Introduction
Cervical cancer is the third most common type of
cancer in the United States. Similarly, in Korea, cervical cancer
is the sixth most common malignancy in female individuals and, more
specifically, the second most common malignancy in females aged
15–44 years (1). The treatment
strategy selected for patients with cervical cancer is based on the
International Federation of Gynecology and Obstetrics (FIGO)
staging system (2). For instance,
surgery is the primary treatment strategy for early stage cervical
cancer (stages IA–IIA), while concurrent chemoradiotherapy (CCRT)
is the primary treatment strategy for more advanced stages of
cervical cancer (stages IIB–IVB) (3).
Despite the application of CCRT resulting in significant
improvements in the disease-free and overall survival rates of
patients with advanced cervical cancer, the survival rates in these
patients remain unsatisfactory (4–7).
Therefore, adjunctive treatment strategies following CCRT may be
considered to improve survival rates for patients with poor
prognostic factors, although there are currently no definite
treatment guidelines. Therefore, it is important to assess
individual prognoses to determine the most effective treatment
method. The prognoses of patients with cervical cancer are
typically estimated using the FIGO staging system. However, the
current FIGO clinical staging system has limited value due to
individual variability in physical examinations and a lack of
consideration of other important factors, including pathological
parameters and lymph node (LN) metastasis (8). Therefore, considering prognostic factors
other than the FIGO stage may be required for more accurate
prediction of individual prognoses.
At present, numerous prognostic factors, including
tumor size, LN involvement, age, pretreatment serum squamous cell
carcinoma antigen (SCC-Ag) levels, parametrial invasion and deep
stromal invasion (9–17), are used to predict the risk of disease
recurrence. However, the majority of previously conducted studies
assessed early stage cervical cancer, while only a relatively small
number of studies were performed in advanced stage cervical cancer
patients.
The aim of the present study was to identify
predictors of survival among various clinical variables and
identify independent prognostic factors in advanced cervical
cancer.
Patients and methods
Patient characteristics
In total, 157 patients diagnosed with stages IIA–IVB
cervical cancer, who were treated between January 2006 and December
2010, were retrospectively included in the present analysis. The
clinical findings and laboratory results of the included patients
were collected from electronic medical records from four Hallym
Medical Centers in South Korea (Hallym University Sacred Heart
Hospital, Anyang; Kangnam Sacred Heart Hospital, Seoul; Chuncheon
Sacred Heart Hospital, Chuncheon; and Kangdong Sacred Heart
Hospital, Seoul, Republic of Korea). The study was approved by the
Institutional Review Boards of Hallym University Sacred Heart
Hospital.
Cases of stage II cervical cancer were subdivided
into stages IIA and IIB, according to the FIGO staging system.
Although the FIGO staging system was revised in 2009 (2), the number of patients exhibiting
cervical cancer of stage IIB or higher was not altered. In
addition, tumor size was included as a variable, and cases of
revised stage IIA cervical cancer were divided by clinically
visible lesion size without affecting the results. The patients
were classified into four serum SCC-Ag groups, including the ≤2,
2–5, 5–15 and >15 ng/ml groups. The tumor size was determined as
the largest diameter of the primary tumor as measured by computed
tomography (CT) or magnetic resonance imaging (MRI), and was
categorized as ≤4, 4–6 or >6 cm. Parametrial involvement was
determined by physical examination and hydronephrosis by CT
scanning or intravenous pyelography. In addition, bladder/rectal
invasion was diagnosed by cystoscopic or sigmoidoscopic biopsy,
while pelvic/para-aortic LN metastasis was pathologically verified
or defined as a nodal size of ≥1 cm using CT or MRI. Patients
exhibiting pelvic and para-aortic LN involvement were included in
the para-aortic LN-positive group. The number of LN metastases
determined from the pathological biopsies was relatively small due
to inoperable advanced cervical cancer cases; thus, imaging
identification of LN metastases was used as a variable. In
addition, high-risk human papilloma virus (HPV) infection was
observed using DNA microarray analysis.
Cancer treatment strategies
Of the 157 patients included in the study, 76 were
treated with CCRT, 26 with surgery and chemotherapy, 23 with
surgery and CCRT and the remaining 32 patients were treated using
alternative methods. The patients treated with alternative methods
were excluded from the current study due to the low sample number
in each treatment group.
Surgical procedures included a radical hysterectomy
with pelvic and/or para-aortic LN dissection for patients with
stage IIA cervical cancer. Patients receiving CCRT were
administered with six weekly infusions of cisplatin (40
mg/m2). Patients with a glomerular filtration rate of
<60 ml/min were treated with carboplatin (120 mg/m2);
however, if the leukocyte count of the patient reduced to
<3,000/m3 or the platelet count decreased to
<100,000/m3, the treatment was terminated. Following
the completion of chemotherapy, the patients undergoing CCRT
received external beam radiation therapy (EBRT) to the entire
pelvis using ≤50.4 Gy with a 10 MeV photon and the four-field box
technique. Daily doses of EBRT were administered in five
fractions/week at 1.8 Gy per fraction. Following EBRT, the patients
undergoing radical CCRT received additional EBRT, as well as
high-dose intracavitary brachytherapy with iridium-192, in which
the dose to point A was 30 Gy in six fractions. When para-aortic LN
metastasis was suspected, the patients received extended field
radiation therapy with a total dose of 55–60 Gy. Furthermore,
patients receiving chemotherapy were administered combination
therapy with paclitaxel (135 mg/m2, 24 h infusion on day
1) and cisplatin (50 mg/m2, 1 h infusion on day 2) every
three weeks.
Following completion of the therapy, the patients
underwent follow-up examinations every three months for the initial
two years, every six months for the next three years and annually
thereafter. The overall survival was assessed from the point of
diagnosis to the point of mortality caused by the specific disease
or the date of the final follow-up visit.
Statistical analysis
Kaplan-Meier analysis was used to calculate the
disease-specific survival rate from the date of the initial
treatment session to the date of disease-specific mortality.
Differences in survival rates between the groups were compared
using the log-rank test for categorical variables. In addition to
the inclusion of age as a continuous covariate, variables
identified as significant by univariate analysis were subsequently
analyzed using the multivariate Cox proportional hazard model to
clarify the association between overall survival and the identified
risk factors. P<0.05 was considered to indicate a statistically
significant difference and all statistical analyses were performed
using the SAS software (version 9.2; SAS Institute Inc., Cary, NC,
USA).
Results
Patient characteristics
The clinical characteristics of the patients
included in the present multi-institutional study are summarized in
Table I. The mean age of the patients
at presentation was 57.52±14.06 years.
 | Table I.Clinical, treatment and outcome
characteristics of 157 patients with cervical cancer treated
between January 2006 and December 2010. |
Table I.
Clinical, treatment and outcome
characteristics of 157 patients with cervical cancer treated
between January 2006 and December 2010.
| Characteristic | Value |
|---|
| Age, years
(n=157) |
|
| Median
(range) | 56 (27–85) |
| Mean ±
standard deviation | 57.52±14.06 |
| Stage, n (%;
n=156) |
|
|
IIA | 55 (35.26) |
|
IIB | 60 (38.46) |
| IIIA
and IIIB | 17 (10.90) |
| IVA and
IVB | 24 (15.38) |
| SCC-Ag, ng/ml (%;
n=149) |
|
| ≤2 | 42 (28.19) |
|
2–5 | 33 (22.15) |
|
5–15 | 36 (24.16) |
|
>15 | 38 (25.50) |
| Tumor size, cm (%;
n=151) |
|
| ≤4 | 61 (40.40) |
|
4–6 | 60 (39.74) |
|
>6 | 30 (19.87) |
| Parametrial
involvement, n (%; n=155) |
|
|
(–) | 39 (25.16) |
|
(+) | 116 (74.84) |
| Lower third vagina
involvement, n (%; n=156) |
|
|
(–) | 153 (98.08) |
|
(+) | 3 (1.92) |
| Hydronephrosis, n
(%; n=156) |
|
|
(–) | 123 (79.35) |
|
(+) | 32 (20.65) |
| Bladder/rectum
involvement, n (%; n=155) |
|
|
(–) | 124 (80.00) |
|
(+) | 31 (20.00) |
| LN status by
imaging, n (%; n=148) |
|
|
(–) | 75 (50.68) |
| (+)
pelvic | 65 (43.92) |
| (+)
para-aortic | 8 (5.41) |
| LN status by
pathological analysis, n (%; n=46) |
|
|
(–) | 31 (67.39) |
|
(+) | 15 (32.61) |
| HPV infection, n
(%; n=65) |
|
|
(–) | 16 (24.62) |
|
(+) | 49 (75.38) |
| Tumor cell type, n
(%; n=157) |
|
|
SCC | 124 (78.98) |
|
Adenocarcinoma/adenosquamous
carcinoma | 18 (11.46) |
| Small
cell carcinoma | 15 (9.55) |
| Treatment modality,
n (%; n=125) |
|
| Surgery
+ chemotherapy | 26 (20.80) |
| Surgery
+ CCRT | 23 (18.40) |
|
CCRT | 76 (60.80) |
Univariate analysis of 12 clinical
factors
The Cox proportional hazards model was applied to
analyze 12 factors, including age, tumor stage, SCC-Ag level, tumor
size, parametrial involvement, lower third vaginal involvement,
hydronephrosis, bladder/rectal involvement, LN metastasis detected
by imaging, LN metastasis detected by pathology, HPV infection and
tumor cell type, which were considered to have a prognostic value.
Of these factors, the following eight variables demonstrated
significantly high relative risk (RR; Table II), in order of decreasing RR: Lower
third vaginal involvement (RR, 12.976), pathological LN metastasis
(RR, 10.971), tumor size of >6 cm (RR, 8.691), LN metastasis
(para-aortic and/or pelvic) detected by imaging (RR, 8.214),
hydronephrosis (RR, 3.740), bladder/rectal involvement (RR, 3.216),
SCC-Ag of >15 ng/ml (RR 2.480) and tumor stage (RR, 2.228).
 | Table II.Cox proportional hazards model for
survival of 12 factors considered to have a prognostic value in
patients with advanced cervical cancer. |
Table II.
Cox proportional hazards model for
survival of 12 factors considered to have a prognostic value in
patients with advanced cervical cancer.
| Variable | RR | 95% CI | P-value |
|---|
| Age | 1.325 | 0.993–1.768 | 0.0558 |
| Stage | 2.228 | 1.620–3.063 | <0.0001 |
| SCC-Ag, ng/ml |
|
|
|
| ≤2 | 1.000 |
|
|
|
2–5 | 0.660 | 0.207–2.106 | 0.4823 |
|
5–15 | 0.733 | 0.250–2.149 | 0.5715 |
|
>15 | 2.480 | 1.097–5.607 | 0.0291 |
| Tumor size, cm |
|
|
|
| ≤4 | 1.000 |
|
|
|
4–6 | 3.528 | 1.282–9.710 | 0.0147 |
|
>6 | 8.691 | 3.088–24.461 | <0.0001 |
| Parametrial
involvement |
|
|
|
|
(–) | 1.000 |
|
|
|
(+) | 2.635 | 0.926–7.501 | 0.0694 |
| Lower third vaginal
involvement |
|
|
|
|
(–) | 1.000 |
|
|
|
(+) | 12.976 | 3.786–44.472 | <0.0001 |
| Hydronephrosis |
|
|
|
|
(–) | 1.000 |
|
|
|
(+) | 3.740 | 1.843–7.589 | 0.0003 |
| Bladder/rectum
involvement |
|
|
|
|
(–) | 1.000 |
|
|
|
(+) | 3.216 | 1.562–6.621 | 0.0015 |
| LN metastasis by
imaging |
|
|
|
|
(–) | 1.000 |
|
|
| (+)
pelvic | 3.505 | 1.604–7.661 | 0.0017 |
| (+)
para-aortic | 8.214 | 2.174–31.036 | 0.0019 |
| LN metastasis by
pathological analysis |
|
|
|
|
(–) | 1.000 |
|
|
|
(+) | 10.971 | 1.279–94.104 | 0.0289 |
| HPV infection |
|
|
|
|
(–) | 1.000 |
|
|
|
(+) | 1.000 | 0.282–3.547 | 1.0000 |
| Tumor cell
type |
|
|
|
|
SCC | 1.000 |
|
Adenoca/adenosq | 1.309 | 0.500–3.426 | 0.5831 |
Furthermore, significant predictive roles were
identified for the following eight factors: i) Stage (P<0.0001);
ii) tumor size (≤4 vs. 4–6 cm, P=0.0147; and ≤4 vs. >6 cm,
P<0.0001); iii) SCC-Ag level of ≤2 vs. >15 ng/ml (P=0.0291);
iv) lower third vaginal involvement (P<0.0001); v)
hydronephrosis (P=0.0003); vi) bladder/rectal involvement
(P=0.0015); vii) pelvic (P=0.0017) or para-aortic (P=0.0019) LN
metastasis detected by imaging vs. no metastasis; and viii) pelvic
LN identified by pathological analysis (P=0.0289).
Multivariate analysis of clinical
factors
Of the eight factors identified as significant by
univariate analysis, lower third vaginal involvement,
hydronephrosis and bladder/rectal involvement were excluded from
multivariate analysis as they are included in the FIGO staging
system. In addition, LN metastasis detected by pathology was
excluded due to the small number of cases available for analysis
(n=46). However, age was included due to its marginal significance
and clinical relevance.
The results of the multivariate analysis are
summarized in Table III. The RR
values were found to be 1.233 [95% confidence interval (CI),
0.918–1.686; P=0.1589] for patient age and 1.460 (95% CI,
0.964–2.210; P=0.0739) for tumor stage. Furthermore, the RR values
for ≤2, 2–5, 5–15 and >15 ng/ml SCC-Ag were 1.000, 0.519 (95%
CI, 0.153–1.760; P=0.2921), 0.458 (95% CI, 0.149–1.402; P=0.1712)
and 0.944 (95% CI, 0.378–2.360; P=0.9022), respectively. In
addition, the RR values for tumor sizes of ≤4, 4–6 and >6 cm
were 1.000, 3.421 (95% CI, 1.077–10.872; P=0.0371) and 6.599 (95%
CI, 1.952–22.311; P=0.0024), respectively. In LN metastasis
detected by imaging, the RR values were 2.319 (95% CI, 1.000–5.376;
P=0.0499) for positive pelvic LN and 3.204 (95% CI, 0.561–18.302;
P=0.1902) for positive para-aortic LN.
 | Table III.Multivariate Cox proportional hazards
model for survival in advanced cervical cancer patients. |
Table III.
Multivariate Cox proportional hazards
model for survival in advanced cervical cancer patients.
| Variable | RR | 95% CI | P-value |
|---|
| Ageb | 1.244 | 0.918–1.686 | 0.1589 |
| Stageb | 1.460 | 0.964–2.210 | 0.0739 |
| SCC-Agb, ng/ml |
|
|
|
| ≤2 | 1.000 |
|
|
|
2–5 | 0.519 | 0.153–1.760 | 0.2921 |
|
5–15 | 0.458 | 0.149–1.402 | 0.1712 |
|
>15 | 0.944 | 0.378–2.360 | 0.9022 |
| Tumor
sizea, cm |
|
|
|
| ≤4 | 1.000 |
|
|
|
4–6 | 3.421 | 1.077–10.872 | 0.0371 |
|
>6 | 6.599 | 1.952–22.311 | 0.0024 |
| LN metastasis by
imaging |
|
|
|
|
(–) | 1.000 |
|
|
| (+)
pelvica | 2.319 | 1.000–5.376 | 0.0499 |
| (+)
para-aorticb | 3.204 | 0.561–18.302 | 0.1902 |
Of the five factors analyzed, the following two were
determined as independent predictive variables by multivariate
analysis: i) Tumor size (≤4 vs. 4–6 cm, P=0.0371; and ≤4 vs. >6
cm, P=0.0024); and ii) pelvic LN metastasis detected by imaging vs.
no metastasis (P=0.0499). By contrast, para-aortic LN metastasis
(P=0.1902), age (P=0.1589), tumor stage (P=0.0739) and serum SCC-Ag
level (P>0.1712) were not found to be independent predictive
factors.
Survival analysis
In the current study, the median follow-up period
was 55 months (range, 12–70 months), the median survival rate was
33.0 months and the mean survival rate was 44.5 months. The
Kaplan-Meier method was applied to determine the duration of
patient survival according to each of the investigated variables.
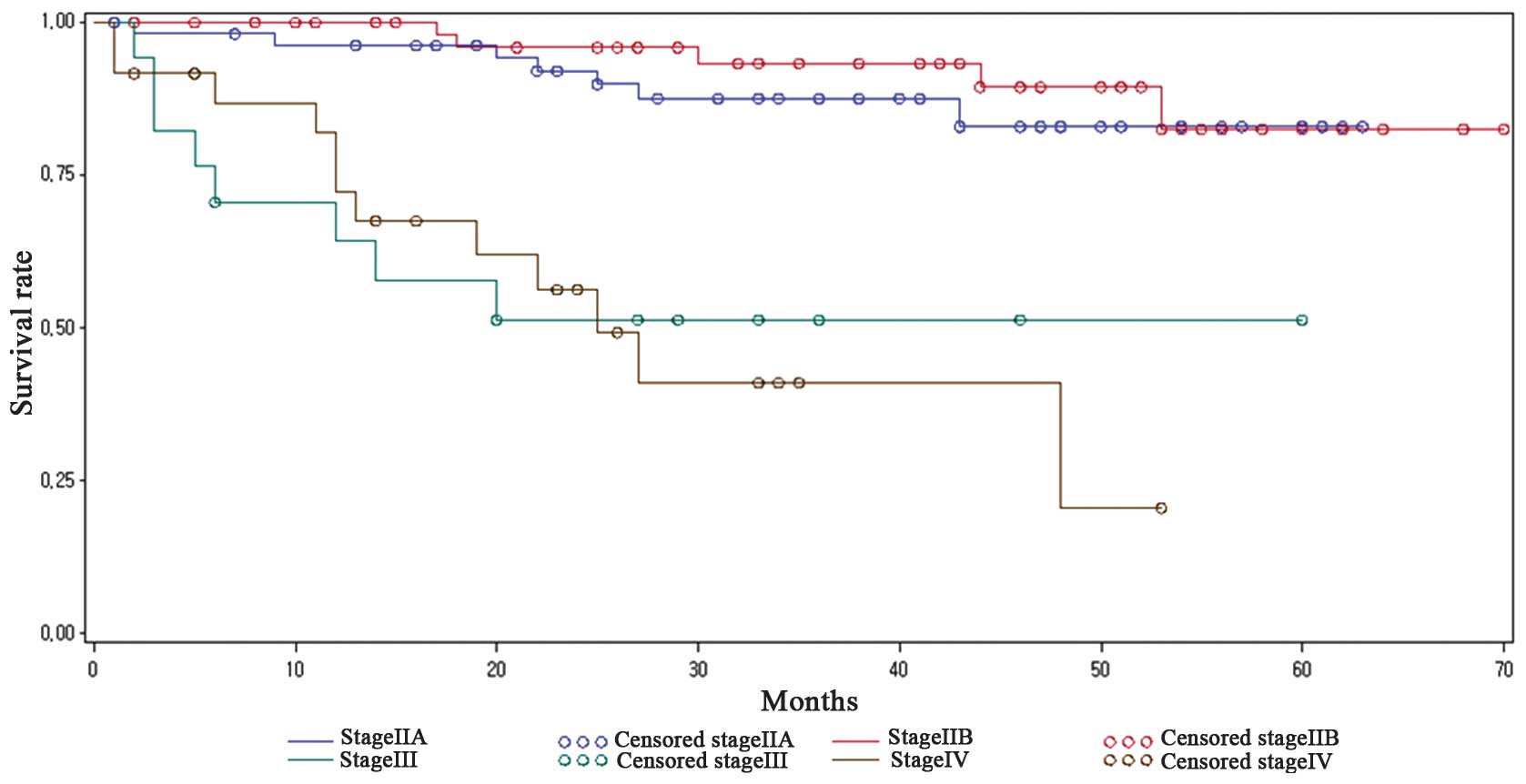
Significantly different mean survival rates were identified among
the various tumor stages (Fig. 1),
including 39.91 months for stage IIA, 50.60 months for stage IIB,
14.34 months for stage III and 28.67 months for stage IV (log-rank
test, P<0.0001). Although the mean survival duration was
significantly different, marked overlapping of the survival
durations were observed between stages IIA and IIB, and stages
IIIA/B and IVA/B of cervical cancer.
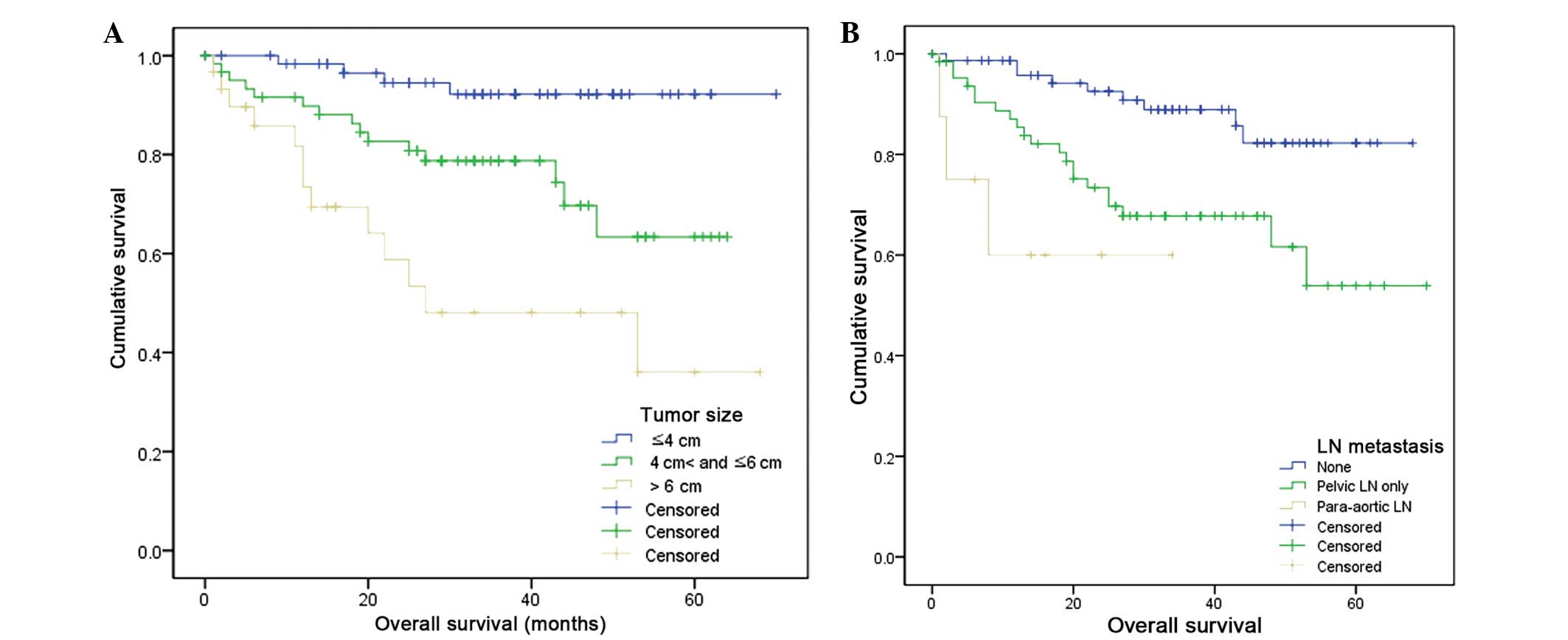
Statistically significant differences in survival
were identified according to tumor size and the presence of LN
metastasis (Fig. 2). For instance,
the mean survival rates of patients with a maximum tumor diameter
of ≤4, 4–6 and >6 cm were 66.1, 50.3 and 38.3 months,
respectively (log-rank test, P<0.001). Furthermore, the mean
survival rates of patients with no LN, pelvic LN and para-aortic LN
metastases were 60.9, 49.3 and 22.0 months, respectively (log-rank
test, P=0.001). No overlapping in survival duration was identified
according to these variables.
Discussion
The FIGO staging system is widely used to select the
appropriate treatment strategy and predict the prognosis for
cervical cancer (2). Accurate staging
of cervical cancer is essential for therapeutic decision-making,
determining the prognosis and comparing the results of different
treatment modalities (2). However,
the current FIGO clinical staging system has limited prognostic
accuracy and an increased possibility of staging errors in patients
with advanced disease (18–20). Thus, the prognosis of patients with
advanced cervical cancer appears to be variable, even among
patients at the same stage (17,21). In
the present study, a significant overlap of survival duration was
observed between cervical cancer stages IIA and IIB, as well as
stages IIIA/B and IVA/B (Fig. 1),
demonstrating the difficulty of accurately predicting survival
using stage alone in advanced cervical cancer cases.
Furthermore, the FIGO staging system does not
include pathological factors, such as tumor size and LN
involvement, which are established prognostic factors (6,13,18–22). Thus,
developing a novel tool for accurate prognostic prediction in
advanced cervical cancer is essential.
As tumor size is closely associated with prognosis,
the preoperative division of stage IIA into substages IIA1 and IIA2
has been attempted based on clinical measurements of the maximum
tumor diameter (2). The tumor size is
reflected in FIGO stages IA–IIA, but not in stage IIB or higher,
from which suffered a number of the subjects in the present study.
As a consequence, tumor size was analyzed as a prognostic factor in
the current study. Classifying tumors into stages IIB1 and IIB2
based on a tumor size of >6 cm was not found to be appropriate
for stage IIB cancer.
The Cox proportional hazards model was used to
analyze factors considered to be associated with the prognosis of
cervical cancer. Significant variables with high RR were identified
as follows: Lower third vaginal involvement (RR, 12.976),
pathological LN metastasis (RR, 10.971), LN metastasis (para-aortic
and/or pelvic) detected by imaging (RR, 8.214), tumor size of >6
cm (RR, 8.691), hydronephrosis (RR, 3.740), bladder/rectal
involvement (RR, 3.216) and tumor stage (RR, 2.228). Among these
variables, lower third vaginal involvement was associated with a
greater risk of poor prognosis compared with bladder/rectal
involvement, resulting in uncertainty regarding the reliability of
lower third vaginal and bladder/rectal involvement as requirements
for FIGO stages IIIB and IVA, respectively. Improvements in
palliative treatment, including percutaneous nephrostomy, diversion
cystostomy/colostomy and neoadjuvant chemotherapy, may have
affected these survival results (23–25).
The involvement of HPV in cervical cancer
development is well-established; however, attempts to determine the
prognostic significance of the presence or absence of HPV DNA in
patients with cervical cancer have yielded conflicting results
(26–29). HPV-negative cervical carcinoma was
associated with poor survival in a number of reports (26–29), but
not in other studies (30,31). Furthermore, high-risk HPV infection is
an important and well-established risk factor for cancer
development, with a previous study identifying high-risk HPV
infection as an independent prognostic factor in early stage
cervical cancer (32). However, the
present study identified no statistically significant correlation
between high-risk HPV infection and cervical cancer prognosis
(P=1.0000). This indicates that HPV infection may be associated
with prognosis in early but not advanced-stage cervical cancer.
The specific tumor cell type of cervical cancer has
been investigated (12). Previous
studies have demonstrated that early and advanced stage
adenocarcinomas are more aggressive and associated with decreased
survival rates (33–36). However, the present study indicated
that the tumor cell type was not significantly associated with
prognosis (P=0.5831).
Based on the results of the present study, the
association between the overall survival and prognostic factors in
stage IIA or higher cervical cancer patients are as follows:
Survival analysis determined that tumor sizes of >6 cm
(P=0.0024) and pelvic LN metastasis detected by imaging (P=0.0499)
were independent factors of advanced-stage cervical cancer.
Preoperative staging or pretreatment evaluation by CT or MRI have
previously been demonstrated as predictive factors (36,37) and
are commonly performed, indicating that it may be clinically
acceptable to investigate tumor size and LN status by imaging.
Thus, based on the present data, it is proposed that the inclusion
of tumor size and imaging detection of pelvic LN metastasis in a
future FIGO staging system may improve the accuracy of survival
prediction. In addition, cancer stage has not been previously
identified as an independent risk factor in early stage cervical
cancer (22). The results of the
present study demonstrated that cancer stage is not an independent
risk factor in advanced-stage cervical cancer (P=0.0739) and, thus,
verifying that the FIGO stage alone is not an accurate method for
predicting the prognosis of advanced cervical cancer. Therefore,
modification of the currently employed advanced cervical cancer
staging system is required. Furthermore, an SCC-Ag level of >15
ng/ml was associated with survival in univariate analysis; however,
it was not identified as an independent variable in the
multivariate analysis. Previous studies have reported that SCC-Ag
levels were associated with FIGO stage, tumor volume and the risk
of developing LN metastasis (38,39);
therefore, SCC-Ag may not be an independent risk factor, but is
associated with survival. In addition, data from the present study
did not support that the age at diagnosis is a prognostic factor,
contrary to previous studies (11–13,40). The
revised FIGO staging system is considered to provide more accurate
details for dividing stage IIA cervical cancer into stages IIA1 and
IIA2, according to a tumor size of 4 cm. In addition, the present
authors consider it necessary for stages III and IV to be divided
into substages according to the tumor size.
However, the current study presents a number of
limitations. The first limitation is that the LN status was
determined by CT or MRI imaging, despite the accuracy of LN
metastasis detection by CT or MRI varying between 75–86 and
75–100%, respectively, and thus potentially biasing the current
results (41). To eliminate this
bias, determination of the LN status by pathological examination is
required. However, staging surgery in patients with advanced stage
cervical cancer is associated with potential morbidity. Recently,
positron emission tomography (PET)-CT has been proven to be a
useful tool for detecting LN metastasis with high sensitivity and
specificity (42); thus, assessment
of LN status using PET-CT may aid in reducing bias. The second
limitation is that the present study included 33 patients with
small cell carcinoma, adenocarcinoma and adenosquamous cell
carcinoma, which are more likely to metastasize compared with other
nonsquamous cell carcinoma histological types. Inclusion of these
patients may have influenced the estimation of other prognostic
variables, including the HPV infection status, SCC-Ag levels and
age. Another limitation is the fact that patients underwent various
treatment strategies (including, surgery plus chemotherapy, surgery
plus CCRT and CCRT alone), which may have potentially influenced
the survival analysis in the present study. In addition, the HPV
types were not analyzed, since HPV typing was not available at
Kangdong Sacred Heart Hospital or Kangnam Sacred Heart Hospital
during the study period. Finally, the significance of the present
study is limited due to its retrospective nature and small number
of patients included. These limitations may be overcome by
conducting a large sample size randomized trial.
In conclusion, accurately predicting survival rates
in advanced cervical cancer is difficult using the stage alone. In
the present study, tumor size and pelvic LN metastasis determined
by CT and/or MRI were identified as independent predictive factors
for the prognosis of stage II–IV cervical cancer. These factors may
provide more clinically significant prognostic data compared with
the currently used FIGO staging system.
Acknowledgements
This abstract was presented at the 15th Biennial
Meeting of the International Gynecologic Cancer Society, November
8-11, 2014, Melbourne, Australia, and published as abstract no.
IGCSM-0886 in the International Journal of Gynecological Cancer,
Volume 24, Issue 9, 2014.
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M, et al:
GLOBOCAN 2008: Cancer incidence, mortality and prevalence
worldwide. IARC Press; Lyon: 2008
|
|
2
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lilic V, Lilic G, Filipovic S, Milosevic
J, Tasic M and Stojilijkovic M: Modern treatment of invasive
carcinoma of the uterine cervix. J BUON. 14:587–592.
2009.PubMed/NCBI
|
|
4
|
Keys HM, Bundy BN, Stehman FB, et al:
Cisplatin, radiation, and adjuvant hysterectomy compared with
radiation and adjuvant hysterectomy for bulky stage IB cervical
carcinoma. N Engl J Med. 340:1154–1161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morris M, Eifel PJ, Lu J, et al: Pelvic
radiation with concurrent chemotherapy compared with pelvic and
para-aortic radiation for high-risk cervical cancer. N Engl J Med.
340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rose PG, Bundy BN, Watkins EB, et al:
Concurrent cisplatin-based radiotherapy and chemotherapy for
locally advanced cervical cancer. N Engl J Med. 340:1144–1153.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whitney CW, Sause W, Bundy BN, et al:
Randomized comparison of fluorouracil plus cisplatin versus
hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA
carcinoma of the cervix with negative para-aortic lymph nodes: a
Gynecologic Oncology Group and Southwest Oncology Group study. J
Clin Oncol. 17:1339–1348. 1999.PubMed/NCBI
|
|
8
|
Kupets R and Covens A: Is the
International Federation of Gynecology and Obstetrics staging
system for cervical carcinoma able to predict survival in patients
with cervical carcinoma?: an assessment of clinimetric properties.
Cancer. 92:796–804. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noguchi H, Shiozawa I, Sakai Y, Yamazaki T
and Fukuta T: Pelvic lymph node metastasis of uterine cervical
cancer. Gynecol Oncol. 27:150–158. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Avall-Lundqvist EH, Sjövall K, Nilsson BR
and Eneroth PH: Prognostic significance of pretreatment serum
levels of squamous cell carcinoma antigen and CA 125 in cervical
carcinoma. Eur J Cancer. 28A:1695–1702. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rutledge FN, Mitchell MF, Munsell M, Bass
S, McGuffee V and Atkinson EN: Youth as a prognostic factor in
carcinoma of the cervix: a matched analysis. Gynecol Oncol.
44:123–130. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kosary CL: FIGO stage, histology,
histologic grade, age and race as prognostic factors in determining
survival for cancers of the female gynecological system: an
analysis of 1973–87 SEER cases of cancers of the endometrium,
cervix, ovary, vulva, and vagina. Semin Surg Oncol. 10:31–46. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brewster WR, DiSaia PJ, Monk BJ, Ziogas A,
Yamada SD and Anton-Culver H: Young age as a prognostic factor in
cervical cancer: results of a population-based study. Am J Obstet
Gynecol. 180:1464–1467. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishikawa H, Nakanishi T, Inoue T and
Kuzuya K: Prognostic factors of adenocarcinoma of the uterine
cervix. Gynecol Oncol. 73:42–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wagenaar HC, Trimbos JB, Postema S, et al:
Tumor diameter and volume assessed by magnetic resonance imaging in
the prediction of outcome for invasive cervical cancer. Gynecol
Oncol. 82:474–482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miller TR and Grigsby PW: Measurement of
tumor volume by PET to evaluate prognosis in patients with advanced
cervical cancer treated by radiation therapy. Int J Radiat Oncol
Biol Phys. 53:353–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tseng JY, Yen MS, Twu NF, et al:
Prognostic nomogram for overall survival in stage IIB-IVA cervical
cancer patients treated with concurrent chemoradiotherapy. Am J
Obstet Gynecol. 202:174. e171–177. 2010.
|
|
18
|
Van Nagell JR Jr, Roddick JW Jr and Lowin
DM: The staging of cervical cancer: inevitable discrepancies
between clinical staging and pathologic findinges. Am J Obstet
Gynecol. 110:973–978. 1971.PubMed/NCBI
|
|
19
|
Averette HE, Ford JH Jr, Dudan RC,
Girtanner RE, Hoskins WJ and Lutz MH: Staging of cervical cancer.
Clin Obstet Gynecol. 18:215–232. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lagasse LD, Creasman WT, Shingleton HM,
Ford JH and Blessing JA: Results and complications of operative
staging in cervical cancer: experience of the Gynecologic Oncology
Group. Gynecol Oncol. 9:90–98. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seo Y, Yoo SY, Kim MS, et al: Nomogram
prediction of overall survival after curative irradiation for
uterine cervical cancer. Int J Radiat Oncol Biol Phys. 79:782–787.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Behtash N, Karimi Zarchi M and Deldar M:
Preoperative prognostic factors and effects of adjuvant therapy on
outcomes of early stage cervical cancer in Iran. Asian Pac J Cancer
Prev. 10:613–618. 2009.PubMed/NCBI
|
|
23
|
Angioli R, Plotti F, Montera R, et al:
Neoadjuvant chemotherapy plus radical surgery followed by
chemotherapy in locally advanced cervical cancer. Gynecol Oncol.
127:290–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lapitan MC and Buckley BS: Impact of
palliative urinary diversion by percutaneous nephrostomy drainage
and ureteral stenting among patients with advanced cervical cancer
and obstructive uropathy: a prospective cohort. J Obstet Gynaecol
Res. 37:1061–1070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sardi JE, Giaroli A, Sananes C, et al:
Long-term follow-up of the first randomized trial using neoadjuvant
chemotherapy in stage Ib squamous carcinoma of the cervix: the
final results. Gynecol Oncol. 67:61–69. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harima Y, Sawada S, Nagata K, Sougawa M
and Ohnishi T: Human papilloma virus (HPV) DNA associated with
prognosis of cervical cancer after radiotherapy. Int J Radiat Oncol
Biol Phys. 52:1345–1351. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riou G, Favre M, Jeannel D, Bourhis J, Le
Doussal V and Orth G: Association between poor prognosis in
early-stage invasive cervical carcinomas and non-detection of HPV
DNA. Lancet. 335:1171–1174. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Higgins GD, Davy M, Roder D, Uzelin DM,
Phillips GE and Burrell CJ: Increased age and mortality associated
with cervical carcinomas negative for human papillomavirus RNA.
Lancet. 338:910–913. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ikenberg H, Sauerbrei W, Schottmüller U,
Spitz C and Pfleiderer A: Human papillomavirus DNA in cervical
carcinoma - correlation with clinical data and influence on
prognosis. Int J Cancer. 59:322–326. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rose BR, Thompson CH, Cossart YE, Elliot
PE and Tattersall MH: Papillomavirus DNA and prognosis in cervical
cancer. Lancet. 337:4891991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lombard I, Vincent-Salomon A, Validire P,
et al: Human papillomavirus genotype as a major determinant of the
course of cervical cancer. J Clin Oncol. 16:2613–2619.
1998.PubMed/NCBI
|
|
32
|
Burger RA, Monk BJ, Kurosaki T, et al:
Human papillomavirus type 18: association with poor prognosis in
early stage cervical cancer. J Natl Cancer Inst. 88:1361–1368.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakanishi T, Ishikawa H, Suzuki Y, Inoue
T, Nakamura S and Kuzuya K: A comparison of prognoses of pathologic
stage Ib adenocarcinoma and squamous cell carcinoma of the uterine
cervix. Gynecol Oncol. 79:289–293. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eifel PJ, Burke TW, Morris M and Smith TL:
Adenocarcinoma as an independent risk factor for disease recurrence
in patients with stage IB cervical carcinoma. Gynecol Oncol.
59:38–44. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sigurdsson K, Hrafnkelsson J, Geirsson G,
Gudmundsson J and Salvarsdóttir A: Screening as a prognostic factor
in cervical cancer: analysis of survival and prognostic factors
based on Icelandic population data, 1964–1988. Gynecol Oncol.
43:64–70. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hricak H, Gatsonis C, Chi DS, et al:
American College of Radiology Imaging Network 6651; Gynecologic
Oncology Group 183: Role of imaging in pretreatment evaluation of
early invasive cervical cancer: results of the intergroup study
American College of Radiology Imaging Network 6651 - Gynecologic
Oncology Group 183. J Clin Oncol. 23:9329–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Subak LL, Hricak H, Powell CB, Azizi L and
Stern JL: Cervical carcinoma: computed tomography and magnetic
resonance imaging for preoperative staging. Obstet Gynecol.
86:43–50. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gadducci A, Tana R, Cosio S and Genazzani
AR: The serum assay of tumour markers in the prognostic evaluation,
treatment monitoring and follow-up of patients with cervical
cancer: a review of the literature. Crit Rev Oncol Hematol.
66:10–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takeda M, Sakuragi N, Okamoto K, et al:
Preoperative serum SCC, CA125, and CA19-9 levels and lymph node
status in squamous cell carcinoma of the uterine cervix. Acta
Obstet Gynecol Scand. 81:451–457. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kapp DS, Fischer D, Gutierrez E, Kohorn EI
and Schwartz PE: Pretreatment prognostic factors in carcinoma of
the uterine cervix: a multivariable analysis of the effect of age,
stage, histology and blood counts on survival. Int J Radiat Oncol
Biol Phys. 9:445–455. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boss EA, Barentsz JO, Massuger LF and
Boonstra H: The role of MR imaging in invasive cervical carcinoma.
Eur Radiol. 10:256–270. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kidd EA, El Naqa I, Siegel BA, Dehdashti F
and Grigsby PW: FDG-PET-based prognostic nomograms for locally
advanced cervical cancer. Gynecol Oncol. 127:136–140. 2012.
View Article : Google Scholar : PubMed/NCBI
|