Introduction
Neurokinins (NKs) are small neuropeptides that are
present in the tumor microenvironment and can act on different
stages of carcinogenesis (1).
Substance P (SP) and NK A (NKA) and B (NKB) are classical members
of the NK family, which exert their biological effects by
activating NK receptors denoted as NK1R, NK2R and
NK3R (also known as TAC1R, TAC2R and
TAC3R) (2). SP, the endogenous
ligand for NK1R, activates responses correlated with tumor
growth in several human cell lines bearing NK1R receptors
(3). Moreover, NKA, the endogenous
ligand for NK2R, is a good predictor of survival in patients
with stage IV well-differentiated small bowel neuroendocrine
neoplasms (4). Numerous tumors that
express NKRs can misuse the NK-induced signaling of normal cells to
promote the proliferation and survival of cancer cells, releasing
cytokines and soluble mediators that favor tumor growth (5). However, the precise involvement and
significance of NK in cancer pathologies remains to be fully
defined.
NKRs belong to the family of seven transmembrane
G-protein coupled receptors. NK1R and NK2R are
dispersed throughout the central and peripheral nervous systems,
while NK3R is distributed mainly in the central nervous
system (6). Human NK1R and
NK2R are 407- and 398-amino acid proteins, respectively
(7). NK2R is expressed in a
variety of organs, including the gastrointestinal tract, while
functional data suggest that NK2R could be significant in
mediating the NK-evoked smooth muscle contraction of organs
(8). The prevalence of the
NK2R mRNA α isoform indicates that NK2R is
significantly involved in regulating human colonic functions
(9).
In the United States, colorectal cancer (CRC) has
the third highest incidence of new cases and the third highest rate
of cancer-associated mortality, with 142,280 and 50,830 cases,
respectively, in 2013 (10). It is
also estimated that the mortality rate for CRC has been gradually
increasing in China (11). The
occurrence and development of this deadly disease may vary among
patients; this may be attributed to variations in genotype
patterns. Recently, a population-based epidemiological study in a
Chinese population found that the NK2R rs4644560 genotype
appeared to be associated with a decreased risk of CRC when
combined with NK1R rs10198644 (12). This finding indicates that these two
subtypes of NKR gene may play an important role in CRC. The
present study used blood samples collected from patients with CRC
to clarify the potential association between the single nucleotide
polymorphisms (SNP) of NK2R rs4644560/NK1Rrs 10198644
and a range of clinical factors important in CRC.
Materials and methods
Participants
The present study was approved by the Ethics
Committee of the First Affiliated Hospital, School of Medicine,
Zhejiang University (Hangzhou, Zhejiang, China). Peripheral blood
samples were collected from 199 patients with CRC following surgery
in the First Affiliated Hospital, School of Medicine, Zhejiang
University, between May and October 2013. All patients provided
written informed consent.
Details of patient demographics and clinical
information were recorded, including age, gender, alcohol
consumption, tobacco exposure, primary tumor site, diagnostic
stage, number of positive lymph nodes, differentiation grade and
carcinoembryonic antigen (CEA) level. Lifestyle factors, including
alcohol consumption and tobacco use, were defined as follows: An
individual who consumed alcohol more than once per day for at least
three months was considered as an alcohol drinker and an individual
who had smoked more than one cigarette per day for over one year
was considered as a tobacco user. Diagnostic stage was confirmed
according to the National Comprehensive Cancer Network guidelines
for CRC (13). Differentiation grade
was defined as poor, moderate and well. An abnormal CEA level was
defined as a level of >5 ng/ml.
Genotyping
Genomic DNA was extracted from whole blood samples
using the QIAamp DNA Mini kit (Qiagen GmbH, Hilden, Germany)
according to the manufacturer's instructions. Genotyping was
performed with polymerase chain reaction (PCR) amplification and
direct-sequencing. Primers were designed with Primer Premier 5.0
software (PREMIER Biosoft, Palo Alto, CA, USA) and synthesized by
Genewiz Biotech (Beijing, China). The primer sequences used for
amplification were as follows: NK1R forward,
5′-AAAGTTGGGATCTGCTTACACT-3′ and reverse,
5′-TTTCTTTTCTCTCACTGTCCCA-3′; and NK2R forward,
5′-ATGCTGCTGTGTCATCTGCT-3′ and reverse, 5′-TATCTTGCCCAGGTTGGTCT-3′.
The PCR was performed with 50 ng of genomic DNA, 0.25 µM of each
primer, 200 µM of each deoxyribonucleotide, 3 mM MgCl2
and 0.2 Units of Taq DNA polymerase (Takara, Dalian, China). The
PCR amplification was performed as follows: Initial denaturation at
95°C for 5 min, followed by 9 cycles of denaturation at 95°C for 45
sec, annealing at 58°C for 45 sec (decreasing by 0.2°C every cycle)
and elongation at 72°C for 30 sec; then 34 cycles of denaturation
at 95°C for 45 sec, annealing at 55°C for 45 sec (decreasing by
0.2°C every cycle) and elongation at 72°C for 30 sec; and a final
elongation at 72°C for 5 min.
The PCR products were purified using the SAP/EXO PCR
kit (New England Biolabs, Inc., Ipswich, MA, USA). Two microliters
of the purified amplicons were directly used for a sequencing
reaction with the Big Dye Terminator cycle sequencing mix v3.1
(Applied Biosystems Life Technologies, Foster City, CA, USA). Dye
purification was performed by alcohol/sodium acetate precipitation.
Sequence analysis was performed on an ABI 3730×1 DNA analyzer
(Applied Biosystems Life Technologies).
Statistical analysis
SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA) was
used for the analysis of the final results. P<0.05 was
considered to indicate a statistically significant difference. A
two-tailed t-test (double samples heteroscedastic hypothesis) was
used to clarify the association between the number of positive
lymph nodes and tobacco exposure. Pearson's correlation was used to
analyze the association between the number of positive lymph nodes
and diagnostic stage. Bartlett's χ2 test was used to
analyze the association between the number of positive lymph nodes
and the genotypes of each NKR. The data was assessed for
homogeneity of variance following logarithmic transformation.
A one-way analysis of variance (ANOVA) was used to
further clarify the impact of different genotype combinations of
NK2R and NK1R on the number of positive lymph nodes.
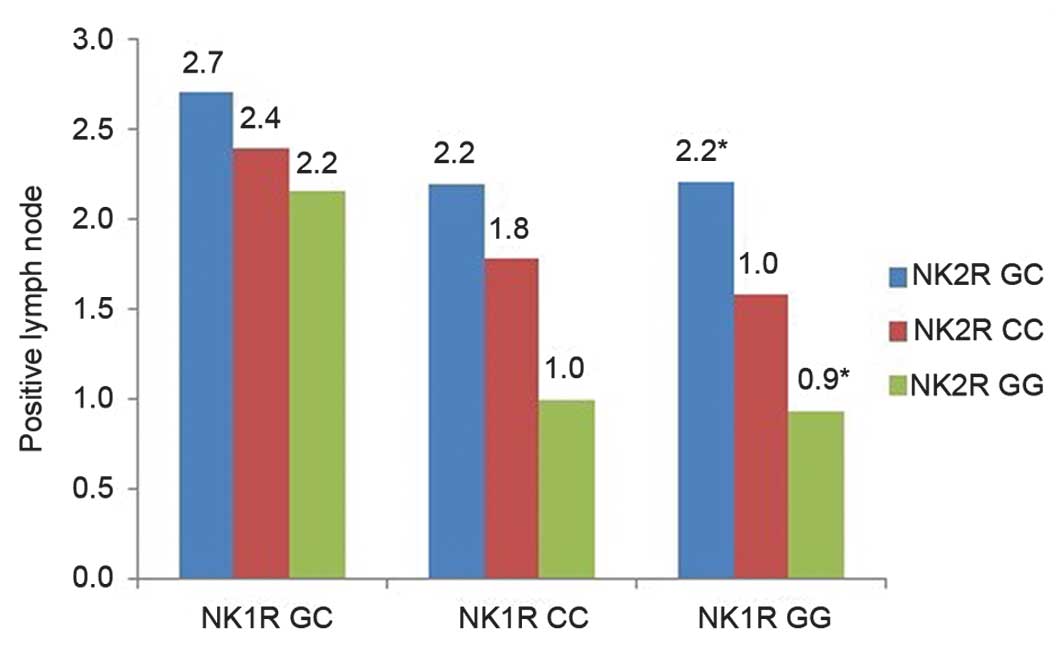
Prior to the analysis, the genotypes of NK2R and NK1R
were first mixed into nine groups (Fig.
1).
Results
A total of 199 patients with pathologically
confirmed CRC were enrolled in the study following surgical
resection. The median age of the patients was 61 years (range,
23–84 years), and the detailed patient characteristics are listed
in Table I. The allele frequencies of
NK1R rs10198644 GC, CC and GG were 52, 17 and 31%,
respectively, while for NK2R rs4644560 GC, CC and GG, the
frequencies were 36, 50 and 14%. A comprehensive analysis of all
patient factors, including demographic, lifestyle and clinical
factors, was performed in order to clarify their association with
the genotypes of NK1R and NK2R, as well as the
interaction between them. The positive outcomes are described
below.
 | Table I.Patient characteristics (n=199). |
Table I.
Patient characteristics (n=199).
| Characteristic | Number (%) |
|---|
| Gender |
|
| Male | 117 (59) |
|
Female | 82 (41) |
| Location of primary
tumor |
|
| Left
colon | 34 (17) |
| Right
colon | 47 (24) |
|
Rectum | 118 (59) |
| Positive lymph
nodes |
|
| 0–1 | 70 (35) |
| 2–3 | 94 (47) |
|
4–6 | 27 (14) |
| ≥7 | 8 (4) |
| Alcohol
drinker |
|
|
Yes | 48 (24) |
| No | 151 (76) |
| Tobacco user |
|
|
Yes | 61 (31) |
| No | 138 (69) |
In total, there were 191 patients for whom
information regarding positive lymph node numbers and tobacco
exposure was available. Among these patients, 53 were in the
tobacco group and 138 were in the non-tobacco group, and the mean
number of positive lymph nodes was 3.2 and 2.0, respectively. The
t-test showed that the tobacco group exhibited significantly more
positive lymph nodes than the non-tobacco group (P=0.04; Table II).
 | Table II.Association between tobacco use and
the number of positive lymph nodes. |
Table II.
Association between tobacco use and
the number of positive lymph nodes.
| Parameter | Tobacco | Non-tobacco | T-value | P-value
(two-tailed) |
|---|
| Mean number of
positive lymph nodes |
3.2 | 2.0 | 2.10 | 0.04a |
| Square
deviation | 16.5 | 3.1 |
|
|
| Number of
patients | 53 | 138 |
|
|
In addition, among the 196 patients for whom
information on stage and number of positive lymph nodes was
available, Pearson's correlation found a positive correlation
between the number of positive lymph nodes and the diagnostic stage
of the tumor (r=0.48; P<0.001).
Finally, information pertaining to the number of
positive lymph nodes and the NKR genotypes was available in
165 patients. Bartlett's χ2 analysis showed that
NK2R rs4644560 GC was associated with significantly more
positive lymph nodes than rs4644560 CC (mean, 2.2 vs. 1.3; P=0.016;
Table III). Furthermore, the
one-way ANOVA highlighted the interaction between NK2R
rs4644560 and NK1R rs10198644, and showed that the number of
positive lymph nodes was increased significantly in the rs4644560
GC/rs10198644 GG group compared with the rs4644560 GG/rs10198644 GG
group (mean, 2.2 vs. 0.9; P=0.04), as shown in Fig. 1.
 | Table III.Comparison of lymph nodes between the
three genotypes of NK2R rs4644560. |
Table III.
Comparison of lymph nodes between the
three genotypes of NK2R rs4644560.
| Genotypes | Number of
patients | Mean number of
positive lymph nodes |
|---|
| GC | 63 | 2.2a |
| CC | 79 | 1.3a |
| GG | 23 | 1.6 |
| F-value |
| 4.27 |
| P-value |
| 0.016a |
Discussion
Based on the identification of the role of NK
pathway genes in the risk of CRC, the allele frequencies of SNPs in
two selected subtypes of NK receptor, NK1R and NK2R,
were investigated and their potential association with various
clinical factors was analyzed. Notably, despite the limitation of
the sample size, tobacco exposure, diagnostic stage and the
selected NKR genotypes were all found to be significantly
associated with the number of metastatic lymph nodes, which is well
known to be one of the most important prognostic factors in CRC
(14,15).
SNPs in receptor-encoded genes can affect a number
of aspects of receptor function (16), and may also result in an increased
susceptibility to disease or produce variable responses to
therapeutic agents (17). There are
several novel gene polymorphisms, including those for
interleukin-10, SDF1α and embryonic ectoderm development, which
have been proven to be associated with lymph node metastasis in CRC
(18,19).
It is well known that lifestyle factors, such as
cigarette smoking and alcohol consumption, may contribute to the
risk of cancer. In the present study, patients exposed to tobacco
exhibited significantly more positive lymph nodes than those
patients not exposed to tobacco; this result is consistent with the
finding that tobacco exposure has a detrimental effect on CRC
(20). In agreement with a previously
published study (21), the present
study also found that diagnostic stage was associated with the
number of metastatic lymph nodes.
As aforementioned, metastatic lymph nodes may be
associated with a variety of clinical factors, therefore, its
potential correlation with NK receptor polymorphisms, and their
mutual interactions, was worthy of investigation. A previous study
has shown that the stimulation of NK2R leads to partial
blunting of the enhanced stimulatory effects mediated by
NK1R. By contrast, stimulatory hematopoietic cytokines
upregulate NK1R expression and downregulate the
constitutively expressed NK2R in bone marrow stroma
(22). In the present study, the role
of NK2R rs4644560 GC in predicting lymph node metastasis was
clarified based on the finding that this heterozygote is associated
with more positive lymph nodes than the homozygote (CC) of
NK2R rs4644560. Notably, despite the complexity of
information on clinical interactions, the interactive model of
NK2R and NK1R was also found to be useful.
NK2R rs4644560 GC was associated with more positive lymph
nodes than the corresponding GG genotype when combined with
NK1R rs10198644 GG. Further research should be performed to
elucidate the potential mechanism underlying this interaction.
Little has been known with regard to the mechanism
of NK2R in CRC carcinogenesis. One study, however, began to
shed light on this area. The study found that activation of
NK2R in afferent neurons led to the protein kinase C
(PKC)-induced phosphorylation of a certain gene (23). PKCδ was known to negatively modulate
the canonical Wnt pathway and cell proliferation in colon tumor
cell lines (24). Moreover, the
connection between NK2R and PKC was confirmed by another
study, which showed that stimulation of NK2R was not only
linked to PKC activation, but that it was also associated with the
activation of a multidrug resistance protein transporter in
vivo (25). Extensive reviews
have also described the neuropeptide receptors as targets for the
treatment and diagnosis of cancer (5). Based on the present findings, one novel
therapeutic strategy for colon cancer in the future may be the
inhibition of the potential signaling pathways associated with
certain NK receptor genotypes.
Pending further research to clarify the potential
mechanism of the malignant inclination of the NK2R rs4644560
GC genotype or its combination with NK1R rs10198644 GG, the
present study indicates that these genotypes may act as a promising
prognostic marker, which may predict lymph node metastasis in CRC
patients.
Acknowledgements
The authors would like to thank Professor Ping Fang
from Zhejiang University (Hangzhou, Zhejiang, China) for providing
statistical technology support, and Aparna Rao (Peter MacCallum
Cancer Centre) who provided so much assistance with perfecting the
language of the manuscript and supplied useful suggestions and
comments. This study was supported by the Major Scientific Project
of Zhejiang Province (grant no. 2012C13014-2). The authors would
also like to express their gratitude to the Consulting Project of
the Chinese Academy of Engineering (grant no. 2012-XY-12-4), the
National Natural Science Foundation of China (grant nos. 81201557,
81201783, 81272679, 81372463 and 31000496), the Zhejiang Natural
Science Foundation (grant no. LY13H160007) and the Zhejiang
Medicines and Health Science and Technology Project (grant no.
201348801) for their support.
References
|
1
|
Palma C: Tachykinins and their receptors
in human malignancies. Curr Drug Targets. 7:1043–1052. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Severini C, Improta G, Falconieri-Erspamer
G, et al: The tachykinin peptide family. Pharmacol Rev. 54:285–322.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mayordomo C, García-Recio S, Ametller E,
et al: Targeting of substance p induces cancer cell death and
decreases the steady state of egfr and her2. J Cell Physiol.
227:1358–1366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diebold AE, Boudreaux JP, Wang YZ, et al:
Neurokinin A levels predict survival in patients with stage iv well
differentiated small bowel neuroendocrine neoplasms. Surgery.
152:1172–1176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reubi JC: Peptide receptors as molecular
targets for cancer diagnosis and therapy. Endocr Rev. 24:389–427.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quartara L and Maggi CA: The tachykinin
nk1 receptor. Part ii: Distribution and pathophysiological roles.
Neuropeptides. 32:1–49. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pennefather JN, Lecci A, Candenas ML, et
al: Tachykinins and tachykinin receptors: a growing family. Life
Sci. 74:1445–1463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lecci A, Santicioli P and Maggi CA:
Pharmacology of transmission to gastrointestinal muscle. Curr Opin
Pharmacol. 2:630–641. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaafari N, Hua G, Adelaide J, et al:
Expression of the tachykinin receptor mrnas in healthy human colon.
Eur J Pharmacol. 599:121–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu M, Zhang SW, Han RQ, et al: Analysis on
the mortality of colorectal and anal cancer in china during
2004–2005. Zhonghua yu Fang Yi Xue Za Zhi. 44:403–407. 2010.(In
Chinese). PubMed/NCBI
|
|
12
|
Yu Y, Pan Y, Jin M, et al: Association of
genetic variants in tachykinins pathway genes with colorectal
cancer risk. Int J Colorectal Dis. 27:1429–1436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benson AB 3rd, Venook AP, Bekaii-Saab T,
et al: Colon cancer, version 3.2014. J Natl Compr Canc Netw.
12:1028–1059. 2014.PubMed/NCBI
|
|
14
|
Zhang B, Lv M, Chen T, et al: The
association between lymph node resection and postoperative survival
in patients with colorectal cancer. Hepatogastroenterology.
60:1922–1926. 2013.PubMed/NCBI
|
|
15
|
Yuan Y, Li MD, Hu HG, et al: Prognostic
and survival analysis of 837 Chinese colorectal cancer patients.
World J Gastroenterol. 19:2650–2659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang CM and Insel PA: Genetic variation in
g-protein-coupled receptors - consequences for g-protein-coupled
receptors as drug targets. Expert Opin Ther Targets. 9:1247–1265.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Castro FA, Försti A, Buch S, et al: Tlr-3
polymorphism is an independent prognostic marker for stage ii
colorectal cancer. Eur J Cancer. 47:1203–1210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang SC, Lin PC, Yang SH, et al:
Sdf-1alpha g801a polymorphism predicts lymph node metastasis in
stage t3 colorectal cancer. Ann Surg Oncol. 16:2323–2330. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seo GS, Yu JI, Chae SC, et al: Eed gene
polymorphism in patients with colorectal cancer. Int J Biol
Markers. 28:274–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Araujo RF Jr, Lira GA, Guedes HG, et al:
Lifestyle and family history influence cancer prognosis in
Brazilian individuals. Pathol Res Pract. 209:753–757. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noura S, Ohue M, Kano S, et al: Impact of
metastatic lymph node ratio in node-positive colorectal cancer.
World J Gastrointest Surg. 2:70–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rameshwar P, Poddar A and Gascón P:
Hematopoietic regulation mediated by interactions among the
neurokinins and cytokines. Leuk Lymphoma. 28:1–10. 1997.PubMed/NCBI
|
|
23
|
Sculptoreanu A, Aura Kullmann F and de
Groat WC: Neurokinin 2 receptor-mediated activation of protein
kinase c modulates capsaicin responses in drg neurons from adult
rats. Eur J Neurosci. 27:3171–3181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hernández-Maqueda JG, Luna-Ulloa LB,
Santoyo-Ramos P, et al: Protein kinase c delta negatively modulates
canonical Wnt pathway and cell proliferation in colon tumor cell
lines. PLoS One. 8:e585402013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun J, Usune S, Zhao Y, et al: Multidrug
resistance protein transporter and ins(1,4,5)P3-sensitive
Ca2+-signaling involved in adenosine triphosphate export via gq
protein-coupled nk2-receptor stimulation with neurokinin a. J
Pharmacol Sci. 114:92–98. 2010. View Article : Google Scholar : PubMed/NCBI
|