Introduction
The majority of patients with an advanced malignancy
develop a range of systemic disorders. Cancer-associated systemic
syndrome (CASS) refers to the cluster of symptoms that typically
includes fever, anemia, endocrine and neurological disorders,
gastrointestinal dysfunction, adipose and muscle atrophy, hepatic
peliosis, ascites and kidney failure (1,2). The
syndrome can also manifest as cachexia and paraneoplastic syndrome
in severe cases (1,3). CASS is the most common cause of
cancer-related mortality, as it confers susceptibility to
infections and other secondary pathologies on cancer patients and
tumor-transplanted mice (4), in
addition to reducing patient responsiveness to chemotherapy
(5).
Angiogenic cytokines, including tumor necrosis
factor α (TNF-α), interleukin (IL)-1 and IL-6, contribute to CASS
and poor survival (6,7). Vascular endothelial growth factor (VEGF)
is an angiogenic factor that has been characterized extensively and
is expressed at high levels in the majority of tumors. In the local
tumor environment, the protein modulates blood vessel growth,
vascular permeability and vascular remodeling, while VEGF produced
by tumors also accumulates in the circulation and induces CASS
(2,8).
Angiogenesis is an important process required for
the growth and metastasis of malignant tumors. Antiangiogenic
drugs, including sunitinib, bevacizumab and sorafenib, cause the
modulation of tumor growth via vascular remodeling and regression,
which improves progression-free survival (9). These drugs may modulate off-target
vasculature in a range of organs and tissues. As a consequence of
these off-tumor target effects, improvements in CASS and
alterations in chemotoxic tolerance may increase overall survival
in patients with cancer (9).
Endostatin, an endogenous angiogenesis inhibitor,
inhibits VEGF-stimulated proliferation, migration and tube
formation by suppressing the VEGF-induced tyrosine phosphorylation
of KDR/Flk-1 [also known as VEGF receptor 2 (VEGFR-2)], overall
VEGFR-2 expression, and the activation of extracellular
signal-regulated kinases, p38 mitogen-activated protein kinase and
AKT (10–12). Furthermore, endostatin suppresses the
expression of VEGF, fibroblast growth factor, TNF-α, matrix
metalloproteinases and vascular cell adhesion molecule-1 (13). Therefore, we hypothesized that
endostatin, as a broad-spectrum antiangiogenic agent, would exhibit
anti-CASS effects. In the present study, an experiment was
conducted in Lewis lung carcinoma (LLC) tumor-bearing mice to test
this hypothesis and investigate any possible mechanisms.
Materials and methods
Cell lines and reagents
LLC cell lines were obtained from the American Type
Culture Collection (Manassas, VA, USA), cultured in Dulbecco's
modified Eagle's medium (Gibco BRL, Life Technologies, Grand
Island, NY, USA) supplemented with 10% fetal bovine serum, and kept
in a humidified 5% CO2 atmosphere at 37°C. Recombinant
human endostatin was obtained from Shandong Simcere Medgenn
Bio-Pharmaceutical Co., Ltd. (Yantai, Shandong, China). A
monoclonal goat anti-mouse cluster of differentiation (CD) 31
antibody (cat. no. sc-1506) was purchased from Santa Cruz
Biotechnology Inc. (Dallas, TX, USA). The TNF-α, VEGF and IL-6
concentrations were detected using an enzyme-linked immunosorbent
assay (ELISA) kit (Neobioscience, Shenzhen, Guangdong, China).
Tumor tissues from the animals were homogenized and solubilized in
RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China).
Tumor models and treatment
protocol
Specific pathogen-free, male, C57BL/6 mice (5–6
weeks old) were purchased from the Laboratory Animal Center of
Sichuan University (Chengdu, Sichuan, China) and maintained in
pathogen-free conditions. All experiments were carried out in
accordance with the guidelines approved by the Animal Care and Use
Committee of Sichuan University. The experimental procedures and
the animal use and care protocols were approved by the Committee on
Ethical Use of Animals of West China Hospital of Sichuan University
(Chengdu, China).
C57BL/6 mice were randomly distributed into 3 groups
(n=10/group); the control group (NS group) was subcutaneously
inoculated with 1×106 LLC cells, the endostatin group
(ES group) was subcutaneously inoculated with 1×106 LLC
cells, and the normal group consisted of non-tumor-bearing mice.
The NS and normal groups received normal saline (NS), but the ES
group received 10 mg/kg endostatin at 8 days post-implantation.
After day 8, 10 mg/kg endostatin or normal saline was intravenously
administered daily (by caudal vein injection) for 12 days. Tumor
volume was estimated every 3 days using the following formula:
Tumor volume = a × b2 / 2, where a and b are the long
and short tumor diameters, respectively. At 20 days
post-implantation, the animals were sacrificed by the cervical
dislocation method in order to harvest tissues and blood samples
for further analysis.
Changes in body and organ weights
Body weight was measured every 3 days after tumor
implantation. Tumors were removed in order to calculate body weight
as the whole body weight minus the tumor weight. Organs were
dissected and weighed for comparison.
Blood sample analysis
Blood samples were collected from the orbital sinus.
Whole blood and serum samples were prepared in the presence or
absence of the ethylenediaminetetraacetic acid anticoagulant.
Hematological parameters, including hemoglobin (HGB) level,
hematocrit (HCT) and red blood cell count, were determined in whole
blood using the MEK6318K automatic blood analyzer (Nihon Kohden,
Tokyo, Japan). Biochemical indicators of liver function and
metabolism, including serum alanine aminotransferase, aspartate
aminotransferase (AST), albumin (ALB), total protein (TP), glucose,
triglycerides, cholesterol (CHO), high-density lipoprotein (HDL)
and low-density lipoprotein (LDL), were determined using a Hitachi
7020 automatic biochemical analyzer (Hitachi High-Tech, Tokyo,
Japan) according to the manufacturer's instructions.
Histological and immunohistochemical
analysis
Tumor-bearing and control mice were sacrificed at
day 20 post-implantation. Necropsies were performed, and various
tissues and organs were removed, weighed and immediately fixed
overnight in 4% paraformaldehyde. Samples of the tissues and organs
were embedded in paraffin. Malignant and non-malignant
paraffin-embedded tissues were sectioned to a 5-µm thickness and
stained with hematoxylin and eosin (H&E). The microvessels were
determined by CD31 immunostaining. Paraffin-embedded tissues
sections were deparaffinized, rehydrated, incubated in 3%
H2O2 and blocked with 5% bovine serum
albumin. The sections were then incubated with CD31 antibody
(dilution, 1:400) at 4°C overnight, followed by incubation with
biotinylated polyclonal rabbit anti-goat antibody (dilution, 1:200;
cat. no. ab124055; Beyotime Institute of Biotechnology). Peroxidase
activity was visualized using a 3,3′-diaminobenzidine substrate kit
(CellChip Biotechnology, Beijing, China). Sections were
counterstained with hematoxylin. The slides were examined using an
Eclipse E600 microscope (Nikon, Tokyo, Japan).
ELISA
Blood samples were collected from all mice after
treatment using non-anticoagulation infertile tubes. Samples were
centrifuged at 2,800 × g for 10 min at 4°C, and separated serum
samples were stored at −80°C until use. The tumor tissues from the
animals were homogenized and solubilized in lysis buffer using a
commercial kit. Samples were centrifuged at 11,000 × g for 30 min
at 4°C, and the separated supernatants were stored at −80°C until
use. TNF-α, VEGF and IL-6 (serum and tumor samples) were assessed
using commercial mouse ELISA kits according to the manufacturer's
instructions. The colorimetric reaction was monitored at 450 nm
using a Benchmark microplate reader (Benchmark Electronics,
Angleton, TX, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using SPSS software (version
16.0; SPSS, Inc., Chicago, IL, USA). Between-group statistical
significance was determined using a one-way analysis of variance,
and a least significant difference test was applied for multiple
comparisons. P<0.05 was used to indicate a statistically
significant difference.
Results
Anticancer effects of endostatin
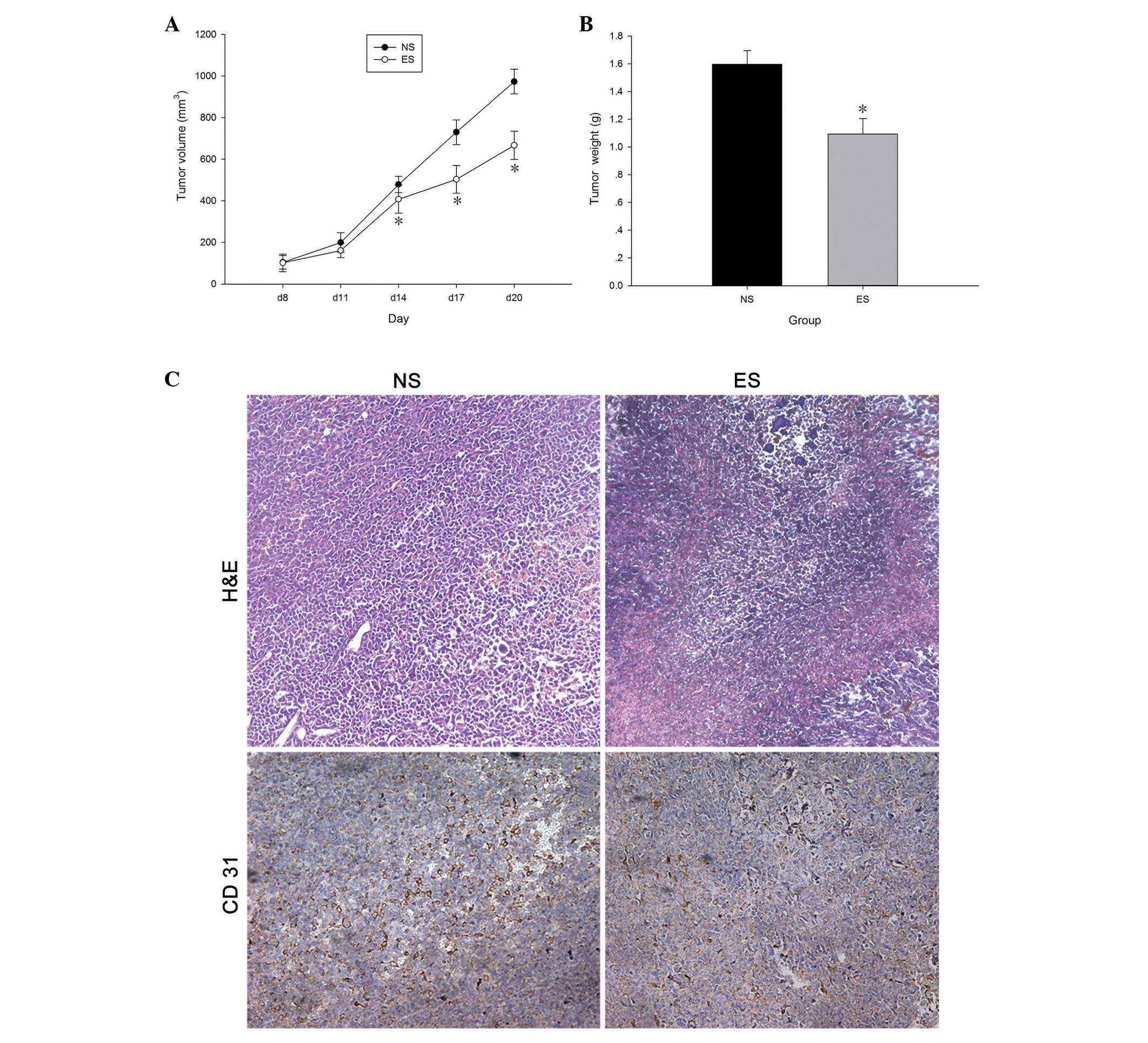
The in vivo results indicated that the tumors
initially became palpable around 7 days after inoculation. The long
and short tumor diameters were estimated after 8 days (Fig. 1A). From day 14 onwards, the tumor
volume of the ES group was significantly lower than the NS group
(P<0.05). Once the mice had been sacrificed on day 20, the
tumors were removed and accurately weighed (Fig. 1B). The tumor weight of the ES group
was significantly lower than the NS group (P<0.05).
Immunohistochemical analysis of the tumor xenografts using the
anti-CD31 antibody showed that the blood vessels appeared as
primitive and dilated sinusoidal vascular structures that consisted
of disorganized and tortuous vascular plexuses (Fig. 1C). The tumor xenografts of the ES
group demonstrated clear blood vessels in comparison with the NS
group, without expanded vascular plexuses, indicating that
endostatin inhibits tumor angiogenesis. In addition, H&E
revealed the prominent visibility of cell necrosis in the tumors of
the ES group (Fig. 1C), which is a
common feature that can be observed in tumors following endostatin
administration (14,15).
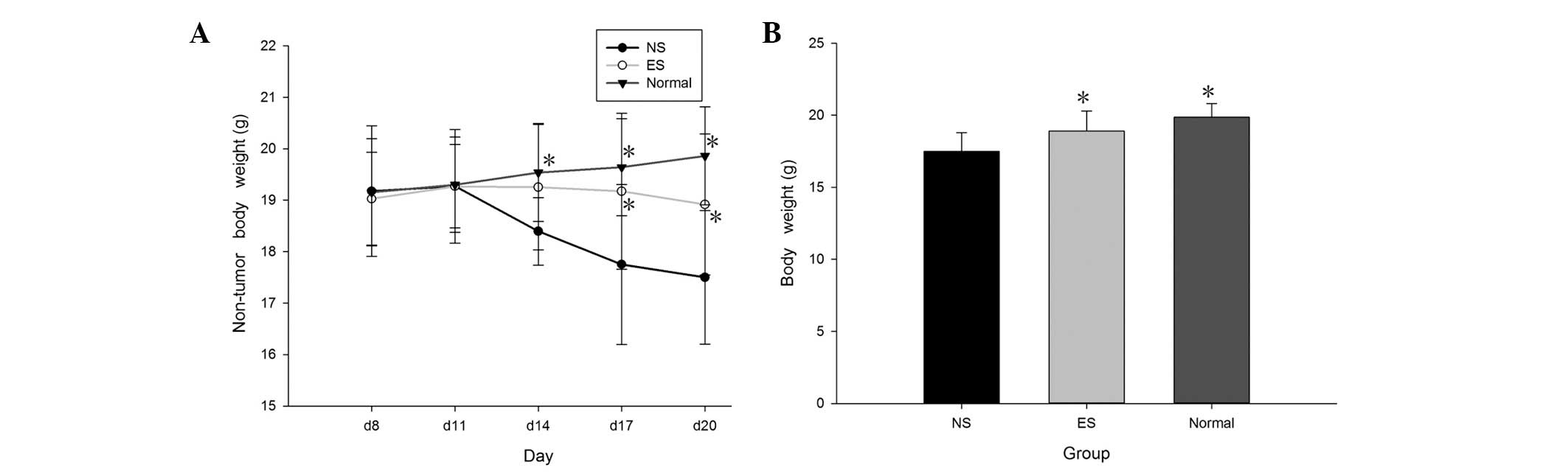
Effect of endostatin on body weight
loss
Body weight was measured every 3 days once the
tumors became palpable (Fig. 2A). The
tumor-bearing mice gained weight early, but their weight
significantly declined after day 14 (P<0.05). However, weight
loss in the endostatin-treated mice was substantially lower than
that in the NS mice (P<0.05). The NS mice weighed significantly
less than the mice in the ES and normal groups (P<0.05). There
was no significant difference in body weight between the
endostatin-treated and normal mice. The tumors were removed, and
the final body weights were measured after sacrifice (Fig. 2B).
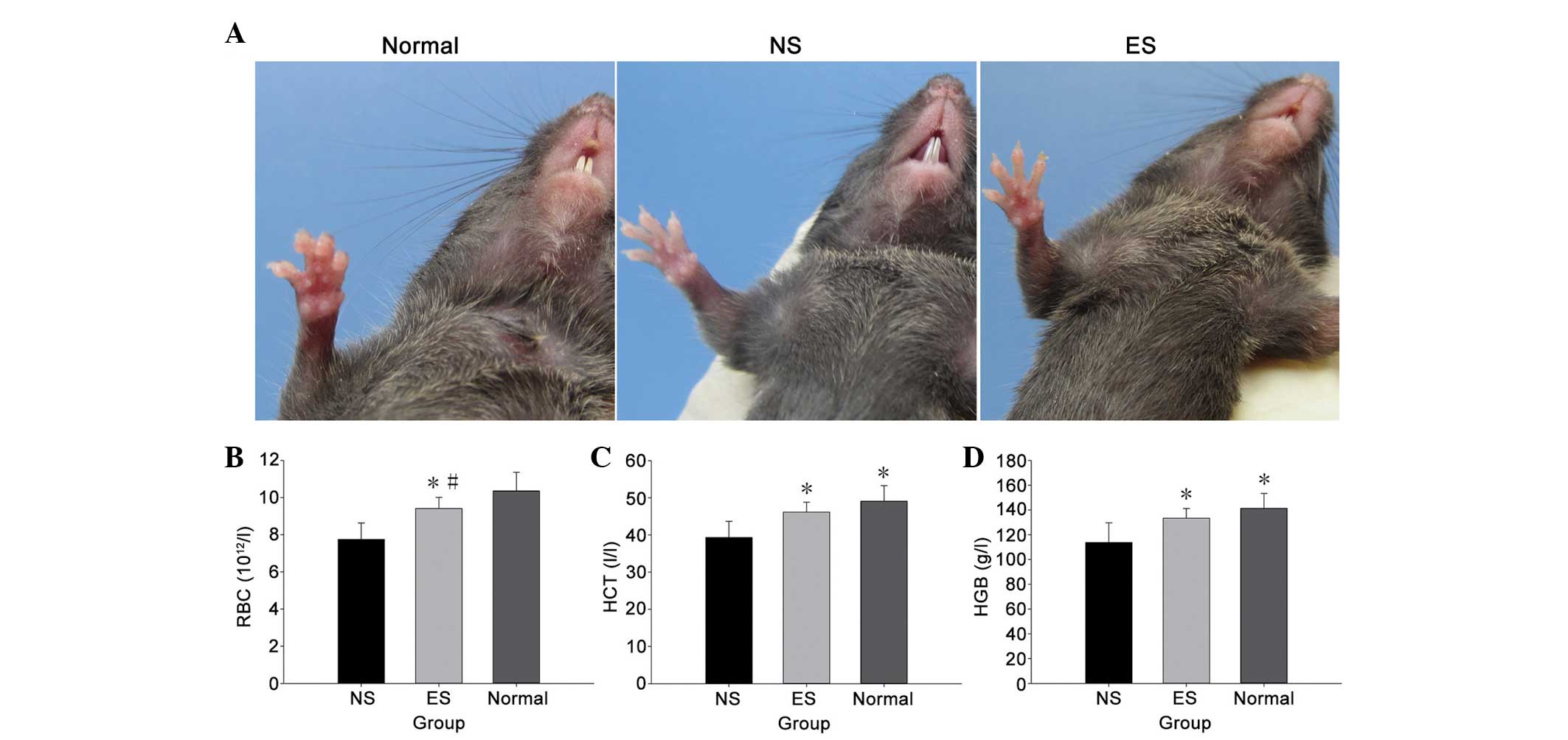
Effect of endostatin on preventing
anemia
Gross examination of the tumor-bearing mice revealed
severe anemia, which manifested as noticeable paleness on the
hairless regions of the paws, mouth, nose and genitals (Fig. 3A). Hematological analysis of the
peripheral blood revealed a significant decrease in HCT at day 20
after tumor implantation (P<0.05) (Fig. 3C). HGB and erythrocytes also
significantly decreased in the peripheral blood (P<0.05)
(Fig. 3B and D). These results showed
that the tumor-bearing mice were severely anemic. However,
endostatin prevented anemia to a certain degree in comparison with
the NS group, and there were no significant differences in HCT or
HGB between the ES group and the normal mice.
Endostatin induces changes in
biochemical parameters
Certain biochemical parameters of liver function and
metabolism were also analyzed. The LLC tumors significantly changed
the majority of biochemical parameters in the NS and ES groups, in
comparison with the normal group (Table
I). For example, in comparison with the normal group, the
levels of serum TP and ALB markedly decreased (P<0.05), and the
levels of CHO and HDL markedly increased in the tumor-bearing mice
(P<0.05). However, these changes in the tumor-bearing mice were
somewhat alleviated by endostatin. There were also significant
differences in terms of AST and CHO levels between the ES and NS
groups.
 | Table I.Biochemical parameters. |
Table I.
Biochemical parameters.
| Parameters | Normal | NS | ES |
|---|
| ALT, IU/l | 51.20±16.35 | 55.50±13.44 | 46.70±7.65 |
| AST, IU/l | 133.20±15.70 |
263.10±69.67a |
198.90±41.30a,b |
| TP, g/l | 54.67±2.90 |
50.59±2.31a |
50.28±3.26a |
| ALB, g/l | 28.57±1.95 |
26.70±1.51a |
26.84±1.81a |
| GLU, mmol/l | 4.58±0.91 |
5.92±1.88a |
6.37±1.06a |
| CHO, mmol/l | 1.23±0.27 |
1.93±0.29a |
1.58±0.29a,b |
| TG, mmol/l | 0.60±0.12 |
1.01±0.52a |
0.97±0.28a |
| HDL, mmol/l | 0.41±0.15 |
0.63±0.10a |
0.60±0.10a |
| LDL, mmol/l | 0.16±0.04 |
0.22±0.08a | 0.17±0.05 |
Effects of endostatin on body weight
and on pathological changes in certain organs
The dissected organs were weighed (Table II), and hepatosplenomegaly and
abnormal kidney weight were noted in the tumor-bearing mice. The
weights of the spleens in the tumor-bearing mice increased almost
2-fold in comparison with the normal group (P<0.05). However,
endostatin improved the hepatosplenomegaly. The liver and kidney
weights were significantly reduced (P<0.05) and reverted almost
to those of the normal mice following endostatin
administration.
 | Table II.Organ weights. |
Table II.
Organ weights.
| Organs | Normal, g | NS, g | ES, g |
|---|
| Liver | 0.803±0.044 |
1.055±0.144a |
0.85±0.079b |
| Spleen | 0.091±0.012 |
0.159±0.037a |
0.143±0.037a |
| Kidney | 0.188±0.008 |
0.209±0.025a | 0.198±0.016 |
H&E staining demonstrated that the hepatic
sinusoid and hepatic central vein in the tumor-bearing mice
exhibited a high degree of dilation architecture. In the spleens of
the tumor-bearing mice, the white pulp (WP) and red pulp (RP)
margins disappeared due to regional necrosis (Fig. 4A). The dilated sinusoidal blood
vessels in the liver appeared normal in the ES group in comparison
with the NS group. Similarly, the structure of the spleen was
normalized by endostatin treatment. The adrenal tissue was dense
without sinusoid dilation, and the bone marrow hematopoietic cells
were rich and evenly distributed; there were no significant
differences between the groups in terms of tissue structure of the
adrenal glands or bone marrow.
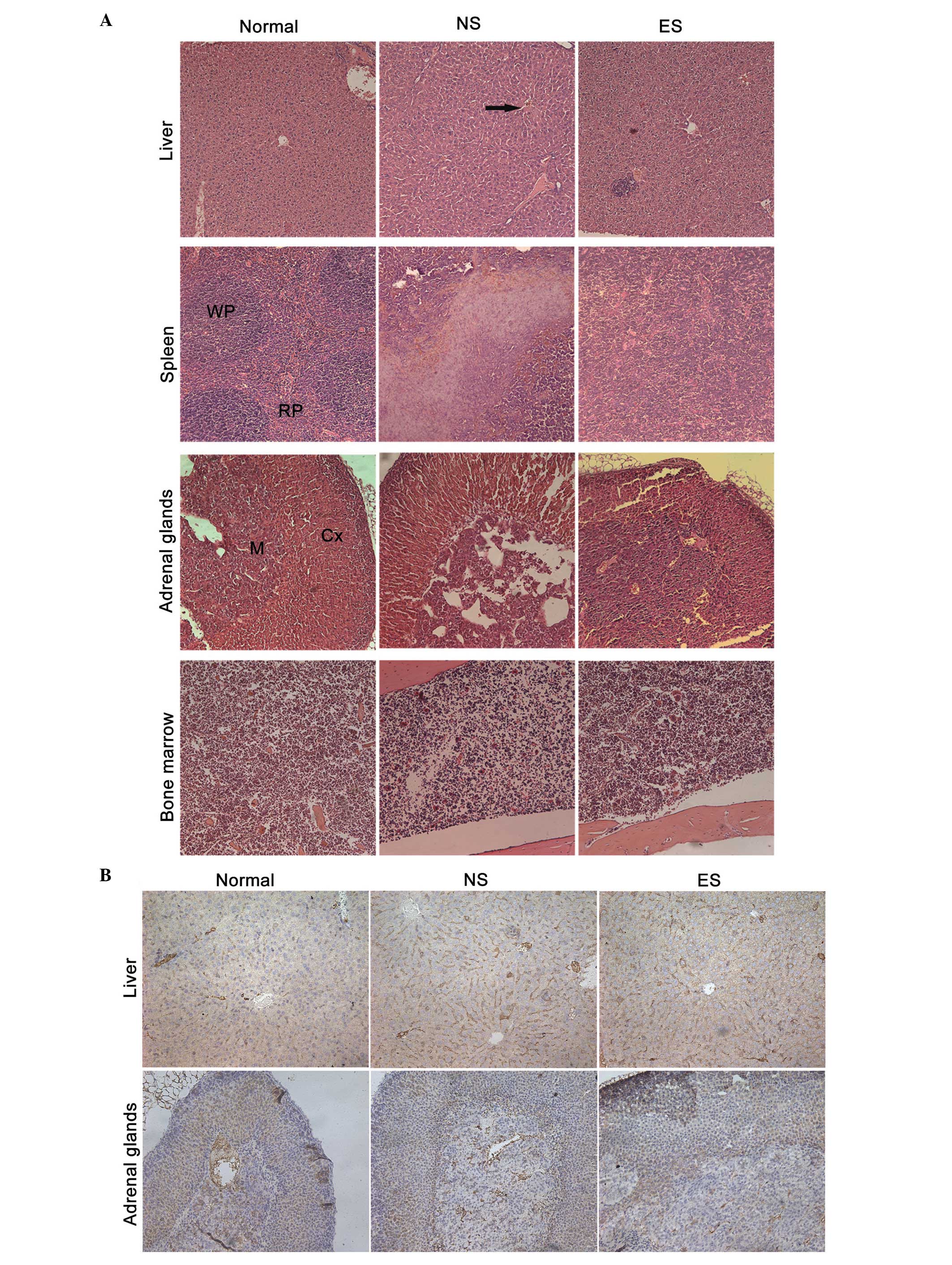 | Figure 4.Histological analysis of the liver,
spleen, adrenal glands and bone marrow (magnification, ×200). (A)
Tissue sections from the liver, spleen, bone marrow and adrenal
glands stained with hematoxylin and eosin. The black arrow
indicates the expanding vessels in the liver. (B) Tissue sections
from the liver and adrenal glands stained with anti-cluster of
differentiation 31 antibody. WP, white pulp; RP, red pulp; Cx,
cortex; M, medulla; ES, endostatin group; NS, normal saline control
group. |
Immunohistochemical analysis using the anti-CD31
antibody showed that the hepatic sinusoid and hepatic central vein
of the tumor-bearing mice in the NS group exhibited primitive and
dilated sinusoidal vascular structures (Fig. 4B). Treating these tumor-bearing mice
with endostatin almost completely reversed the dilation of the
sinusoidal blood vessels. The adrenal tissue structure was dense
without sinusoid dilation, and there were no significant
differences between the groups.
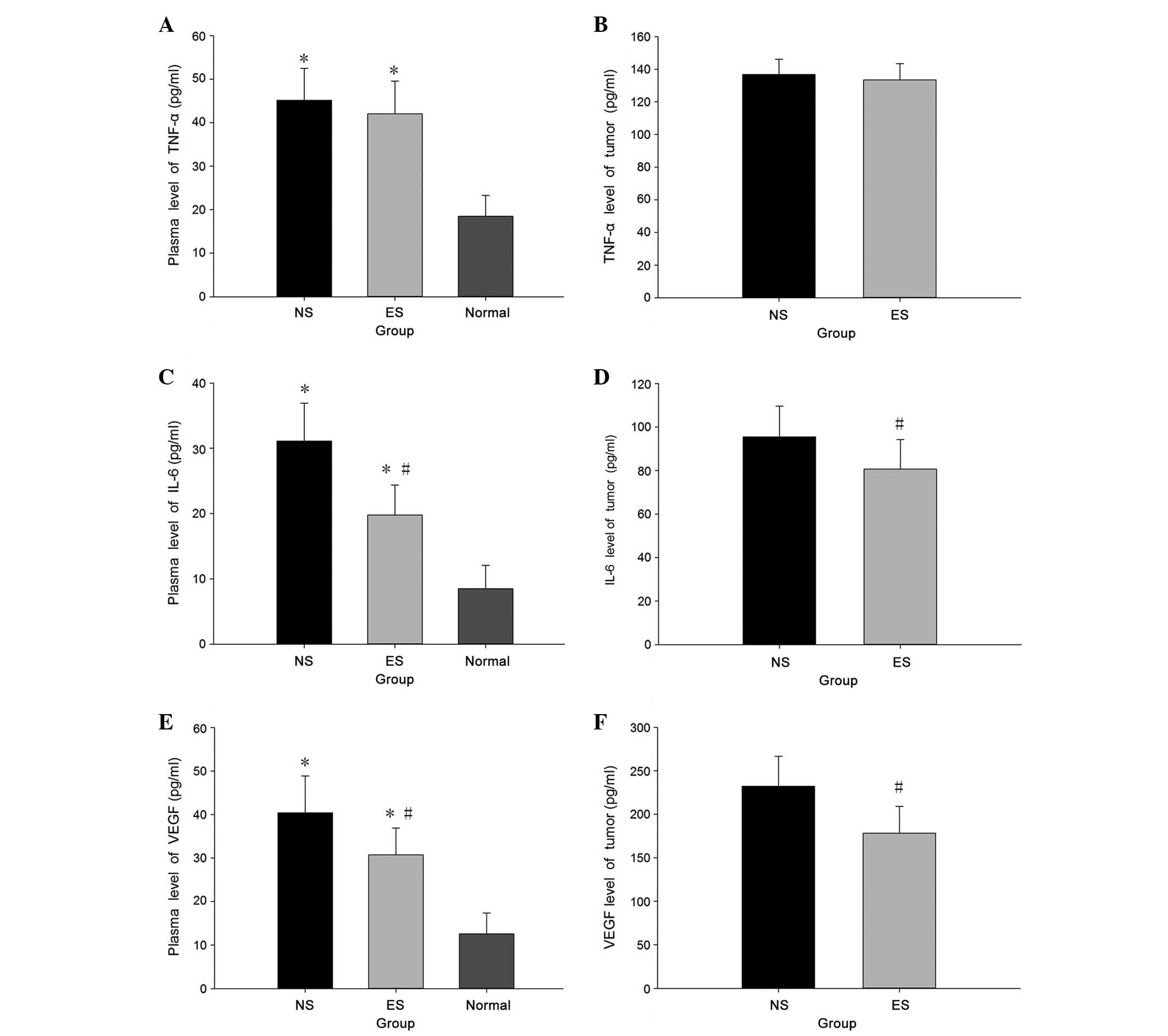
Endostatin inhibits IL-6 and VEGF
production
TNF-α, IL-6 and VEGF expression in the sera and
tumors were detected using ELISA. TNF-α, IL-6 and VEGF levels were
significantly higher in the tumor-bearing mice compared with the
normal group (P<0.05) (Fig. 5).
IL-6 and VEGF production were significantly inhibited by
endostatin, with no significant effect on TNF-α expression.
Discussion
The present study found that the body weights of LLC
tumor-bearing mice quickly decline at 14 days post-tumor
implantation. Gross examination revealed severe anemia, which
manifested as considerable paleness in several hairless regions,
including the paws, mouth, nose and genitals. Hematological
analysis of the peripheral blood samples revealed a significant
decrease in HCT at day 20 post-tumor implantation. HGB level and
RBC count was also significantly decreased in the peripheral blood.
These results showed that the tumor-bearing mice had developed
severe anemia. Serum parameters that reflect hepatic function and
carbohydrate, lipid and protein metabolism were already
considerably disordered, however, the tumor-bearing mice also
developed hepatosplenomegaly. Histological analysis demonstrated
that high-density sinusoidal vasculature filled the entire liver.
In the spleen, the WP and RP margins disappeared, accompanied by
regional necrosis. In short, all these findings showed that the
tumor-bearing mice suffered from CASS.
Endostatin, an endogenous broad-spectrum
angiogenesis inhibitor, can selectively target neovascular
endothelial cells and suppress tumor growth (16). The present study found that the tumor
burden in the endostatin-treated mice was significant lower than in
the placebo-treated mice. Furthermore, the CASS-alleviating effect
of endostatin in the LLC tumor-bearing mice was evident by the
weight gain, improvement in blood biochemical parameters and
anemia, and the preservation of organ function. All these findings
confirmed that endostatin can effectively affect off-tumor organs
and improve CASS in an LLC tumor-induced murine model. Other
antiangiogenic drugs, including bevacizumab, sunitinib and
sorafenib, may also physiologically alter organ function and
vascular remodeling, thereby improving CASS and progression-free
survival in tumor-bearing mice (9).
However, as the drugs are exogenous angiogenesis inhibitors, a
large number of cancer patients develop adverse effects in response
to antiangiogenic agents, including impaired wound healing,
hypothyroidism, hypertension, hemorrhage, proteinuria and
cardiovascular disorders (9,17). Therefore, endostatin, which does not
cause these adverse effects, could be used to treat patients with
CASS.
Although certain cytokines, such as VEGF, TNF-α and
IL-6, contribute to CASS, its underlying molecular mechanisms
remain unknown (18,19). For example, CASS is associated with an
elevated serum IL-6 level, and anti-IL-6 monoclonal antibody
therapy decreases the incidence of cancer-related anorexia and
cachexia (1). TNF-α treatment can
impair the oxidation of long-chain fatty acids (20). Cancer cachexia is partly mediated by
spleen-secreted IL-6 (21), and can
be ameliorated by L-carnitine via the regulation of serum TNF-α and
IL-6 levels, and the modulation of carnitine palmitoyltransferase
expression and activity in the liver (22). Consistent with the results from these
aforementioned studies, the present study found that serum VEGF,
TNF-α, and IL-6 levels markedly increase with the progression of
CASS, and that serum VEGF and IL-6 levels notably decrease
following administration of endostatin. Therefore, it is possible
that endostatin ameliorates CASS by attenuating serum VEGF and IL-6
levels, although the exact mechanism remains to be elucidated.
The majority of preclinical and clinical studies on
anti-VEGF agents focus on tumor vasculature or growth, and little
is known with regard to the systemic effects of these therapeutic
agents. In preclinical studies, the systemic administration of
antiangiogenic agents in non-tumor-bearing mice has been reported
to alter vascular density and architecture in numerous organs and
tissues, particularly endocrine organs containing fenestrated
vessels (23). A previous study
reported that the level of circulating VEGF correlates with the
severity of CASS in tumor-bearing mice and human cancer patients,
and that anti-VEGF agents could improve organ function (8). As a consequence of the off-tumor target
effects, improvements to organ function and alterations in
chemotoxic tolerance may be the most important mechanisms with a
survival benefit in cancer patients with CASS (8,24).
In summary, the present data showed that in an LLC
tumor-induced CASS model, endostatin effectively reduced the tumor
burden, preserved body weight and improved CASS, possibly by
reducing the serum VEGF and IL-6 levels. Further studies are
required to further explore the application of this endogenous
angiogenesis inhibitor in the treatment of advanced cancer patients
with CASS.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (nos. 81071864 and
81372560). The authors would like to acknowledge the animal care
provided by Mr. Yan-yang Liu and Mr. Li Wang.
Abbreviations:
|
CASS
|
cancer-associated systemic
syndrome
|
|
LLC
|
Lewis lung carcinoma
|
|
VEGF
|
vascular endothelial growth factor
|
|
TNF-α
|
tumor necrosis factor α
|
|
IL
|
interleukin
|
|
HGB
|
hemoglobin
|
|
HCT
|
hematocrit
|
|
AST
|
aspartate aminotransferase
|
|
ALB
|
albumin
|
|
TP
|
total protein
|
|
CHO
|
cholesterol
|
|
HDL
|
high-density lipoprotein
|
|
LDL
|
low-density lipoprotein
|
|
H&E
|
hematoxylin and eosin
|
References
|
1
|
Nathanson L and Hall TC: Introduction:
paraneoplastic syndromes. Semin Oncol. 24:265–268. 1997.PubMed/NCBI
|
|
2
|
Nakamura I, Shibata M, Gonda K, et al:
Serum levels of vascular endothelial growth factor are increased
and correlate with malnutrition, immunosuppression involving MDSCs
and systemic inflammation in patients with cancer of the digestive
system. Oncol Lett. 5:1682–1686. 2013.PubMed/NCBI
|
|
3
|
Tisdale MJ: Cachexia in cancer patients.
Nat Rev Cancer. 2:862–871. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inui A: Cancer anorexia-cachexia syndrome:
current issues in research and management. CA Cancer J Clin.
52:72–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi Y, Yasumoto K and Mai M:
Chemotherapy under cachectic conditions and the possibility of
cachexia-controlled chemotherapy. Oncol Rep. 14:135–140.
2005.PubMed/NCBI
|
|
6
|
Krzystek-Korpacka M, Matusiewicz M,
Diakowska D, et al: Impact of weight loss on circulating IL-1,
IL-6, IL-8, TNF-alpha, VEGF-A, VEGF-C and midkine in
gastroesophageal cancer patients. Clin Biochem. 40:1353–1360. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ebos JM, Lee CR, Christensen JG, et al:
Multiple circulating proangiogenic factors induced by sunitinib
malate are tumor-independent and correlate with antitumor efficacy.
Proc Natl Acad Sci USA. 104:17069–17074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xue Y, Religa P, Cao R, et al: Anti-VEGF
agents confer survival advantages to tumor-bearing mice by
improving cancer-associated systemic syndrome. Proc Natl Acad Sci
USA. 105:18513–18518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao Y: Off-tumor target - beneficial site
for antiangiogenic cancer therapy? Nat Rev Clin Oncol. 7:604–608.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ling Y, Yang Y, Lu N, et al: Endostar, a
novel recombinant human endostatin, exerts antiangiogenic effect
via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of
endothelial cells. Biochem Biophys Res Commun. 361:79–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Zhang J, Jiang D, et al:
Inhibition of T-type Ca2+ channels by endostatin
attenuates human glioblastoma cell proliferation and migration. Br
J Pharmacol. 166:1247–1260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim J, Duong T, Lee G, et al: The effect
of intracellular protein delivery on the anti-tumor activity of
recombinant human endostatin. Biomaterials. 34:6261–6271. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu W, Ye P, Li Z, et al: Endostar, a
recently introduced recombinant human endostatin, inhibits
proliferation and migration through regulating growth factors,
adhesion factors and inflammatory mediators in choroid-retinal
endothelial cells. Mol Biol (Mosk). 44:664–670. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huszthy PC, Brekken C, Pedersen TB, et al:
Antitumor efficacy improved by local delivery of species-specific
endostatin. J Neurosurg. 104:118–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Yang G, Zhang Y, et al: An
experimental research into endostatin microbubble combined with
focused ultrasound for anti-tumor angiogenesis in colon cancer.
Gastroenterol Rep (Oxf). 2:44–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ning T, Jiang M, Peng Q, et al: Low-dose
endostatin normalizes the structure and function of tumor
vasculature and improves the delivery and anti-tumor efficacy of
cytotoxic drugs in a lung cancer xenograft murine model. Thoracic
Cancer. 3:229–238. 2012. View Article : Google Scholar
|
|
17
|
Jain RK: Normalization of tumor
vasculature: an emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kayacan O, Karnak D, Beder S, et al:
Impact of TNF-alpha and IL-6 levels on development of cachexia in
newly diagnosed NSCLC patients. Am J Clin Oncol. 29:328–335. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Q, Wan L, Zhou Z, et al: Parthenolide
from Parthenium integrifolium reduces tumor burden and alleviate
cachexia symptoms in the murine CT-26 model of colorectal
carcinoma. Phytomedicine. 20:992–998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noguchi Y, Makino T, Yoshikawa T, et al:
The possible role of TNF-alpha and IL-2 in inducing
tumor-associated metabolic alterations. Surg Today. 26:36–41. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barton BE and Murphy TF: Cancer cachexia
is mediated in part by the induction of IL-6-like cytokines from
the spleen. Cytokine. 16:251–257. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu S, Wu HJ, Zhang ZQ, et al: L-carnitine
ameliorates cancer cachexia in mice by regulating the expression
and activity of carnitine palmityl transferase. Cancer Biol Ther.
12:125–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamba T, Tam BY, Hashizume H, et al:
VEGF-dependent plasticity of fenestrated capillaries in the normal
adult microvasculature. Am J Physiol Heart Circ Physiol.
290:H560–H576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rein DT, Volkmer AK, Volkmer J, et al:
Systemic administration of bevacizumab prolongs survival in an in
vivo model of platinum pre-treated ovarian cancer. Oncol Lett.
3:530–534. 2012.PubMed/NCBI
|