Introduction
It is a well-established fact that rapid cell
division in solid tumors leads to depletion of the oxygen levels
and variable levels of hypoxia across the tumor. The latter is a
major driving force for the process of endothelial proliferation
and tumor angiogenesis. Squamous cell carcinoma of the larynx is
the most common neoplasm of the head and neck. It is well-known
that the rapid proliferation of malignant cells and the irregular
local vasculature jointly favor the formation of hypoxic areas
within human solid tumors including laryngeal cancer. Despite the
vast amount of papers, there is a lack of quantitative studies
reporting the levels of expression of hypoxia-inducible factors in
this neoplasm. Hypoxia-inducible factors (HIFs) are essential in
the primary transcriptional responses to hypoxic stress in normal
and neoplastic cells. These molecules are heterodimeric
transcription factors that activate a large number of target genes,
including phosphoglycerate kinase and vascular endothelial growth
factor (VEGF) A. This leads to increased glycolysis, endothelial
proliferation and angiogenesis, which facilitates the adaptation of
the tumor to hypoxia. HIFs are composed of α and β subunits; three
α isoforms exist, which are normally rapidly degraded in an
oxygen-dependent manner (1–5), while the β subunit is expressed at
constant level under normoxic conditions. HIF-1α and HIF-2α (also
known as EPAS1) are structurally similar, and activate the
transcription of target genes by binding to hypoxia response
elements (HREs) or similar sequence elements. The presence of HREs
has been demonstrated in a number of angiogenic genes, including
VEGFA (6,7), VEGF receptor 1/fms-related tyrosine
kinase 1 (8–10), erythropoietin (2,11–14) and endothelial nitric oxide synthase
(15–18). Little is known about the third HIFα
isoform. It has been demonstrated that a number of splice variants
of HIF-3α may act as dominant-negative regulators of the other two
α isoforms; however, its primary function, and the regulatory
mechanism through which HIF-3α and its variants exert their
effects, remains unclear based on currently available evidence
(19).
VEGF-A is a key regulator of angiogenesis, but has
also been identified to be a multi-functional factor involved in
tumor progression, immunosuppression and immune tolerance (20). Endothelial cells are the primary
targets of VEGF-A, which acts as a survival factor for these cells,
and prevents endothelial apoptosis induced by serum starvation
(21–25). In addition, VEGF induces expression of
Bcl-2, an anti-apoptotic protein (22).
Materials and methods
Patient recruitment and
assessment
The study was conducted in the Ear, Nose and Throat
Department of University Hospital ‘Queen Jovanna’ (Sofia,
Bulgaria), in cooperation with the Molecular Medicine Center at the
Medical University of Sofia (Sofia, Bulgaria), over the period
between 2010 and 2013. A total of 63 patients with
histopathologically verified carcinoma of the larynx were enrolled
in the study. Informed consent was obtained from each patient, and
the protocol of the study was approved by the Ethics Committee of
the Medical University of Sofia. A standardized history was
obtained for each patient. Detailed descriptions of the
endoscopic/microscopic direct laryngoscopy findings were recorded,
in addition to the computed tomography examination results. All
patients underwent surgical intervention consisting of total
laryngectomy or organ saving surgery, depending on the extent of
the disease. Tumor and normal laryngeal tissue samples were
obtained from each patient during the surgery and immediately
frozen in liquid nitrogen. The tissue samples were stored at −80°C
until use.
Genetic testing
Total RNA extraction and cDNA synthesis
Total RNA was isolated from normal and tumor fresh
frozen tissue samples of each patient using an RNeasy Mini kit
(Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's
protocol. The quality of RNA was assessed by denaturing
electrophoresis on a formaldehyde gel. The amount of RNA was
determined spectrophotometrically using a NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Wilmington, DE,
USA).
From each sample, 1 µg RNA underwent reverse
transcription using a High-Capacity cDNA Reverse Transcription (RT)
kit (Applied Biosystems Life Technologies, Foster City, CA, USA)
according to manufacturer's recommendations. In brief, 2X RT master
mix, prepared according to the manufacturer's instructions, was
added to RNA in a total volume of 20 µl. Reverse transcription was
performed in three steps: 25°С for 10 min, 37°С for 120 min and
85°С for 5 min.
Quantitative polymerase chain reaction (PCR)
In the present study, the expression of four genes,
HIF-1α, HIF-2α, HIF-3α and VEGF-A, was analyzed. Quantitative PCR
was performed in a 25-µl total volume of 1X RotorGene SYBR Green
PCR Mix (Qiagen), 1X QuantiTect Primer Assay (Qiagen) for the
respective gene (Hs_HIF1A_1_SG, Hs_EPAS1_1_SG, Hs_HIF3A_1_SG,
Hs_VEGFA_1_SG) and 100 ng cDNA. The conditions were as follows:
Initial denaturation at 95°С for 5 min, followed by 45 cycles of
denaturation at 95°С for 15 sec, primer annealing at 55°С for 30
sec, and synthesis with data acquisition at 72°С for 30 sec. Each
sample was examined in triplicate, and the mean threshold cycle
(Ct) values from the three repeats were used for the data analysis.
Negative and no template controls were also evaluated. β-actin
(Hs_ACTB_1_SG; QuantiTect Primer Assay) was used as a reference
gene for normalization. To determine the relative expression of
each gene in the tumor, the 2-ΔΔCt method was applied (26). Briefly, mean Ct values for the gene of
interest (GOI) and a reference gene in tumor (CtT,GOI
and CtT,Ref, respectively) and normal
(CtN,GOI and CtN,Ref, respectively) tissues
were used to calculate ΔCt (CtGOI - CtRef)
for each tissue, and then to derive the relative quantity (RQ) of
the gene in the tumor compared with the normal tissue: RQ =
2ΔΔCt, where ΔΔCt = ΔCtT - ΔCtN.
An RQ of >2 was defined as overexpression, and an RQ of <0.5
was defined as underexpression of the gene, in agreement with
previous studies (27,28).
Statistical analysis
IBM SPSS Statistics 21 (IBM SPSS, Armonk, NY, USA)
was used for all statistical analyses. A two-sided t-test was used
to calculate the statistical significance of the results. The
χ2 test was used to evaluate differences in mRNA
expression levels of HIF-1α and VEGF-A. Spearman analysis was used
to determine correlations. P<0.05 was considered to indicate a
statistically significant difference.
Results
The mean age of the study group was 60.5 years, with
a standard deviation of 7.8 years (range, 41–84 years). The cohort
comprised 2 female and 61 male patients, all of whom had
histologically verified squamous cell carcinoma of the larynx.
Distribution according to tumor-node-metastasis classification was
as follows: Stage T1, 2 patients (3.17%); T2, 7 patients (11.11%);
T3, 23 patients (36.51%); and T4, 31 patients (49.21%) (29). Histologically verified lymph node
metastases were present in 14 patients (22.22%) at the time of
surgery.
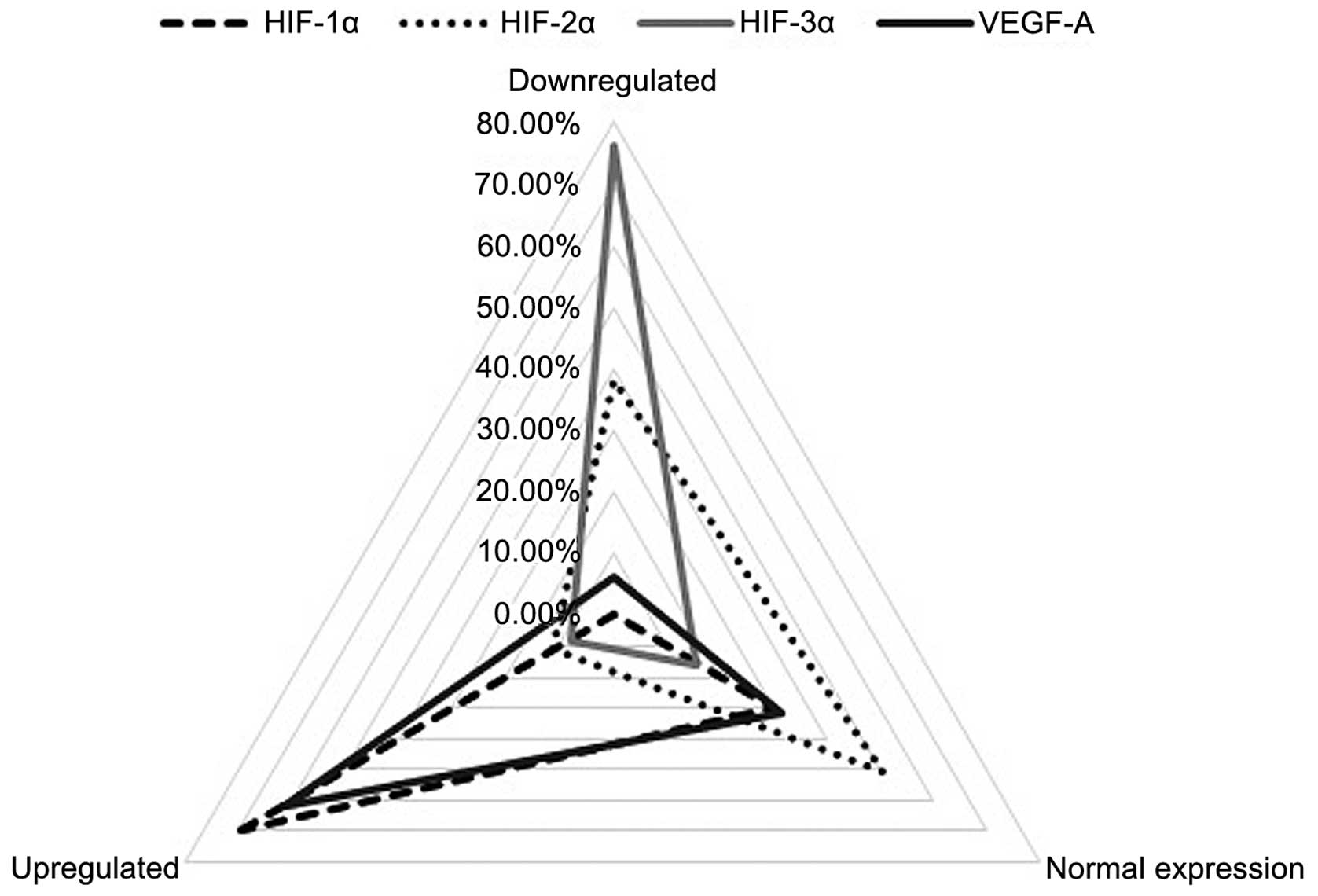
HIF-1α was upregulated (RQ>2) in the majority of
patients (44 patients, 69.84%) and normally expressed
(0.5<RQ<2) in the remaining 19 (30.16%) patients. By
contrast, HIF-2α overexpression (RQ>2) was only identified in 7
patients (11.11%); of the remaining 56 (88.89%) patients, 24
patients exhibited almost silenced HIF-2α expression (RQ<0.5),
and the other 32 patients exhibited expression similar to that of
the matched normal laryngeal samples (0.5<RQ<2). The HIF-3α
isoform was markedly downregulated (RQ<0.5) in the majority of
patients (48 patients, 76.19%). Normal levels of HIF-3α mRNA
expression (0.5<RQ<2) were registered in 10 (15.87%) patients
and a small group of 5 (7.94%) patients exhibited significant
overexpression of the HIF-3α isoform (RQ>2). For VEGF-A, 61.90%
(39 patients) showed overexpression (RQ>2), 6.35% (4 patients)
displayed low expression (RQ<0.5) and 31.75% (20 patients)
exhibited normal expression levels (0.5<RQ<2) (Fig. 1).
Quantitative analysis of the study group revealed
mean values of HIF-1α mRNA expression that were 2.71 times higher
than the corresponding normal laryngeal epithelium, while the
expression levels of HIF-2α, HIF-3α and VEGF-A were 0.92, 0.50 and
2.98 times that of the normal epithelium, respectively. One patient
was excluded as an outlier after the mRNA expression level of
VEGF-A was measured to be 955 times higher in the tumor tissue
compared with the corresponding normal laryngeal tissue (testing
was repeated five times).
A χ2 test for association was conducted
between patients with upregulated and without upregulated mRNA
expression of HIF-1α and VEGF-A. There was a statistically
significant association between the overexpression of HIF-1α and
VEGF-A (χ21=7.246, P=0.008).
Spearman's rank correlation coefficient was used to
assess the correlation between mRNA expression levels of HIF-1α and
VEGF-A. Preliminary analyses revealed the association to be
monotonic, as assessed by visual inspection of a scatterplot.
Pearson's correlation could not be used, as the variables were not
normally distributed, as assessed by a Shapiro-Wilk test
(P>0.05). Six outliers were recognized and removed from the
analyzed group. There was a moderate positive correlation between
mRNA expression levels of HIF-1α and VEGF-A (rs=0.392,
P<0.005).
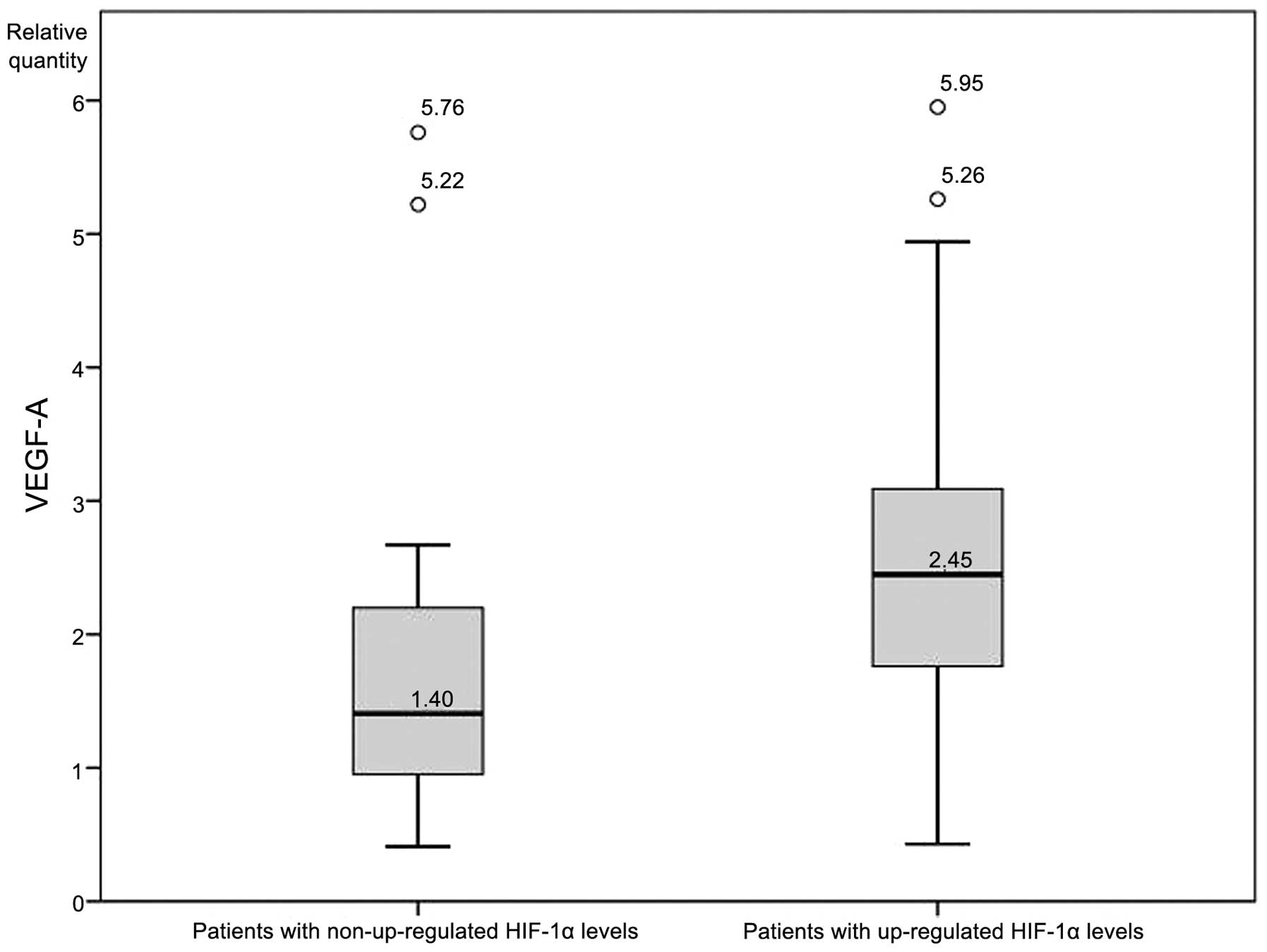
An independent-samples t-test was conducted to
determine whether expression levels of VEGF-A differed
significantly between patients with and without upregulated HIF-1α.
VEGF-A expression was significantly higher (P<0.05; Fig. 2) in patients with upregulated HIF-1α
(2.72±1.41 RQ) compared with patients without upregulated HIF-1α
(1.86±1.46 RQ). There was homogeneity of variances, as assessed by
Levene's test for equality of variances (P=0.813), while no
significant differences in VEGF-A levels between patients with and
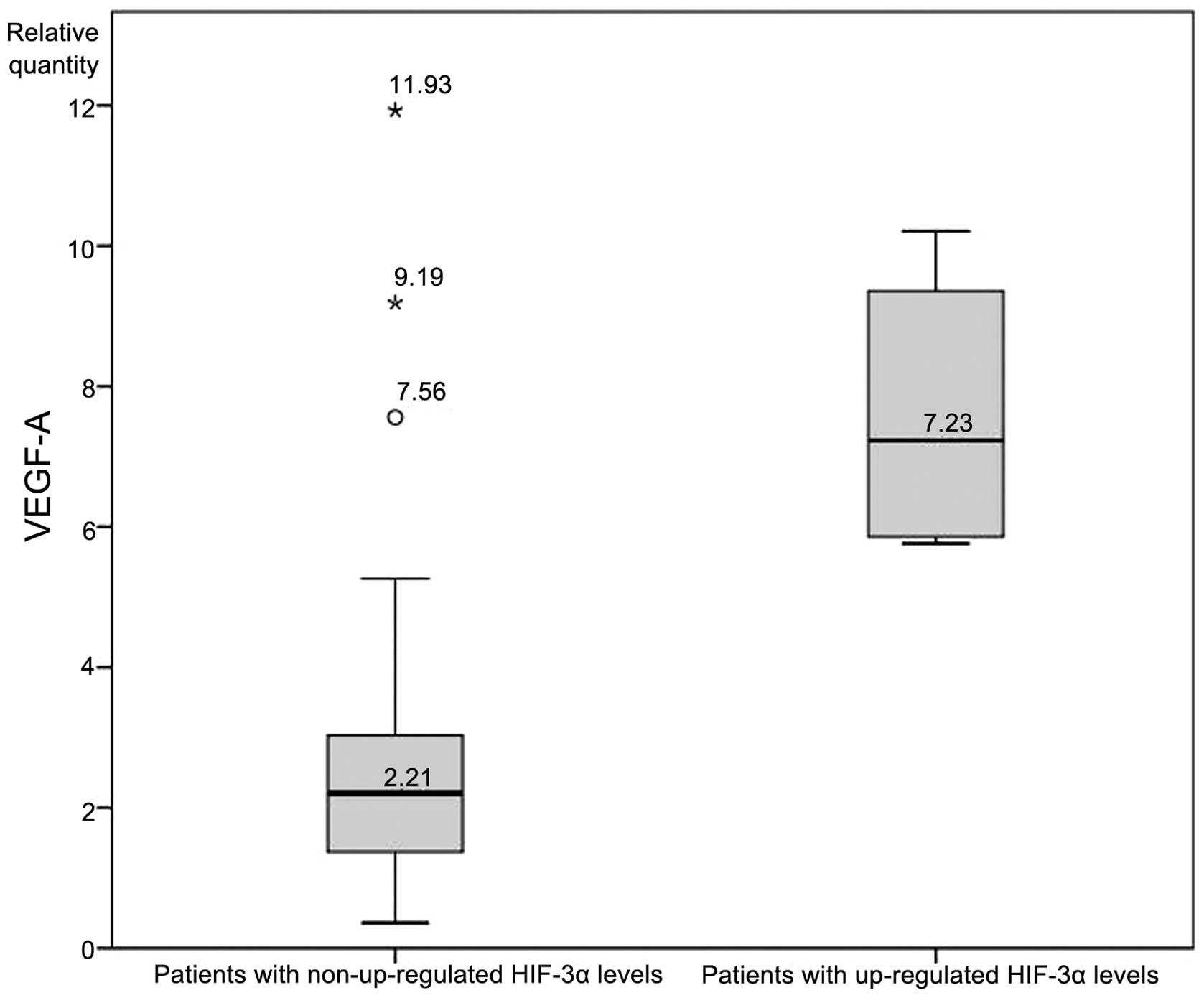
without upregulated HIF-2α were identified. Finally, an analysis of
VEGF-A levels between patients with and without upregulated HIF-3α
revealed a statistically significant difference (7.61±2.14 vs.
2.66±2.13, respectively; Fig. 3).
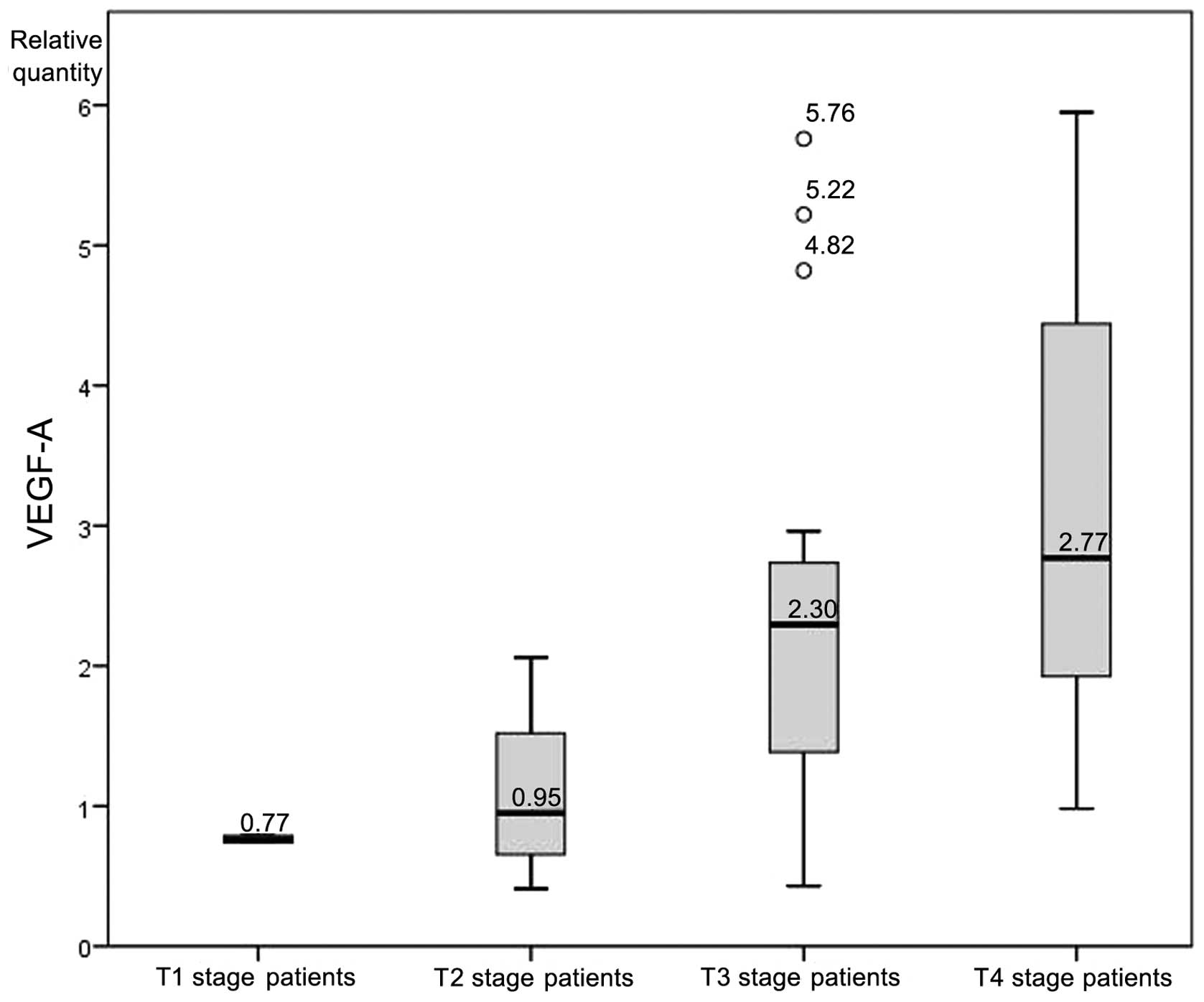
A one-way analysis of variance was conducted to
determine whether the levels of VEGF-A mRNA expression differed
between patients of different T stages. Participants were
classified into four groups: T1 stage (n=2), T2 stage (n=7), T3
stage (n=22) and T4 stage (n=31). Six outliers were recognized and
removed from the analyzed group. There was homogeneity of
variances, as assessed by Levene's test of homogeneity of variances
(P=0.120). Data is presented as the mean ± standard deviation. The
VEGF-A expression rate was significantly different between the
groups (F=4.79, P=0.005). VEGF-A expression increased from T1 stage
patients (0.77±0.03), to T2 stage patients (1.11±0.61), to T3 stage
patients (2.37±1.46) to T4 stage patients (2.98±1.40) (Fig. 4).
Discussion
To the best of our knowledge, the presents study is
the first to investigate the mRNA expression levels of all three
isoforms of the HIF family in carcinoma tissue samples. Following a
review of the literature, only 13 studies on the topic of laryngeal
carcinoma and HIF were identified (Table
I). These studies all examined the expression of HIF-1α only,
and none investigated HIF-1α mRNA expression levels from in
vivo samples (30–42). The current study is the first to
present full quantitative data regarding the mRNA expression levels
of HIF-1α and HIF-2α in laryngeal carcinoma samples, and is the
first to report on the mRNA expression levels of HIF-3α in an in
vivo study of a malignant neoplasm.
 | Table I.Summary of previous studies regarding
HIF expression and laryngeal carcinoma. |
Table I.
Summary of previous studies regarding
HIF expression and laryngeal carcinoma.
| First author
(ref.) | Year | n | Type of studied
specimen | Isoforms
studied | Method |
|---|
| Moreno-Galindo
et al (30) | 2014 | 41 | Paraffin-embedded
surgical tissue specimens | HIF-1α | IHC |
| Wachters et
al (31) | 2013 | 60 | Paraffin-embedded
surgical tissue specimens | HIF-1α | IHC |
| Li et al
(32) | 2013 | - | Laryngeal cancer
cell line culture | HIF-1α | RT-PCR and western
blotting |
| Xie et al
(33) | 2013 | 86 | Paraffin-embedded
surgical tissue specimens and laryngeal cancer cell line
culture | HIF-1α | IHC and
RT-qPCR |
| Wu et al
(34) | 2013 | 49 | Paraffin-embedded
surgical tissue specimens | HIF-1α | IHC |
| Li et al
(35) | 2013 | 86 | Paraffin-embedded
surgical tissue specimens and laryngeal cancer cell line
culture | HIF-1α | IHC, RT-qPCR and
western blotting |
| Douglas et
al (36) | 2013 | 286 | Paraffin-embedded
surgical tissue specimens | HIF-1α | IHC |
| Wu et al
(37) | 2010 | 40 | Paraffin-embedded
surgical tissue specimens | HIF-1α | IHC |
| Moon et al
(38) | 2009 | - | Laryngeal cancer
cell line culture | HIF-1α | Western blotting
and immunofluorescence |
| Cabanillas et
al (39) | 2009 | 106 | Paraffin-embedded
surgical tissue specimens | HIF-1α | IHC |
| Wildeman et
al (40) | 2009 | 26 | Paraffin-embedded
surgical tissue specimens | HIF-1α | IHC |
| Kyzas et al
(41) | 2005 | 81 | Paraffin-embedded
surgical tissue specimens | HIF-1α | IHC |
| Yu et al
(42) | 2004 | N/A | Paraffin-embedded
surgical tissue specimens | HIF-1α | IHC |
Analysis of the results revealed a distinctive
expression pattern among the majority of the patients:
Overexpression of HIF-1α (69.84%), normal or downregulated levels
of HIF-2α, and normal or downregulated levels of HIF-3α (92.06%).
HIF-2α was demonstrated to be stabilized at moderate oxygen levels
(2–5% O2), whereas HIF-1α is upregulated only at lower
oxygen levels (0-2% O2) (reviewed in 43). Additionally,
Holmquist-Mengelbier et al (30) demonstrated that HIF-1α is most active
during short periods (2–24 h) of intense hypoxia or anoxia
(<0.1% O2), whereas HIF-2α may be active for a longer
period of mild hypoxia (<5% O2). This phenomenon is
described in the literature as the HIF switch: HIF-1α drives the
initial response to hypoxia, and HIF-2 subsequently takes over the
major role during chronic hypoxic exposure (44–46). The
HIF switch is particularly evident during the development of renal
cell carcinoma, where there is a gradual shift from HIF-1α to
HIF-2α expression with increasing tumor grade (46–48). In
contrast to these findings, the results of the present study
display a lack of such a HIF switch in laryngeal carcinoma: Of the
7 patients with upregulated HIF-2α, 4 also exhibited an
upregulation in HIF-1α levels. Thus only three patients
demonstrated a distinct HIF switch, despite 85.7% of the cohort
having advanced-stage disease (T3 or T4 stage).
The statistically significant association between
overexpression of HIF-1α and VEGF-A is expected, as this is
consistent with the canonical HIF pathway: Overexpression of HIF-1α
triggers the expression of VEGF-A (49). This is also supported by the fact that
VEGF-A levels in the current study were significantly higher in
patients with upregulated HIF-1α expression compared with those
without upregulated HIF-1α expression. Additionally, there was a
statistically significant correlation between the levels of
expression of the two molecules, i.e., there was a quantitative
association between the level of mRNA expression of HIF-1α and
VEGF-A.
Another notable result from the present study was
with regard to the mRNA expression of HIF-3α. HIF-3α is the most
poorly studied isoform of the three, and in the review of the
literature, no other studies were found that investigated in
vivo expression in any malignant neoplasm. In the present
study, the majority of the patients display silenced expression of
HIF-3α, with the exception of a small group of five patients who
exhibited upregulated mRNA expression levels. Compared with the
remaining patients, significantly higher levels of VEGF-A
expression were detected in this group (mean RQ, 7.61±2.14 vs.
2.66±2.13; Fig. 3). Various possible
effects of HIF-3α have been reported due to its multiple splice
variants. Of major significance is the downregulatory function of
HIF-3α on HIF-1α and HIF-2α activity (reviewed in 43). This
indicates that the overexpression of VEGF-A may be a driving force
for the upregulation of HIF-3α, as the latter would act a negative
feedback regulator of the canonical HIF pathway. Despite this,
there were also a few cases in the present cohort of patients in
which a significant upregulation of VEGF-A plus silenced HIF-3α was
detected; other regulatory factors must play a role in these
cases.
Finally, the clinical correlation between VEGF-A
expression and the stage of the tumor may be explained by the
growing size of the lesion and the expansion of the process of
neoangiogenesis, in which VEGF-A is essential (49).
The present study reports, for the first time, full
quantitative data on the expression of all three isoforms of the
HIFs in malignant neoplasms. The findings indicate a specific
phenotype of HIF expression in laryngeal carcinoma, where the HIF
switch is absent. Further investigations are required to uncover
the obscure nature of HIF-3α, and the factors that determine which
isoform, HIF-1α or HIF-2α, would be the major driving force of the
canonical HIF pathway in neoplasms.
Acknowledgements
The present study was partially funded by a grant
from the Medical University of Sofia (grant no. 2-D/2012).
References
|
1
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wiesener MS, Turley H, Allen WE, et al:
Induction of endothelial PAS domain protein-1 by hypoxia:
Characterization and comparison with hypoxia-inducible
factor-1alpha. Blood. 92:2260–2268. 1998.PubMed/NCBI
|
|
3
|
Heidbreder M, Fröhlich F, Jöhren O, et al:
Hypoxia rapidly activates HIF-3alpha mRNA expression. FASEB J.
17:1541–1543. 2003.PubMed/NCBI
|
|
4
|
Li QF, Wang XR, Yang YW and Lin H: Hypoxia
upregulates hypoxia-inducible factor (HIF)-3alpha expression in
lung epithelial cells: Characterization and comparison with
HIF-1alpha. Cell Res. 16:548–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pugh CW, O'Rourke JF, Nagao M, et al:
Activation of hypoxia-inducible factor-1; definition of regulatory
domains within the alpha subunit. J Biol Chem. 272:11205–11214.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ikeda E, Achen MG, Breier G and Risau W:
Hypoxia-induced transcriptional activation and increased mRNA
stability of vascular endothelial growth factor in C6 glioma cells.
J Biol Chem. 270:19761–19766. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forsythe JA, Jiang BH, Iyer NV, et al:
Activation of vascular endothelial growth factor gene transcription
by hypoxia-inducible factor 1. Mol Cell Biol. 16:4604–4613.
1996.PubMed/NCBI
|
|
8
|
Takeda N, Maemura K, Imai Y, et al:
Endothelial PAS domain protein 1 gene promotes angiogenesis through
the transactivation of both vascular endothelial growth factor and
its receptor, Flt-1. Circ Res. 95:146–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gerber HP, Condorelli F, Park J and
Ferrara N: Differential transcriptional regulation of the two
vascular endothelial growth factor receptor genes. Flt-1, but not
Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem.
272:23659–23667. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marti HH and Risau W: Systemic hypoxia
changes the organ-specific distribution of vascular endothelial
growth factor and its receptors. Proc Natl Acad Sci USA.
95:15809–15814. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992.PubMed/NCBI
|
|
12
|
Kertesz N, Wu J, Chen TH, et al: The role
of erythropoietin in regulating angiogenesis. Dev Biol.
276:101–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jaquet K, Krause K, Tawakol-Khodai M,
Geidel S and Kuck KH: Erythropoietin and VEGF exhibit equal
angiogenic potential. Microvasc Res. 64:326–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morita M, Ohneda O, Yamashita T, et al:
HLF/HIF-2alpha is a key factor in retinopathy of prematurity in
association with erythropoietin. EMBO J. 22:1134–1146. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirota K and Semenza GL: Regulation of
angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol.
59:15–26. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu J, de Muinck ED, Zhuang Z, et al:
Endothelial nitric oxide synthase is critical for ischemic
remodeling, mural cell recruitment, and blood flow reserve. Proc
Natl Acad Sci USA. 102:10999–11004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coulet F, Nadaud S, Agrapart M and
Soubrier F: Identification of hypoxia-response element in the human
endothelial nitric-oxide synthase gene promoter. J Biol Chem.
278:46230–46240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao X, Lu X and Feng Q: Deficiency in
endothelial nitric oxide synthase impairs myocardial angiogenesis.
Am J Physiol Heart Circ Physiol. 283:H2371–H2378. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maynard MA, Qi H, Chung J, et al: Multiple
splice variants of the human HIF-3 alpha locus are targets of the
von Hippel-Lindau E3 ubiquitin ligase complex. J Biol Chem.
278:11032–11040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strauss L, Volland D, Kunkel M and
Reichert TE: Dual role of VEGF family members in the pathogenesis
of head and neck cancer (HNSCC): Possible link between angiogenesis
and immune tolerance. Med Sci Monit. 11:BR280–BR292.
2005.PubMed/NCBI
|
|
21
|
Alon T, Hemo I, Itin A, Pe'er J, Stone J
and Keshet E: Vascular endothelial growth factor acts as a survival
factor for newly formed retinal vessels and has implications for
retinopathy of prematurity. Nat Med. 1:1024–1028. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gerber HP, Dixit V and Ferrara N: Vascular
endothelial growth factor induces expression of the antiapoptotic
proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem.
273:13313–13316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gerber HP, McMurtrey A, Kowalski J, et al:
Vascular endothelial growth factor regulates endothelial cell
survival through the phosphatidylinositol 3′-kinase/Akt signal
transduction pathway. Requirement for Flk-1/KDR activation. J Biol
Chem. 273:30336–30343. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benjamin LE, Golijanin D, Itin A, Pode D
and Keshet E: Selective ablation of immature blood vessels in
established human tumors follows vascular endothelial growth factor
withdrawal. J Clin Invest. 103:159–165. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan F, Chen Y, Dellian M, Safabakhsh N,
Ferrara N and Jain RK: Time-dependent vascular regression and
permeability changes in established human tumor xenografts induced
by an anti-vascular endothelial growth factor/vascular permeability
factor antibody. Proc Natl Acad Sci USA. 93:14765–14770. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scrideli CA, Carlotti CG Jr, Okamoto OK,
et al: Gene expression profile analysis of primary glioblastomas
and non-neoplastic brain tissue: Identification of potential target
genes by oligonucleotide microarray and real-time quantitative PCR.
J Neurooncol. 88:281–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Borel F, Han R, Visser A, Petry H, van
Deventer SJ, Jansen PL and Konstantinova P: Réseau Centre de
Ressources Biologiques Foie (French Liver Biobanks Network),
France: Adenosine triphosphate-binding cassette transporter genes
up-regulation in untreated hepatocellular carcinoma is mediated by
cellular microRNAs. Hepatology. 55:821–832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
International Union Against Cancer: Head
and Neck Tumours. In: TNM Classification of Malignant Tumours
(7th). Sobin LH, Gospodarowicz MK and Wittekind C: Wiley-Blackwell.
39–46. 2009.
|
|
30
|
Moreno-Galindo C, Hermsen M,
Garcia-Pedrero JM, Fresno MF, Suarez C and Rodrigo JP: p27 and BCL2
expression predicts response to chemotherapy in head and neck
squamous cell carcinomas. Oral Oncol. 50:128–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wachters JE, Schrijvers ML,
Slagter-Menkema L, Mastik M, de Bock GH, Langendijk JA, et al:
Prognostic significance of HIF-1a, CA-IX, and OPN in T1-T2
laryngeal carcinoma treated with radiotherapy. Laryngoscope.
123:2154–2160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li DW, Dong P, Wang F, Chen XW, Xu CZ and
Zhou L: Hypoxia induced multidrug resistance of laryngeal cancer
cells via hypoxia-inducible factor-1α. Asian Pac J Cancer Prev.
14:4853–4858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie J, Li DW, Chen XW, Wang F and Dong P:
Expression and significance of hypoxia-inducible factor-1α and
MDR1/P-glycoprotein in laryngeal carcinoma tissue and hypoxic Hep-2
cells. Oncol Lett. 6:232–238. 2013.PubMed/NCBI
|
|
34
|
Wu XH, Chen SP, Mao JY, Ji XX, Yao HT and
Zhou SH: Expression and significance of hypoxia-inducible factor-1α
and glucose transporter-1 in laryngeal carcinoma. Oncol Lett.
5:261–266. 2013.PubMed/NCBI
|
|
35
|
Li DW, Zhou L, Jin B, Xie J and Dong P:
Expression and significance of hypoxia-inducible factor-1α and
survivin in laryngeal carcinoma tissue and cells. Otolaryngol Head
Neck Surg. 148:75–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Douglas CM, Bernstein JM, Ormston VE, et
al: Lack of prognostic effect of carbonic anhydrase-9, hypoxia
inducible factor-1α and bcl-2 in 286 patients with early squamous
cell carcinoma of the glottic larynx treated with radiotherapy.
Clin Oncol (R Coll Radiol). 25:59–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu XH, Lu YF, Hu XD, Mao JY, Ji XX, Yao
HT, et al: Expression of hypoxia inducible factor-1α and its
significance in laryngeal carcinoma. J Int Med Res. 38:2040–2046.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moon SY, Chang HW, Roh JL, et al: Using
YC-1 to overcome the radioresistance of hypoxic cancer cells. Oral
Oncol. 45:915–919. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cabanillas R, Rodrigo JP, Secades P, et
al: The relation between hypoxia-inducible factor (HIF)-1alpha
expression with p53 expression and outcome in surgically treated
supraglottic laryngeal cancer. J Surg Oncol. 99:373–378. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wildeman MA, Gibcus JH, Hauptmann M, et
al: Radiotherapy in laryngeal carcinoma: can a panel of 13 markers
predict response? Laryngoscope. 119:316–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kyzas PA, Stefanou D, Batistatou A and
Agnantis NJ: Hypoxia-induced tumor angiogenic pathway in head and
neck cancer: an in vivo study. Cancer Lett. 225:297–304. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu L, Liu Y and Cui Y: Expression of
hypoxia inducible factor-1alpha and its relationship to apoptosis
and proliferation in human laryngeal squamous cell carcinoma. J
Huazhong Univ Sci Technolog Med Sci. 24:636–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: Sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2012.
|
|
44
|
Holmquist-Mengelbier L, Fredlund E,
Löfstedt T, et al: Recruitment of HIF-1alpha and HIF-2alpha to
common target genes is differentially regulated in neuroblastoma:
HIF-2alpha promotes an aggressive phenotype. Cancer Cell.
10:413–423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Koh MY, Lemos R Jr, Liu X and Powis G: The
hypoxia-associated factor switches cells from HIF-1α- to
HIF-2α-dependent signaling promoting stem cell characteristics,
aggressive tumor growth and invasion. Cancer Res. 71:4015–4027.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Koh MY and Powis G: Passing the baton: The
HIF switch. Trends Biochem Sci. 37:364–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Raval RR, Lau KW, Tran MG, Sowter HM,
Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL and Ratcliffe
PJ: Contrasting properties of hypoxia-inducible factor 1 (HIF-1)
and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol
Cell Biol. 25:5675–5686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mandriota SJ, Turner KJ, Davies DR, et al:
HIF activation identifies early lesions in VHL kidneys: Evidence
for site-specific tumor suppressor function in the nephron. Cancer
Cell. 1:459–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fong GH: Mechanisms of adaptive
angiogenesis to tissue hypoxia. Angiogenesis. 11:121–140. 2008.
View Article : Google Scholar : PubMed/NCBI
|