Introduction
Second mitochondria-derived activator of
caspases/direct inhibitor of apoptosis-binding protein with low pI
(Smac/DIABLO) is a mitochondrial apoptogenic molecule that is
released from the mitochondria in response to apoptotic stress.
Smac/DIABLO is known to antagonize the function of inhibitors of
apoptosis proteins (IAPs), including X-linked inhibitor of
apoptosis (XIAP), cellular IAP1/2 and survivin (1–7). The
lack of Smac/DIABLO expression may inhibit apoptosis in cancer
cells, promoting survival. Previous studies have indicated that
decreased levels of Smac/DIABLO correlate with cancer progression.
Smac/DIABLO is considered a potent therapeutic target (8–11).
However, the clinical significance of Smac/DIABLO in various
cancers remains unclear.
Survivin, a member of the IAP family, exhibits a
dual cellular function as an inhibitor of apoptosis and a regulator
of mitosis. The anti-apoptotic effect of survivin is associated
with the inhibition of caspase activity. Furthermore, survivin also
acts as a chromosome passenger protein, regulating the
G2 and M phases of the cell cycle (12–17).
High levels of survivin expression are found in numerous embryonic
tissues and in the majority of human tumors. By contrast, extremely
low level or undetectable levels of expression are found in
differentiated adult tissues. As a result, survivin may prove to be
a marker for tumor progression and prognosis (18–20).
Apoptosis is a primary biochemical cell-death
pathway that is critical for normal tissue homeostasis, cellular
differentiation and development (21,22).
Apoptosis is regulated by the activity of caspases via two possible
pathways, the death receptor-mediated apoptotic pathway (extrinsic
pathway, involving caspase 8) and the mitochondrial-mediated
apoptotic pathway (intrinsic pathway, involving caspase 9)
(21–23). Generally, IAPs suppress apoptosis
via the inhibition of caspase activation.
The expression of cleaved caspase (CC)8 and 9,
microtubule-associated protein 1 light chain 3 (LC3), an autophagy
marker, and survivin in gastric and colorectal carcinomas has been
investigated to elucidate the cell death pathway (24,25).
Results from our previous studies revealed that the expression of
CC9 in colorectal carcinoma was significantly lower than that in
gastric carcinoma (P<0.0001) (24). By contrast, the expression of LC3
and survivin in colon carcinoma was significantly higher than that
in gastric carcinoma (P<0.0001 and P<0.01, respectively)
(24,25). These results indicated that
different cellular death pathways are activated in gastric and
colorectal carcinomas (24).
In the present study, the expression of Smac/DIABLO
in gastric and colorectal carcinoma was examined using
immunohistochemistry. Furthermore, the association between
Smac/DIABLO expression and clinicopathological parameters was
investigated. Additionally, the correlation between the expression
of Smac/DIABLO and survivin was elucidated.
Materials and methods
Tissue samples
Surgically resected tumor tissues were collected
from the archives of the Department of Diagnostic Pathology of the
Osaka Red Cross Hospital (Osaka, Japan) and the Kobe Central
Hospital of Social Insurance (Kobe, Japan). A total of 72 advanced
gastric adenocarcinomas (36 well- to moderately-differentiated and
36 poorly-differentiated) and 78 colorectal adenocarcinomas (68
well- to moderately-differentiated and 10 poorly-differentiated)
were analyzed for Smac/DIABLO expression by immunohistochemistry.
The study was approved by the ethics committee of Kobe University
Graduate School of Health Sciences (Kobe, Japan). The tumors were
classified according to the tumor-node-metastasis (TNM)
classification of malignant tumors (TNM 2009) (26). All specimens were preserved in 10%
formalin and embedded in paraffin. Sections that were 3-μm thick
were cut consecutively and mounted on
aminopropyltriethoxysilane-coated slides.
Immunohistochemical staining
Smac/DIABLO expression was analyzed using
immunohistochemistry. The tissue sections were deparaffinized with
xylene (Nacalai Tesque, Inc., Kyoto, Japan) and dehydrated using a
graded series of ethanol solutions. Antigen retrieval was performed
by immersing the slides in 10 mM citrate buffer (pH 7.0; Nacalai
Tesque, Inc.) and heating for 10 min in a pressure cooker (Tefal,
Haute-Savoie, France). The sections were then cooled at room
temperature in a soaking solution (10 mM citrate buffer; pH 7.0;
Nacalai Tesque, Inc.) for 30 min. Next, the sections were washed
with water, followed by 10 mM phosphate-buffered saline (PBS; pH
7.2). Following blocking with 0.25% casein in PBS (Dako, Glostrup,
Denmark), the sections were incubated with a mouse monoclonal
antibody against Smac/DIABLO (1:1,000; Cell Signaling Technology,
Inc., Danvers, USA) overnight at room temperature. The sections
were then rinsed with PBS. To detect Smac/DIABLO, the sections were
incubated with Histofine Simple Stain MAX-PO (Nichirei Bioscience
Inc., Tokyo, Japan) for 1 h at room temperature. The reaction
products were then developed using 3,3′-diaminobenzidine (Dako,
Glostrup, Denmark) and counterstained using Mayer’s hematoxylin
(Merck KGaA, Darmstadt, Germany). For the negative control, a
section was treated as described above, but PBS was used instead of
the primary antibody.
Evaluation of immunostaining
Sections were considered positive for Smac/DIABLO if
cytoplasmic staining was observed. A mean percentage of positive
tumor cells was determined in ≥5 areas at a magnification of ×400
and assigned to the following categories: 0, negative; 1, <30%;
2, 30–69%; and 3, ≥70%. The intensity of Smac/DIABLO immunostaining
was scored as follows: 1, weak; 2, moderate; and 3, intense. A
final score was obtained by calculating the sum of these two
scores. Cases with final scores of <5 were considered to exhibit
low expression, whereas cases with final scores of ≥5 were
considered to exhibit high expression.
Statistical analysis
Differences in the expression of Smac/DIABLO between
gastric and colorectal adenocarcinomas, well- to
moderately-differentiated and poorly-differentiated
adenocarcinomas, patient age and gender, as well as lymphatic and
vascular invasion were evaluated using the χ2 and
Fisher’s exact tests. The correlation between marker expression and
tumor location, depth of invasion, lymph node metastasis and
pathological stage were analyzed using the Kruskal-Wallis test.
Spearman’s rank correlation was used to assess the correlation
between the different markers. P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression of Smac/DIABLO in gastric and
colorectal carcinomas
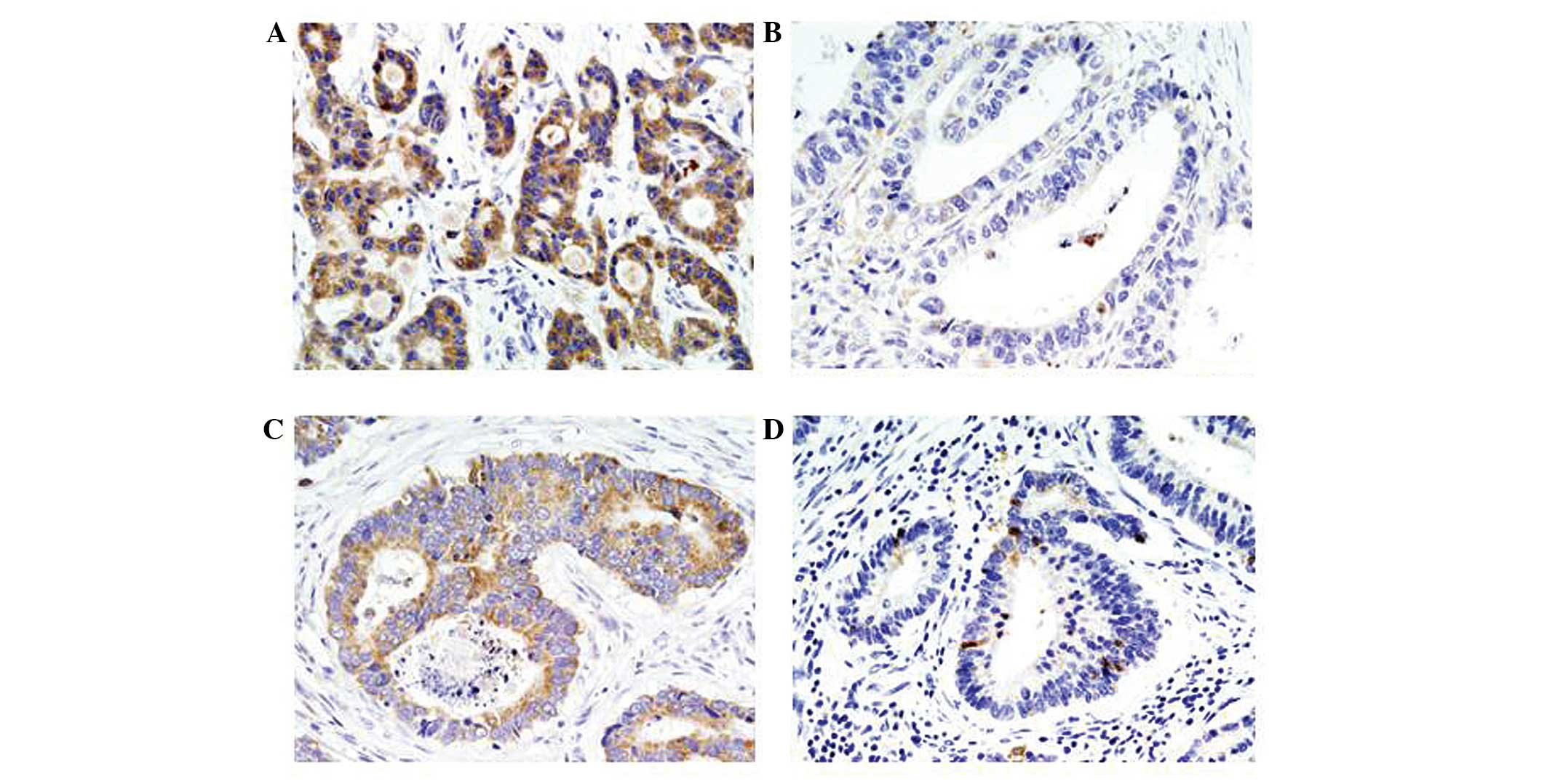
Smac/DIABLO expression was predominantly observed in
the cytoplasm (Fig. 1).
Smac/DIABLO-positive staining was observed in 46% (33/72) of
gastric carcinomas. Smac/DIABLO expression was higher in well- to
moderately-differentiated specimens (50%) compared with
poorly-differentiated specimens (42%), however, no significant
differences were identified (Table
I).
 | Table IExpression of Smac/DIABLO in
gastrointestinal adenocarcinomas. |
Table I
Expression of Smac/DIABLO in
gastrointestinal adenocarcinomas.
| Adenocarcinoma
type | Total, n | High expression, n
(%) | Low expression, n
(%) |
|---|
| Gastric
adenocarcinoma | 72 | 33 (46)a | 39 (54) |
| Well- to
moderately-differentiated | 36 | 18 (50) | 18 (50) |
|
Poorly-differentiated | 36 | 15 (42) | 21 (58) |
| Colorectal
adenocarcinoma | 78 | 54 (69)a | 24 (31) |
| Well- to
moderately-differentiated | 68 | 48 (71) | 20 (29) |
|
Poorly-differentiated | 10 | 6 (60) | 4 (40) |
Smac/DIABLO-positive staining
Smac/DIABLO-positive staining was observed in 69%
(54/78) of colorectal carcinomas. The expression of Smac/DIABLO was
significantly higher in colorectal carcinoma than in gastric
carcinoma (P<0.01). Smac/DIABLO expression was higher in well-
to moderately-differentiated specimens (71%) compared with
poorly-differentiated specimens (60%), however, no significant
differences were identified.
Correlation between Smac/DIABLO
expression and clinicopathological parameters
Tables II and
III present the associations
between Smac/DIABLO protein expression and the clinicopathological
parameters. In gastric carcinomas, none of the parameters of
patient age, gender, tumor location, depth of invasion, lymph-node
metastasis, lymphatic invasion, vascular invasion or pathological
stage were found to be associated with the positive expression of
Smac/DIABLO. In colorectal carcinomas, the level of Smac/DIABLO
expression was significantly higher in cases without vascular
invasion than in the cases with vascular invasion (P<0.05).
However, none of the other parameters of patient age, gender, tumor
location, depth of invasion, lymph-node metastasis, lymphatic
invasion, or pathological stage were found to be associated with
positive Smac/DIABLO expression. Although no significant difference
was identified between the majority of the clinicopathological
parameters and the expression of Smac/DIABLO, a trend towards an
association between a decreased expression level of Smac/DIABLO and
tumor stage was identified.
 | Table IICorrelation between Smac/DIABLO
expression and clinicopathological parameters in gastric
adenocarcinomas. |
Table II
Correlation between Smac/DIABLO
expression and clinicopathological parameters in gastric
adenocarcinomas.
| Clinicopathological
parameters | Total, n | Smac/DIABLO
expression, n (%) |
|---|
|
|---|
| High | Low |
|---|
| Age, years |
| <60 | 10 | 4 (40) | 6 (60) |
| >60 | 62 | 29 (47) | 33 (53) |
| Gender |
| Male | 51 | 23 (45) | 28 (55) |
| Female | 21 | 10 (48) | 11 (52) |
| Location |
| Cardia | 17 | 7 (41) | 10 (59) |
| Fundus | 31 | 15 (48) | 16 (52) |
| Antrum | 24 | 11 (46) | 13 (54) |
| Depth of
invasion |
| pT2 | 17 | 11 (65) | 6 (35) |
| pT3 | 20 | 9 (45) | 11 (55) |
| pT4 | 35 | 13 (37) | 22 (63) |
| Lymph node
metastasis |
| pN0 | 23 | 14 (61) | 9 (39) |
| pN1 | 15 | 4 (27) | 11 (73) |
| pN2 | 11 | 4 (36) | 7 (64) |
| pN3 | 23 | 11 (48) | 12 (52) |
| Lymphatic
invasion |
| Negative | 16 | 7 (44) | 9 (56) |
| Positive | 56 | 26 (46) | 30 (54) |
| Vascular
invasion |
| Negative | 38 | 14 (37) | 24 (63) |
| Positive | 34 | 19 (56) | 15 (44) |
| UICC p-Stage |
| IB | 11 | 8 (73) | 3 (27) |
| IIA and IIB | 22 | 9 (41) | 13 (59) |
| IIIA, IIIB and
IIIC | 39 | 16 (41) | 23 (59) |
 | Table IIICorrelation between Smac/DIABLO
expression and clinicopathological parameters in colorectal
adenocarcinomas. |
Table III
Correlation between Smac/DIABLO
expression and clinicopathological parameters in colorectal
adenocarcinomas.
| Clinicopathological
parameters | Total, n | Smac/DIABLO
expression, n (%) |
|---|
|
|---|
| High | Low |
|---|
| Age, years |
| <60 | 22 | 16 (73) | 6 (27) |
| >60 | 56 | 38 (68) | 18 (32) |
| Gender |
| Male | 44 | 29 (66) | 15 (34) |
| Female | 34 | 25 (74) | 9 (26) |
| Location |
| Right colon | 24 | 16 (67) | 8 (33) |
| Left colon | 24 | 19 (79) | 5 (21) |
| Rectum | 30 | 19 (63) | 11 (37) |
| Depth of
invasion |
| pT2 | 7 | 5 (71) | 2 (29) |
| pT3 | 46 | 31 (67) | 15 (33) |
| pT4 | 25 | 18 (72) | 7 (28) |
| Lymph node
metastasis |
| pN0 | 35 | 25 (71) | 10 (29) |
| pN1 | 31 | 23 (74) | 8 (26) |
| pN2 | 12 | 6 (50) | 6 (50) |
| Lymphatic
invasion |
| Negative | 24 | 16 (67) | 8 (33) |
| Positive | 54 | 38 (70) | 16 (30) |
| Vascular
invasion |
| Negative | 40 | 32 (80)a | 8 (20) |
| Positive | 38 | 22 (58)a | 16 (42) |
| UICC p-Stage |
| IB | 6 | 5 (83) | 1 (17) |
| IIA and IIB | 28 | 20 (71) | 8 (29) |
| IIIA, IIIB and
IIIC | 44 | 29 (66) | 15 (34) |
Association between Smac/DIABLO
expression in gastric and colorectal carcinomas
A correlation was identified between Smac/DIABLO and
nuclear survivin (r=0.245; P<0.01) in well- to
moderately-differentiated colorectal adenocarcinomas. However, in
all cases, the expression of Smac/DIABLO was not significantly
associated with survivin expression (P=0.08).
Discussion
In the present study, the expression of Smac/DIABLO
in gastric and colorectal carcinomas was investigated and compared
by immunohistochemistry. Smac/DIABLO-positive staining was observed
in 46% (33/72) and 69% (54/78) of gastric and colorectal
carcinomas, respectively. These results revealed that the
expression of Smac/DIABLO was significantly higher in colorectal
carcinoma than in gastric carcinoma (P<0.01). Regarding the
association between Smac/DIABLO expression and tumor type, Yoo
et al (8) analyzed archival
tissues of 100 carcinomas and 50 sarcomas from various origins
using immunohistochemistry. The study reported that Smac/DIABLO was
differentially expressed among cancer types and indicated that
gastric, colorectal and ovarian carcinomas exhibited a high
frequency of Smac/DIABLO expression, whereas Smac/DIABLO expression
in prostate carcinoma and non-small cell lung carcinoma was low.
According to previous studies, the positive rates of expression in
gastric and colorectal carcinomas are 13–70% and 66–90%,
respectively (9,10,27–29).
Taken together, these studies indicate that Smac/DIABLO expression
in colorectal carcinoma is higher than that in gastric carcinoma,
however, differences may be observed due to the different counting
methods used.
The results of the present study demonstrated that
Smac/DIABLO expression in well- to moderately-differentiated
gastric and colorectal carcinomas was higher than that in
poorly-differentiated adenocarcinomas. However, no statistically
significant difference was identified. Kim et al (27) reported that Smac/DIABLO expression
was associated with a higher proportion of diffuse histology types
than overall cases (intestinal, diffuse and mixed types). By
contrast, Shibata et al (28) identified a significant correlation
between Smac/DIABLO expression and tumor differentiation
(P<0.0001), whereby patients with high Smac/DIABLO expression
presented more differentiated tumors. Yoo et al (8) identified no correlation between
histological subtype (diffuse type vs. intestinal type) and the
expression of Smac/DIABLO (8).
Based on these results, we hypothesized that there is no
association between the expression of Smac/DIABLO and tumor
differentiation in gastric and colorectal carcinomas.
Furthermore, the association between Smac/DIABLO
expression and clinicopathological parameters was investigated in
the present study. In colorectal carcinomas, the level of
Smac/DIABLO expression was significantly higher in the cases
without vascular invasion than in the cases with vascular invasion
(P<0.05). However, this association was not identified in
gastric carcinoma. Previous studies have demonstrated that
Smac/DIABLO expression is associated with a good prognosis and low
tumor stage (8–11). In the present study, a trend towards
an association between decreased Smac/DIABLO expression and
pathological stage in gastric and colorectal carcinomas was
observed, however, no statistically significant difference was
identified. Endo et al (9)
reported that no significant difference was present between the two
Smac/DIABLO-positive and -negative tumor groups with respect to
tumor size, tumor location, histological differentiation and
lymphatic and venous invasion. The clinical significance of
Smac/DIABLO expression in various cancers remains unclear.
Therefore, additional comprehensive studies are required to
elucidate the clinical significance of Smac/DIABLO expression in
gastric and colorectal carcinomas.
In the present study, the expression of Smac/DIABLO
and nuclear survivin were found to correlate in well- to
moderately-differentiated colorectal adenocarcinomas (r=0.245;
P<0.01). De Oliveira Lima et al (30) identified a correlation between the
expression of survivin and Smac/DIABLO in colorectal carcinoma
using immunohistochemistry. The results of the present study only
identified a correlation in well- to moderately-differentiated
cases, however, the correlation was not observed in all colorectal
cancer cases. By contrast, Kim et al (27) revealed that Smac/DIABLO expression
was not associated with survivin, whereas Smac/DIABLO expression
was found to inversely correlate with the expression of XIAP, an
IAP, using immunohistochemistry. To the best of our knowledge, only
a small number of studies have demonstrated the association between
Smac/DIABLO and survivin expression using immunohistochemistry.
Endo et al (9) proposed that
the decrease of Smac/DIABLO expression is an independent factor of
poor prognosis for colorectal cancer patients, while other studies
have indicated that survivin may be a marker for tumor progression
and prognosis (5,31,32).
Survivin is found in the nucleus and cytoplasm. Nuclear survivin is
considered a promoter of cell proliferation, whereas cytoplasmic
survivin is considered to exhibit cytoprotective effects (16,33),
however, the mechanisms involved remain unclear. Previous studies
have indicated that nuclear and cytoplasmic survivin expression is
associated with an improved prognosis in gastric carcinomas. By
contrast, in colorectal carcinoma, the upregulation of cytoplasmic
survivin is associated with a poor prognosis (31,32,34–37).
However, there is no disagreement between the results of previous
studies and the present study. At present, the association between
survivin or Smac/DIABLO and the clinical prognosis remains
controversial. Thus, further studies are required to confirm the
association between Smac/DIABLO expression and nuclear or
cytoplasmic survivin expression.
In conclusion, the present study demonstrated that
the expression of Smac/DIABLO was significantly higher in
colorectal carcinoma than in gastric carcinoma. Additionally, a
correlation was found between the expression of Smac/DIABLO and
nuclear survivin in well- to moderately-differentiated colorectal
adenocarcinomas (r=0.245; P<0.01). Based on these results, we
hypothesized that gastric and colorectal carcinomas differ in their
levels of Smac/DIABLO expression. These results, in addition to our
previous results, indicate that not only LC3 and survivin
expression levels, but also Smac/DIABLO expression levels, are
significantly higher in colorectal carcinoma than in gastric
carcinoma (24,25). We hypothesized that the expression
of Smac/DIABLO in colorectal carcinoma may be upregulated to
suppress the anti-apoptotic effect of survivin. Furthermore, the
results of this study indicate that the analysis of Smac/DIABLO,
survivin and LC3 expression in colorectal carcinoma is likely to
aid cancer therapy due to the involvement of these markers in
apoptosis and/or autophagy.
Acknowledgements
The authors would like to thank Dr Masayuki Shintaku
and Dr Toshihiko Miyake for their kind support. This study was
supported by a Grant-in-Aid for Scientific Research (grant no.
23590396) from the Japan Society for the Promotion of Science.
References
|
1
|
Shiozaki EN and Shi Y: Caspases, IAPs and
Smac/DIABLO: mechanisms from structural biology. Trends Biochem
Sci. 29:486–494. 2004.
|
|
2
|
Cheung HH, LaCasse EC and Korneluk RG:
X-linked inhibitor of apoptosis antagonism: strategies in cancer
treatment. Clin Cancer Res. 12:3238–3242. 2006.
|
|
3
|
Srinivasula SM, Hegde R, Saleh A, et al: A
conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO
regulates caspase activity and apoptosis. Nature. 410:112–116.
2001.
|
|
4
|
Song Z, Yao X and Wu M: Direct interaction
between survivin and Smac/DIABLO is essential for the
anti-apoptotic activity of survivin during taxol-induced apoptosis.
J Biol Chem. 278:23130–23140. 2003.
|
|
5
|
Miura K, Fujibuchi W, Ishida K, et al:
Inhibitor of apoptosis protein family as diagnostic markers and
therapeutic targets of colorectal cancer. Surg Today. 41:175–182.
2011.
|
|
6
|
Grzybowska-Izydorczyk O, Cebula B, Robak T
and Smolewski P: Expression and prognostic significance of the
inhibitor of apoptosis protein (IAP) family and its antagonists in
chronic lymphocytic leukaemia. Eur J Cancer. 46:800–810. 2010.
|
|
7
|
Wrzesień-Kuś A, Smolewski P, Sobczak-Pluta
A, Wierzbowska A and Robak T: The inhibitor of apoptosis protein
family and its antagonists in acute leukemias. Apoptosis.
9:705–715. 2004.
|
|
8
|
Yoo NJ, Kim HS, Kim SY, et al:
Immunohistochemical analysis of Smac/DIABLO expression in human
carcinomas and sarcomas. APMIS. 111:382–388. 2003.
|
|
9
|
Endo K, Kohnoe S, Watanabe A, et al:
Clinical significance of Smac/DIABLO expression in colorectal
cancer. Oncol Rep. 21:351–355. 2009.
|
|
10
|
Yan H, Yu J, Wang R, Jiang S, Zhu K, Mu D
and Xu Z: Prognostic value of Smac expression in rectal cancer
patients treated with neoadjuvant therapy. Med Oncol. 29:168–173.
2012.
|
|
11
|
Zheng LD, Tong QS, Wang L, Liu J and Qian
W: Stable transfection of extrinsic Smac gene enhances
apoptosis-inducing effects of chemotherapeutic drugs on gastric
cancer cells. World J Gastroenterol. 11:79–83. 2005.
|
|
12
|
Li F: Survivin study: what is the next
wave? J Cell Physiol. 197:8–29. 2003.
|
|
13
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997.
|
|
14
|
Liston P, Fong WG and Korneluk RG: The
inhibitors of apoptosis: there is more to life than Bcl2. Oncogene.
22:8568–8580. 2003.
|
|
15
|
Li F, Ackermann EJ, Bennett CF, et al:
Pleiotropic cell-division defects and apoptosis induced by
interference with survivin function. Nat Cell Biol. 1:461–466.
1999.
|
|
16
|
Knauer SK, Mann W and Stauber RH:
Survivin’s dual role: an export’s view. Cell Cycle. 6:518–521.
2007.
|
|
17
|
Sah NK, Khan Z, Khan GJ and Bisen PS:
Structural, functional and therapeutic biology of survivin. Cancer
Lett. 244:164–171. 2006.
|
|
18
|
Andersen MH, Svane IM, Becker JC and
Straten PT: The universal character of the tumor-associated antigen
survivin. Clin Cancer Res. 13:5991–5994. 2007.
|
|
19
|
Choi J and Chang H: The expression of MAGE
and SSX, and correlation of COX2, VEGF, and survivin in colorectal
cancer. Anticancer Res. 32:559–564. 2012.
|
|
20
|
Wang TT, Qian XP and Liu BR: Survivin:
potential role in diagnosis, prognosis and targeted therapy of
gastric cancer. World J Gastroenterol. 13:2784–2790. 2007.
|
|
21
|
Kerr JF, Winterford CM and Harmon BV:
Apoptosis. Its significance in cancer and cancer therapy. Cancer.
73:2013–2026. 1994.
|
|
22
|
Reed JC: Mechanisms of apoptosis. Am J
Pathol. 157:1415–1430. 2000.
|
|
23
|
Lavrik IN, Golks A and Krammer PH:
Caspases: pharmacological manipulation of cell death. J Clin
Invest. 115:2665–2672. 2005.
|
|
24
|
Shintani M, Sangawa A, Yamao N, Miyake T
and Kamoshida S: Immunohistochemical analysis of cell death
pathways in gastrointestinal adenocarcinoma. Biomed Res.
32:379–386. 2011.
|
|
25
|
Shintani M, Sangawa A, Yamao N and
Kamoshida S: Immunohistochemical expression of nuclear and
cytoplasmic survivin in gastrointestinal carcinoma. Int J Clin Exp
Pathol. 6:2919–2927. 2013.
|
|
26
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; New Jersey: 2009
|
|
27
|
Kim MA, Lee HE, Lee HS, Yang HK and Kim
WH: Expression of apoptosis-related proteins and its clinical
implication in surgically resected gastric carcinoma. Virchows
Arch. 459:503–510. 2011.
|
|
28
|
Shibata T, Noguchi T, Takeno S, Gabbert
HE, Ramp U and Kawahara K: Disturbed XIAP and XAF1 expression
balance is an independent prognostic factor in gastric
adenocarcinomas. Ann Surg Oncol. 15:3579–3587. 2008.
|
|
29
|
DA Silva LC, Forones NM, Ribeiro DA, Ihara
SS, Gomes TS, Neto RA and Oshima CT: Immunoexpression of DIABLO,
AIF and cytochrome C in gastric adenocarcinoma assessed by tissue
Microarray. Anticancer Res. 33:647–653. 2013.
|
|
30
|
De Oliveira Lima F, De Oliveira Costa H,
Barrezueta LF, et al: Immunoexpression of inhibitors of apoptosis
proteins and their antagonist SMAC/DIABLO in colorectal carcinoma:
correlation with apoptotic index, cellular proliferation and
prognosis. Oncol Rep. 22:295–303. 2009.
|
|
31
|
Fang YJ, Lu ZH, Wang GQ, et al: Elevated
expressions of MMP7, TROP2, and survivin are associated with
survival, disease recurrence, and liver metastasis of colon cancer.
Int J Colorectal Dis. 24:875–884. 2009.
|
|
32
|
Okada E, Murai Y, Matsui K, Isizawa S,
Cheng C, Masuda M and Takano Y: Survivin expression in tumor cell
nuclei is predictive of a favorable prognosis in gastric cancer
patients. Cancer Lett. 163:109–116. 2001.
|
|
33
|
Stauber RH, Mann W and Knauer SK: Nuclear
and cytoplasmic survivin: molecular mechanism, prognostic, and
therapeutic potential. Cancer Res. 67:5999–6002. 2007.
|
|
34
|
Ponnelle T, Chapusot C, Martin L, et al:
Cellular localisation of survivin: impact on the prognosis in
colorectal cancer. J Cancer Res Clin Oncol. 131:504–510. 2005.
|
|
35
|
Qi G, Tuncel H, Aoki E, et al:
Intracellular localization of survivin determines biological
behavior in colorectal cancer. Oncol Rep. 22:557–562. 2009.
|
|
36
|
Lee YY, Yu CP, Lin CK, Nieh S, Hsu KF,
Chiang H and Jin JS: Expression of survivin and cortactin in
colorectal adenocarcinoma: association with clinicopathological
parameters. Dis Markers. 26:9–18. 2009.
|
|
37
|
Vallböhmer D, Drebber U, Schneider PM, et
al: Survivin expression in gastric cancer: Association with
histomorphological response to neoadjuvant therapy and prognosis. J
Surg Oncol. 99:409–413. 2009.
|