Introduction
Thyroid cancer is the most common endocrine
malignancy (1), with an increasing
incidence rate worldwide (2).
Differentiated thyroid cancer (DTC) accounts for 80–90% of all
cases of thyroid cancer and is primarily responsible for the
increased incidence of this disease (3). By contrast, poorly-differentiated
thyroid cancer (PDTC) represents only 5% of all cases of thyroid
cancer cases (4); however, PDTC is
more aggressive and is associated with worse outcomes compared with
DTC. For patients with advanced DTC that is not suitable for
radioiodine therapy, surgical resection or local therapy with
external-beam radiation, there are only a limited number of
therapeutic strategies available (5).
One-third of all patients with PDTC develop
recurrent disease, and only 30% of patients with distant metastases
may achieve complete remission with radioiodine therapy (6,7). As
recurrent PDTC is aggressive and often associated with poor
outcomes, these patients would primarily benefit from the
development of novel therapeutic strategies (8). Recent studies have revealed information
regarding the biological and molecular mechanisms underlying
thyroid cancer, facilitating the identification of novel molecular
targets for the treatment of the patients with PDTC. At present,
the use of suntinib for the treatment of thyroid cancers is under
investigation (ClinicalTrials.gov identifiers, NCT00510640,
NCT00381641 and NCT00668811), however, there are very few reports
evaluating sunitinib for PDTC.
The present study describes a case of recurrent PDTC
that was not amenable to any currently available therapeutic
strategies, but was successfully treated with sunitinib. Written
informed consent for this case report was obtained from the
patient's family.
Case report
A 49-year-old female was referred to Tianjin Huanhu
Hospital (Tianjin, China) in October 2013 due to the post-operative
recurrence of PDTC and lung metastases.
The patient was initially admitted to the Affiliated
Hospital of Inner Mongolia Medical College (Hohhot, Mongolia) in
September 2012 with neck masses. The patient underwent resection of
the right lobe of the thyroid and isthmus, as well as subtotal
resection of the left lobe. A pathological examination revealed
that the 40×30×40-mm tumor in the right lobe was a
poorly-differentiated papillary thyroid carcinoma with partial
neuroendocrine large-cell undifferentiated carcinoma and
Hashimoto's thyroiditis, a type of PDTC. The tumor was
immunoreactive to thyroid transcription factor 1, thyroglobulin and
cytokeratin 19, with a Ki-67 labeling index of <70%. Otherwise,
the left thyroid lobe and isthmus lesions were benign. The patient
opted for treatment with herbal and conservative traditional
Chinese medicine alone (drug name and dose unknown), which was
administered by a family doctor, as opposed to radiotherapy and/or
chemotherapy due to the patient's concerns regarding possible
side-effects. Two and a half months after surgery, fluorine-18
fluorodeoxyglucose positron emission tomography/computed tomography
(CT) revealed tumor recurrence in the resection site and metastases
in the posterior basal segment of the lower lobe of the right lung.
However, the patient insisted on the continuation of traditional
Chinese medicine. The right neck mass became ulcerous in August
2013, with dyspnea appearing one month later and gradually
worsening. The patient was eventually admitted to Tianjin Huanhu
Hospital for further diagnosis and treatment. Despite weight loss
of 10 kg over two months, the patient's disposition, diet and
overall physical condition were adequate. In additional to
traditional Chinese medicine, the patient had been taking thyroid
tablets (dose, 120 mg daily) for hypothyroidism since undergoing
surgery. Furthermore, the patient's diabetes had been managed with
routine medication for three years, and no other medical problems
or family history of malignancy were noted.
The patient's voice sounded hoarse with orthopnea
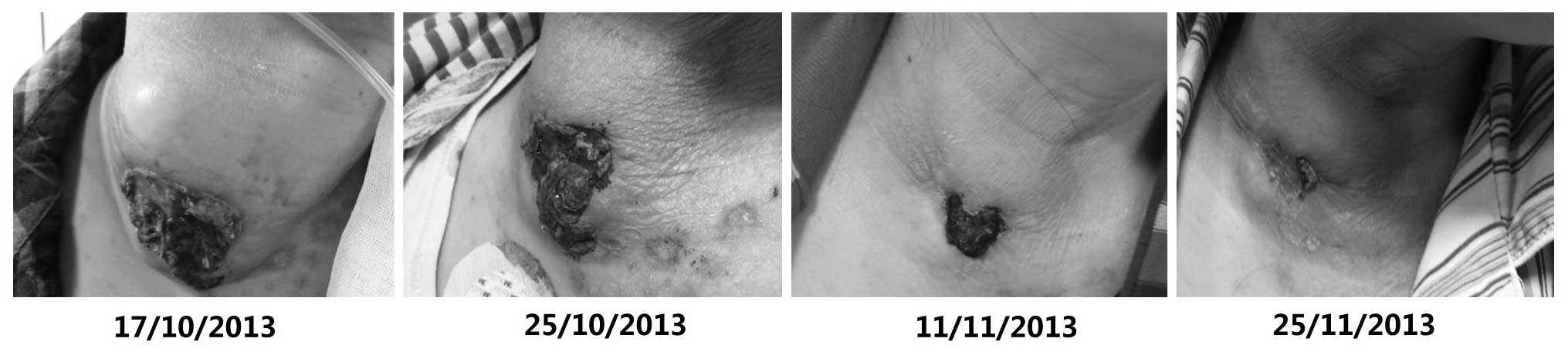
upon physical examination. A large right neck mass was clearly
visible, measuring 10×10 cm, and an area of skin ulceration of ~5×5
cm was present on the surface in the middle of the mass. A decrease
in respiratory sounds was found for each lung and edema was notable
in the right upper limb.
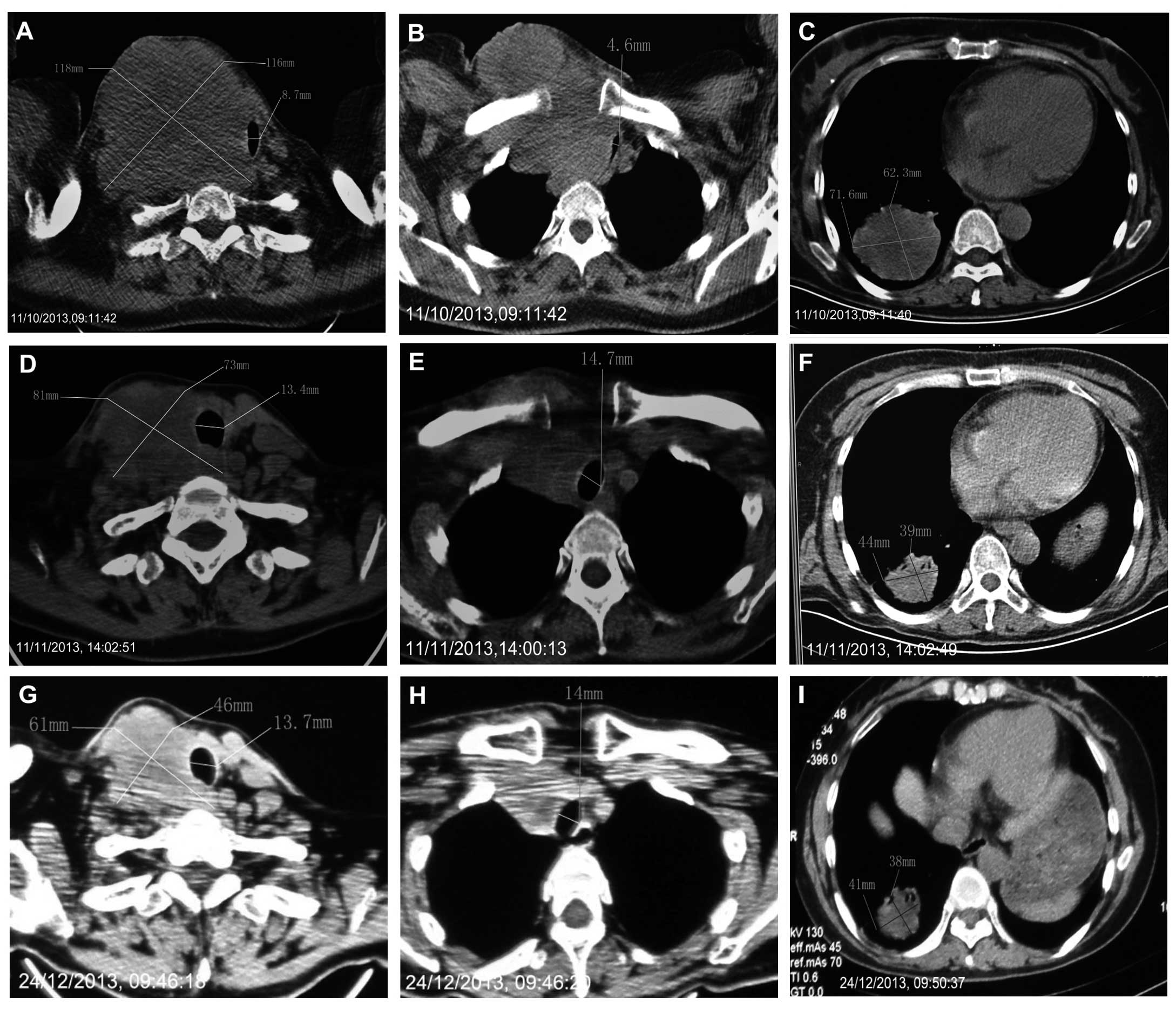
A CT scan revealed a large mass with uneven density
in the right lobe of the thyroid. The trachea was compressed to the
left in the neck, with CT indicating a right supraclavicular
conglomerate of lymph nodes and multiple enlarged lymph nodes
compressed in the tracheoesophageal groove and the right
paratracheal space. Additionally, multiple metastases were present
in the lungs (Fig. 1).
The patient experienced dyspnea and the inability to
lie down. The local recurrence that developed following tumor
resection was considered to be unresectable, therefore, the patient
was selected to undergo treatment with sunitinib. Following
approval by the Institutional Review Board of Tianjin Huanhu
Hospital and receipt of written informed consent from the patient,
sunitinib malate (Sutent®; Pfizer, Inc., New York, NY, USA)
treatment commenced in October 2013 at a dose of 37.5 mg/day.
After only seven days of treatment, the dyspnea
symptoms improved and the patient was able to sleep in a
semi-supine position. The dyspnea completely disappeared one month
later. A lasting response was observed over three months of
therapy, with sunitinib successfully inducing the shrinkage of the
large right neck mass and lung metastases (Figs. 1 and 2).
CT indicated evidence of an objective response, according to the
response evaluation criteria in solid tumors (RECIST) (9), indicating that the current patient
exhibited a partial response to treatment with sunitinib (Fig. 1). During treatment, the patient
experienced mild fatigue and the development of hand/foot syndrome
was unavoidable. Grade III leukopenia was observed one month after
therapy commenced, resulting in the temporary discontinuation of
treatment for four weeks. At the end of January 2014, grade III
thrombocytopenia was observed, thus, sunitinib treatment was
terminated. The tumor eventually progressed and the patient
succumbed to the disease in February 2014. The time to progression
was 3.25 months.
Discussion
Primary thyroid cancer presents as one of four
histological subtypes: Papillary thyroid cancer (PTC), follicular
thyroid cancer (FTC), medullary thyroid cancer (MTC) and anaplastic
thyroid cancer (ATC), with PTC and FTC typically grouped together
as DTC. DTC is conventionally treated using a combined approach of
total thyroidectomy and radioactive iodine (RAI) therapy prior to
thyroid-stimulating hormone suppression. Such an approach generally
results in a good prognosis for patients with DTC, with a five-year
disease-associated survival rate of 97.8% (2).
Despite the good initial outcome of surgery for the
vast majority of patients, 10–15% experience recurrent disease,
with ~5% exhibiting distant metastases at presentation (10). The clinical management of patients
with recurrent and advanced disease is often challenging. Their
response to conventional chemotherapy is typically poor, with
commonly used cytostatic chemotherapeutic agents resulting in
limited and transient response rates of 0–22%, and typically
causing significant toxicity (11).
In certain cases, repeated surgery may control locoregional
disease, however, it is not a viable option in cases of anaplastic
cancer, for which possible treatment strategies are limited due to
almost universally poor outcomes (12). The tumor pathology of the current
patient was complex, consisting of poorly-differentiated papillary
thyroid carcinoma combined with partial neuroendocrine large-cell
undifferentiated carcinoma. Due to the possibly difficult complete
resection of the tumor and the patient's orthopnea, a second
surgical procedure, RAI, external-beam radiation or chemotherapy
were not ideal treatment strategies.
The management of patients with PDTC is challenging
and controversial, with no effective treatment strategies available
prior to the recent development of multi-targeted tyrosine kinase
inhibitors (13). Recent advances in
the understanding of the biology of thyroid cancer, including DTC,
MTC and ATC, have enabled the identification of various important
molecular changes in thyroid cancer cells. These changes provide
the basis for developing targeted therapies that have been
considered for the treatment of thyroid cancer (12).
Various diagnostic and prognostic molecular markers,
including BRAF and RAS point mutations,
RET/PTC and PAX8/PPARγ gene
rearrangements, mitogen-activated protein kinase (MAPK),
phosphoinositide 3-kinase (PI3K), tumor protein p53, Wnt-β catenin,
hypoxia-inducible factor 1α and nuclear factor-κB signaling
pathways, microRNA profiles, and aberrant methylation, have been
identified in >70% of DTC cases (14). Ras/Raf/MEK/ERK (MAPK) and
PI3K/Akt/mammalian target of rapamycin are the two major signaling
pathways indicated to be involved in thyroid cancer, and are
closely associated with the promotion of cell survival, cell cycle
progression, migration, proliferation, metabolism, tumorigenesis
and angiogenesis (15). Furthermore,
the most frequently observed nucleotide substitutions in DTC
involve BRAF, RAS (H-, N- and K-RAS)
and RET (16,17).
BRAF, an important regulator of thyroid
protein expression and cell proliferation, and the most potent
activator of the MAPK pathway, is commonly mutated in thyroid
cancer. Previously, the presence of BRAF V600E has been
associated with a higher prevalence of persistent and recurrent
disease (18). As BRAF has
been indicated to be involved in PTC and ATC, it is the focus of
various studies regarding the development of targeted therapies for
thyroid cancer (12). In addition,
BRAF is associated with the overexpression of vascular
endothelial growth factor (VEGF), a protein with a role in
angiogenesis. VEGF and VEGF receptor (VEGFR), as well as various
growth factors, including platelet-derived growth factor (PDGF) and
fibroblast growth factor, are often overexpressed in thyroid cancer
cells, constituting a second group of possible targets for the
treatment of thyroid cancer (12).
The Ras family of small GTPases is composed of K-,
H- and N-Ras. These G-proteins are fundamental in the transduction
of intracellular signals from the cell membrane, however,
constitutive activation of these three genes has been observed in
all thyroid tumors originating from follicular cells (FTC).
Furthermore, RAS substitutions are frequently detected in
ATC and PDTCs. The most commonly reported RAS mutations in
thyroid tumors are those in codon 61 of N-RAS and in
H-RAS, however, others have been described (19,20).
RET is a proto-oncogene that encodes a
transmembrane tyrosine-kinase receptor. The gene is located on
chromosome 10q11.2 and its activation stimulates various signaling
pathways, including the MAPK cascade. RET is not typically
expressed in the follicular cells of the thyroid gland, however,
germline point mutations of RET have been identified in ~98%
of all hereditary MTC cases and somatic RET mutations are
present in ~40% (range, 30–70%) of sporadic cases (21).
In the current case, RAS, BRAF and
RET mutation status was unavailable, as the patient did not
wish to undergo tumor biopsies. We propose that these genes should
be routinely surveyed in future cases for individualized
therapy.
Sunitinib is an oral tyrosine kinase inhibitor that
targets VEGFR-1, −2 and −3, RET, KIT, PDGF receptors α and β,
FMS-like tyrosine kinase 3 and colony-stimulating factor receptor
type 1 (22). In an initial
open-label, phase II study (23), 43
patients with metastatic, iodine-refractory thyroid carcinoma of
all histological subtypes (37 DTCs and 6 MTCs) exhibited
biochemical and objective RECIST responses to six-week cycles (four
weeks on and two weeks off) of sunitinib at a single daily dose of
50 mg. Of the 31 patients with DTC who completed two cycles of
sunitinib therapy, 13% exhibited a partial response, 68%
experienced stable disease, 10% experienced progressive disease and
9% were not evaluable. Thus, the objective response rate was 13%
and the disease control rate was 81% (23). A second phase II trial (24) of sunitinib (37.5 mg daily) in patients
with progressive DTC (n=28) or MTC (n=7) reported a complete
response in 3% of patients, a partial response in 28% of patients
and stable disease in 46% of patients. Toxicities included fatigue,
neutropenia, hand-foot syndrome, diarrhea and leukopenia. In
addition, one patient who was administered with anticoagulation
medication succumbed due to gastrointestinal bleeding (24). A third open-label, phase II trial
(ClinicalTrials.gov identifier,
NCT00510640) has been completed, however, the study results have
yet to be published. Furthermore, no phase III trial of sunitinib
for the treatment of thyroid cancer has been conducted thus
far.
In the present case, treatment with sunitinib
resulted in a significant reduction in tumor size and caused
symptom amelioration. A noticeable response was observed in the
neck tumor, potentially providing opportunities for additional
treatments. Therefore, the current study indicates that sunitinib
may be a good treatment strategy for similar cases of PDTC.
However, genetic testing should be considered for more
individualized therapy and attention should be focused on possible
adverse reactions, particularly hematological toxicity.
Acknowledgements
The authors would like to thank Dr Li Xiao Long,
Department of Head and Neck Tumors at Tianjin Medical University
Cancer Institute and Hospital (Tianjin, China), for providing
technical assistance. The authors would also like to thank Dr Fu Le
Jun from the Imaging Department at Tianjin Huanhu Hospital
(Tianjin, China) for preparing the tumor images.
References
|
1
|
Agate L, Lorusso L and Elisei R: New and
old knowledge on differentiated thyroid cancer epidemiology and
risk factors. J Endocrinol Invest. 35(Suppl): 3–9. 2012.PubMed/NCBI
|
|
2
|
National Cancer Institute, . Surveillance
Epidemiology and End Results Program: SEER stat fact sheets:
Thyroid cancer. http://seer.cancer.gov/statfacts/html/thyro.html#incidence-mortality15th–
June. 2013
|
|
3
|
Cooper DS, Doherty GM, Haugen BR, et al:
American Thyroid Association (ATA) Guidelines Taskforce on Thyroid
Nodules and Differentiated Thyroid Cancer: Revised American Thyroid
Association management guidelines for patients with thyroid nodules
and differentiated thyroid cancer. Thyroid. 19:1167–1214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brose M, Nutting C, Jarzab B, et al:
Sorafenib in locally advanced or metastatic patients with
radioactive iodine-refractory differentiated thyroid cancer: The
phase III DECISION trial. J Clin Oncol. 31(Suppl): Abstract 4.
2013.
|
|
5
|
DeSantis CE, Lin CC, Mariotto AB, et al:
Cancer treatment and survivorship statistics, 2014. CA Cancer J
Clin. 64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dohán O, De la Vieja A, Paroder V, et al:
The sodium/iodide symporter (NIS): characterization, regulation and
medical significance. Endocr Rev. 24:48–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schlumberger MJ: Diagnostic follow-up of
well-differentiated thyroid carcinoma: historical perspective and
current status. J Endocrinol Invest. 22(Suppl): 3–7.
1999.PubMed/NCBI
|
|
8
|
Durante C, Haddy N, Baudin E, et al:
Long-term outcome of 444 patients with distant metastases from
papillary and follicular thyroid carcinoma: benefits and limits of
radioiodine therapy. J Clin Endocrinol Metab. 91:2892–2899. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perez CA, Santos ES, Arango BA, Raez LE
and Cohen EE: Novel molecular targeted therapies for refractory
thyroid cancer. Head Neck. 34:736–745. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baudin E and Schlumberger M: New
therapeutic approaches for metastatic thyroid carcinoma. Lancet
Oncol. 8:148–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nixon IJ, Shaha AR and Tuttle MR: Targeted
therapy in thyroid cancer. Curr Opin Otolaryngol Head Neck Surg.
21:130–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Antonelli A, Fallahi P, Ferrari SM, et al:
New targeted therapies for thyroid cancer. Curr Genomics.
12:626–631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Omur O and Baran Y: An update on molecular
biology of thyroid cancers. Crit Rev Oncol Hematol. 90:233–252.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barollo S, Bertazza L, Baldini E, et al:
The combination of RAF265, SB590885, ZSTK474 on thyroid cancer cell
lines deeply impact on proliferation and MAPK and PI3K/Akt
signaling pathways. Invest New Drugs. 32:626–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel KN and Shaha AR: Poorly
differentiated thyroid cancer. Curr Opin Otolaryngol Head Neck
Surg. 22:121–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sherman SI: Targeted therapy of thyroid
cancer. Biochem Pharmacol. 80:592–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kebebew E, Weng J, Bauer J, et al: The
prevalence and prognostic value of BRAF mutation in thyroid cancer.
Ann Surg. 246:466–470; discussion 470–461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nikiforova MN, Lynch RA, Biddinger PW, et
al: RAS point mutations and PAX8-PPAR gamma rearrangement in
thyroid tumors: evidence for distinct molecular pathways in thyroid
follicular carcinoma. J Clin Endocrinol Metab. 88:2318–2326. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vasko V, Ferrand M, Di Cristofaro J,
Carayon P, Henry JF and de Micco C: Specific pattern of RAS
oncogene mutations in follicular thyroid tumors. J Clin Endocrinol
Metab. 88:2745–2752. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castellone MD and Santoro M: Dysregulated
RET signaling in thyroid cancer. Endocrinol Metab Clin North Am.
37:363–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sherman SI: Early clinical studies of
novel therapies for thyroid cancers. Endocrinol Metab Clin North
Am. 37:511–524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen EE, Needles BM, Cullen KJ, et al:
Phase 2 study of sunitinib in refractory thyroid cancer. In: ASCO
Meeting Abstracts. 26. pp. 60252008;
|
|
24
|
Carr LL, Mankoff DA, Goulart BH, et al:
Phase II study of daily sunitinib in FDG-PET-positive,
iodine-refractory differentiated thyroid cancer and metastatic
medullary carcinoma of the thyroid with functional imaging
correlation. Clin Cancer Res. 16:5260–5268. 2010. View Article : Google Scholar : PubMed/NCBI
|