sada E, et al: Adult
patient with Epstein-Barr virus (EBV)-associated
lymphoproliferative disorder: chronic active EBV infection or de
novo extranodal natural killer (NK)/T-cell lymphoma, nasal type?
Intern Med. 48:471–474. 2009.
|
|
14
|
Kim JE, Kim YA, Jeon YK, Park SS, Heo DS
and Kim CW: Comparative analysis of NK/T-cell lymphoma and
peripheral T-cell lymphoma in Korea: Clinicopathological
correlations and analysis of EBV strain type and 30-bp deletion
variant LMP1. Pathol Int. 53:735–743. 2003.
|
|
15
|
Iqbal J, Kucuk C, Deleeuw RJ, et al:
Genomic analyses reveal global functional alterations that promote
tumor growth and novel tumor suppressor genes in natural
killer-cell malignancies. Leukemia. 23:1139–1151. 2009.
|
|
16
|
Nakashima Y, Tagawa H, Suzuki R, et al:
Genome-wide array-based comparative genomic hybridization of
natural killer cell lymphoma/leukemia: different genomic alteration
patterns of aggressive NK-cell leukemia and extranodal Nk/T-cell
lymphoma, nasal type. Genes Chromosomes Cancer. 44:247–255.
2005.
|
|
17
|
Quintanilla-Martinez L, Franklin JL,
Guerrero I, et al: Histological and immunophenotypic profile of
nasal NK/T cell lymphomas from Peru: high prevalence of p53
overexpression. Hum Pathol. 30:849–855. 1999.
|
|
18
|
Takakuwa T, Dong Z, Nakatsuka S, et al:
Frequent mutations of Fas gene in nasal NK/T cell lymphoma.
Oncogene. 21:4702–4705. 2002.
|
|
19
|
Wang TT, Xu C, Liu SL, et al:
Clinicopathology, immunophenotype, T cell receptor gene
rearrangement, Epstein-Barr virus status and p53 gene mutation of
cutaneous extranodal NK/T-cell lymphoma, nasal-type. Chin Med J
(Engl). 126:1281–1287. 2013.
|
|
20
|
Qi Yingjie LY: Development and prospect of
nasal type NK/T cell lymphoma and clinical treatment of Ren
adorable. Chinese Medicine Guide. 9:205–206. 2011.
|
|
21
|
Yasuda H, Sugimoto K, Imai H, et al:
Expression levels of apoptosis-related proteins and Ki-67 in nasal
NK/T-cell lymphoma. Eur J Haematol. 82:39–45. 2009.
|
|
22
|
Kim SJ, Kim BS, Choi CW, et al: Ki-67
expression is predictive of prognosis in patients with stage I/II
extranodal NK/T-cell lymphoma, nasal type. Ann Oncol. 18:1382–1387.
2007.
|
|
23
|
Huang Y, De Reyniès A, De Leval L, et al:
Gene expression profiling identifies emerging oncogenic pathways
operating in extranodal NK/T-cell lymphoma, nasal type. Blood.
115:1226–1237. 2010.
|
|
24
|
Liu X, Wang B, Ma X and Guo Y: NF-kappaB
activation through the alternative pathway correlates with
chemoresistance and poor survival in extranodal NK/T-cell lymphoma,
nasal type. Jpn J Clin Oncol. 39:418–424. 2009.
|
|
25
|
Piccaluga PP, Gazzola A, Agostinelli C,
Bacci F, Sabattini E and Pileri SA: Pathobiology of Epstein-Barr
virus-driven peripheral T-cell lymphomas. Semin Diagn Pathol.
28:234–244. 2011.
|
|
26
|
Su ZL, Mo XL, Feng ZY, Lin HL and Ding YG:
UBE1 expression in extranodal NK/T cell lymphoma, nasal type. Leuk
Lymphoma. 49:1821–1822. 2008.
|
|
27
|
Yagi H, Seo N, Ohshima A, et al: Chemokine
receptor expression in cutaneous T cell and NK/T-cell lymphomas:
immunohistochemical staining and in vitro chemotactic assay. Am J
Surg Pathol. 30:1111–1119. 2006.
|
|
28
|
Wood PB, Parikh SR and Krause JR:
Extranodal NK/T-cell lymphoma, nasal type. Proc (Bayl Univ Med
Cent). 24:251–254. 2011.
|
|
29
|
Liang DN, Yang ZR, Wang WY, et al:
Extranodal nasal type natural killer/T-cell lymphoma of testis:
report of seven cases with review of literature. Leuk Lymphoma.
53:1117–1123. 2012.
|
|
30
|
Ng SB, Lai KW, Murugaya S, et al:
Nasal-type extranodal natural killer/T-cell lymphomas: a
clinicopathologic and genotypic study of 42 cases in Singapore. Mod
Pathol. 17:1097–1107. 2004.
|
|
31
|
Yang S: Clinical and pathological
characteristics of nasal type NK/T cell lymphoma. Acta Academiae
Medicinae cPAPF. 21:305–309. 2012.(In Chinese).
|
|
32
|
Mraz-Gernhard S, Natkunam Y, Hoppe RT,
LeBoit P, Kohler S and Kim YH: Natural killer/natural killer-like
T-cell lymphoma, CD56+, presenting in the skin: an
increasingly recognized entity with an aggressive course. J Clin
Oncol. 19:2179–2188. 2001.
|
|
33
|
Yanagi H, Nakamura Y, Takagi D and Kubota
K: Extranodal natural killer/T-cell lymphoma: a diagnostic dilemma.
Rhinology. 50:325–331. 2012.
|
|
34
|
Ren X, Jia Q, Xiang G, Zhao Z, Xu K and Du
W: Clinical study of 34 patients with extranodal NK/T cell
lymphoma-nasal type. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za
Zhi. 21:361–362. 2007.(In Chinese).
|
|
35
|
Lou LL, Cen XN, Ou JP, et al: Clinical and
pathological analysis of 236 patients with primary extranodal
lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 22:85–92. 2014.(In
Chinese).
|
|
36
|
Shuyun Y: Clinical and pathological
characteristics of nasal type NK/T cell lymphoma. Armed Police
Logistics College (Medical Edition). 21:305–308. 2012.
|
|
37
|
Lee WJ, Jung JM, Won CH, et al: Cutaneous
extranodal natural killer/T-cell lymphoma: a comparative
clinicohistopathologic and survival outcome analysis of 45 cases
according to the primary tumor site. J Am Acad Dermatol.
70:1002–1009. 2014.
|
|
38
|
Li LL, Chai Y, Zhang LS, Dong C and Hao
ZD: A case of blastic plasmacytoid dendritic cell neoplasm and
literatures review. Zhonghua Xue Ye Xue Za Zhi. 34:553–554.
2013.(In Chinese).
|
|
39
|
Cheung MM, Chan JK, Lau WH, Ngan RK and
Foo WW: Early stage nasal NK/T-cell lymphoma: clinical outcome,
prognostic factors, and the effect of treatment modality. Int J
Radiat Oncol Biol Phys. 54:182–190. 2002.
|
|
40
|
Kim TM and Heo DS: Extranodal NK/T-cell
lymphoma, nasal type: new staging system and treatment strategies.
Cancer Sci. 100:2242–2248. 2009.
|
|
41
|
Koom WS, Chung EJ, Yang WI, et al:
Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic
viewpoints. Int J Radiat Oncol Biol Phys. 59:1127–1137. 2004.
|
|
42
|
Shikama N, Ikeda H, Nakamura S, et al:
Localized aggressive non-Hodgkin’s lymphoma of the nasal cavity: a
survey by the Japan Lymphoma Radiation Therapy Group. Int J Radiat
Oncol Biol Phys. 51:1228–1233. 2001.
|
|
43
|
Zhao J and Yi C: Advances in the clinical
treatment of nasal and nasal type NK/T cell lymphoma. Chinese
Journal of Clinical Oncology. 33:836–839. 2006.(In Chinese).
|
|
44
|
Kim SJ, Kim K, Kim BS, et al: Phase II
trial of concurrent radiation and weekly cisplatin followed by VIPD
chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal
NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma
study. J Clin Oncol. 27:6027–6032. 2009.
|
|
45
|
Avilés A, Neri N, Fernández R, Calva A,
Huerta-Guzmán J and Nambo MJ: Nasal NK/T-cell lymphoma with
disseminated disease treated with aggressive combined therapy. Med
Oncol. 20:13–17. 2003.
|
|
46
|
You JY, Chi KH, Yang MH, et al: Radiation
therapy versus chemotherapy as initial treatment for localized
nasal natural killer (NK)/T-cell lymphoma: a single institute
survey in Taiwan. Ann Oncol. 15:618–625. 2004.
|
|
47
|
Yang Yong ZY, Ying Sun, et al: Early
extranodal nasal type NK/T cell lymphoma chemotherapy efficacy
analysis. Chinese Journal of Clinical Oncology. 19:378–381.
2012.
|
|
48
|
Sasaki M, Matsue K, Takeuchi M, Mitome M
and Hirose Y: Successful treatment of disseminated nasal NK/T-cell
lymphoma using double autologous peripheral blood stem cell
transplantation. Int J Hematol. 71:75–78. 2000.
|
|
49
|
Reimer P: Impact of autologous and
allogeneic stem cell transplantation in peripheral T-cell
lymphomas. Adv Hematol. 2010:3206242010.
|
|
50
|
Niu T, Chen XC, Xue HL, et al: Aggressive
NK/T lymphoma with autologous hematopoietic stem cell
transplantation. Hua Xi Yi Xue. 12:1763–1766. 2011.(In
Chinese).
|
|
51
|
Shen L, Au WY, Guo T, et al: Proteasome
inhibitor bortezomib-induced apoptosis in natural killer (NK)-cell
leukemia and lymphoma: an in vitro and in vivo preclinical
evaluation. Blood. 110:469–470. 2007.
|
|
52
|
Kim SJ, Yoon DH, Kang HJ, et al:
Bortezomib in combination with CHOP as first-line treatment for
patients with stage III/IV peripheral T-cell lymphomas: a
multicentre, single-arm, phase 2 trial. Eur J Cancer. 48:3223–3231.
2012.
|
|
53
|
Jaccard A, Petit B, Girault S, et al:
L-asparaginase-based treatment of 15 western patients with
extranodal NK/T-cell lymphoma and leukemia and a review of the
literature. Ann Oncol. 20:110–116. 2009.
|
|
54
|
Chim CS, Ma SY, Au WY, et al: Primary
nasal natural killer cell lymphoma: long-term treatment outcome and
relationship with the International Prognostic Index. Blood.
103:216–221. 2004.
|
|
55
|
Liu XL and Zou L: Treatment and research
status of the cutaneous NK/T cell lymphoma. Hua Xi Yi Xue.
27:1745–1748. 2012.(In Chinese).
|
|
56
|
Lee J, Suh C, Park YH, et al: Extranodal
natural killer T-cell lymphoma, nasal-type: a prognostic model from
a retrospective multicenter study. J Clin Oncol. 24:612–618.
2006.
|